Can Transcranial Direct Current Stimulation Enhance Functionality in Older Adults? A Systematic Review
Abstract
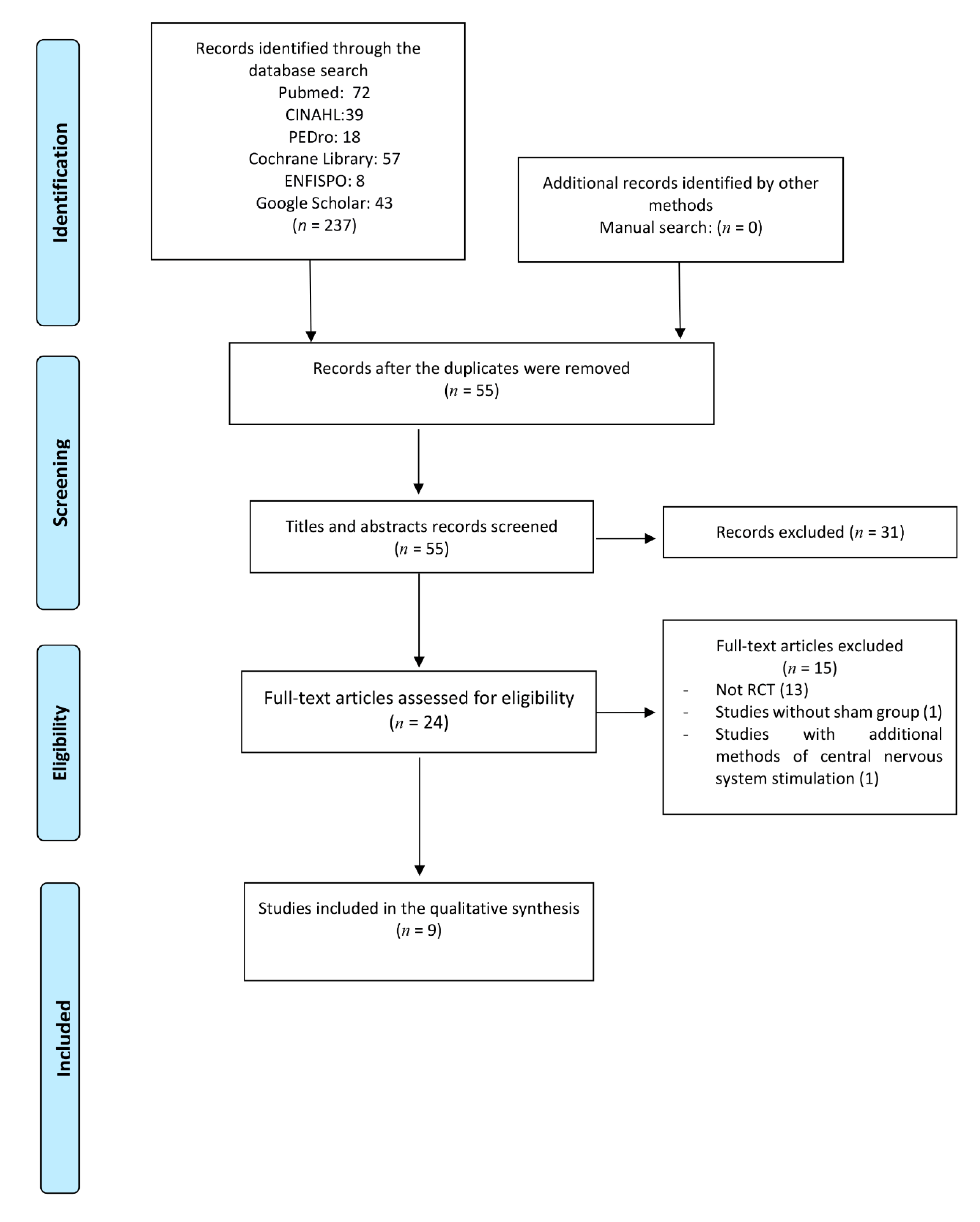
:1. Introduction
2. Materials and Methods
2.1. Study Inclusion Criteria
2.2. Literature Databases and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
3.1. Characteristics of Participants
3.2. Stimulation Patterns and Parameters
3.3. Number of Sessions and Duration of Treatment
3.4. Recorded Variables and tDCS Effect
3.4.1. Effect on Static and Dynamic Stability
3.4.2. Effect on Fine Manual Dexterity
3.4.3. Muscle Strength of Elbow Flexors
3.4.4. Effect on Cognitive Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Paulus, W. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [Green Version]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Pascual-Leone, A. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Pascual-Leone, A. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef]
- Metuki, N.; Sela, T.; Lavidor, M. Enhancing cognitive control components of insight problems solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimul. 2012, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hecht, D.; Walsh, V.; Lavidor, M. Transcranial Direct Current Stimulation Facilitates Decision Making in a Probabilistic Guessing Task. J. Neurosci. 2010, 30, 4241–4245. [Google Scholar] [CrossRef] [Green Version]
- Ragert, P.; Vandermeeren, Y.; Camus, M.; Cohen, L.G. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin. Neurophysiol. 2008, 119, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Reis, J.; Fritsch, B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr. Opin. Neurol. 2011, 24, 590–596. [Google Scholar] [CrossRef]
- Kinsella, K.; Phillips, D.R. Global aging: The challenge of success. Popul. Bull. 2005, 60, 4–7. [Google Scholar]
- Craik, F.I.M.; Bialystok, E. Cognition through the lifespan: Mechanisms of change. Trends Cogn. Sci. 2006, 10, 131–138. [Google Scholar] [CrossRef]
- Kearney, F.C.; Harwood, R.H.; Gladman, J.R.; Lincoln, N.; Masud, T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement. Geriatr. Cogn. Disord. 2013, 36, 20–35. [Google Scholar] [CrossRef]
- Schoene, D.; Valenzuela, T.; Lord, S.R.; de Bruin, E.D. The effect of interactive cognitive-motor training in reducing fall risk in older people: A systematic review. BMC Geriatr. 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, L.; Yaseen, Z.; Kopylev, L.; Cohen, L.G. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2003, 53, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Szameitat, A.J.; Schubert, T.; Müller, K.; Von Cramon, D.Y. Localization of Executive Functions in Dual-Task Performance with fMRI. J. Cogn. Neurosci. 2002, 14, 1184–1199. [Google Scholar] [CrossRef] [Green Version]
- Cutson, T.M. Falls in the elderly. Am. Fam. physician 1994, 49, 149–156. [Google Scholar]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk Factors for Falls among Elderly Persons Living in the Community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Piirtola, M.; Era, P. Force Platform Measurements as Predictors of Falls among Older People—A Review. Gerontology 2006, 52, 1–16. [Google Scholar] [CrossRef]
- Gusi, N.A. Balance training reduces fear of falling and improves dynamic balance and isometric strength in institutionalised older people: A randomised trial. J. Physiother. 2012, 58, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Judge, J.O.; Lindsey, C.; Underwood, M.; Winsemius, D. Balance Improvements in Older Women: Effects of Exercise Training. Phys. Ther. 1993, 73, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Castell, S. Effect of exercise on balance, strength and reaction time in older people. Aust. J. Physiother. 1994, 40, 83–88. [Google Scholar] [CrossRef] [Green Version]
- van Tulder, M.; Furlan, A.; Bombardier, C.M.D.F.; Bouter, L. Editorial Board of the Cochrane Collaboration Back Review GrouUpdated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003, 28, 1290–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharlouei, H.; Sadeghi-Demneh, E.; Mehravar, M.; Manzari, P.; Yazdi, M.J.S.; Joghataei, M.T.; Jaberzadeh, S. Comparison of Transcranial Direct Current Stimulation of the Primary Motor Cortex and Cerebellum on Static Balance in Older Adults. Iran. Red Crescent Med. J. 2020, 22. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, E.; Hoff, M.; Rjosk, V.; Steele, C.J.; Gundlach, C.; Sehm, B.; Ragert, P. Anodal Transcranial Direct Current Stimulation Does Not Facilitate Dynamic Balance Task Learning in Healthy Old Adults. Front. Hum. Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Ljubisavljevic, M.R.; Oommen, J.; Filipovic, S.; Bjekic, J.; Szolics, M.; Nagelkerke, N. Effects of tDCS of Dorsolateral Prefrontal Cortex on Dual-Task Performance Involving Manual Dexterity and Cognitive Task in Healthy Older Adults. Front. Aging Neurosci. 2019, 11, 144. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Harrison, R.; Lo, O.Y.; Travison, T.G.; Hausdorff, J.M.; Lipsitz, L. Transcranial Direct Current Stimulation May Improve Cognitive-Motor Function in Functionally Limited Older Adults. Neurorehabilit. Neural Repair 2018, 32, 788–798. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Jor’dan, A.; Zhang, J.; Fang, J.; Pascual-Leone, A. Reduction of Dual-task Costs by Noninvasive Modulation of Prefrontal Activity in Healthy Elders. J. Cogn. Neurosci. 2016, 28, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Oki, K.; Clark, L.A.; Amano, S.; Clark, B.C. Effect of Anodal Transcranial Direct Current Stimulation of the Motor Cortex on Elbow Flexor Muscle Strength in the Very Old. J. Geriatr. Phys. Ther. 2019, 42, 243–248. [Google Scholar] [CrossRef]
- Yosephi, M.H.; Ehsani, F.; Zoghi, M.; Jaberzadeh, S. Multi-session anodal tDCS enhances the effects of postural training on balance and postural stability in older adults with high fall risk: Primary motor cortex versus cerebellar stimulation. Brain Stimul. 2018, 11, 1239–1250. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, J.; Chen, H.; Manor, B.; Lin, J.; Zhang, J. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp. Brain Res. 2015, 233, 2401–2409. [Google Scholar] [CrossRef]
- Zhou, J.; Lo, O.Y.; Lipsitz, L.A.; Zhang, J.; Fang, J.; Manor, B. Transcranial direct current stimulation enhances foot sole somatosensation when standing in older adults. Exp. Brain Res. 2018, 236, 795–802. [Google Scholar] [CrossRef]
- Seeck, M.; Koessler, L.; Bast, T.; Leijten, F.; Michel, C.; Baumgartner, C.; Beniczky, S. The standardized EEG electrode array of the IFCN. Clin. Neurophysiol. 2017, 128, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, S.S.Y.; Wang, J.; Chen, Y.; Chua, E. Transcranial Direct Current Stimulation to Primary Motor Area Improves Hand Dexterity and Selective Attention in Chronic Stroke. Am. J. Phys. Med. Rehabil. 2014, 93, 1057–1064. [Google Scholar] [CrossRef]
- Kan, B.; Dundas, J.E.; Nosaka, K. Effect of transcranial direct current stimulation on elbow flexor maximal voluntary isometric strength and endurance. Appl. Physiol. Nutr. Metab. 2013, 38, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Ranganathan, R.; Kantak, S.S.; Dhaher, Y.Y.; Rymer, W.Z. Anodal Transcranial Direct Current Stimulation Alters Elbow Flexor Muscle Recruitment Strategies. Brain Stimul. 2014, 7, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.M.; Edwards, D.J.; Ruffini, G.; Labar, D.; Stampas, A.; Pascual-Leone, A.; Cortes, M. Intensity Dependent Effects of Transcranial Direct Current Stimulation on Corticospinal Excitability in Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2015, 96, S114–S121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Hanakawa, T.; Honda, M.; Watanabe, K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp. Brain Res. 2009, 196, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Takeda, K.; Otaka, Y.; Kita, K.; Osu, R.; Honda, M.; Watanabe, K. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabilit. Neural Repair 2011, 25, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, E.P.; Santos, L.; Claflin, E.S.; Krishnan, C. Low-level intermittent quadriceps activity during transcranial direct current stimulation facilitates knee extensor force-generating capacity. Neuroscience 2016, 329, 93–97. [Google Scholar] [CrossRef]
- Marquez, J.; Conley, A.; Karayanidis, F.; Lagopoulos, J.; Parsons, M. Anodal direct current stimulation in the healthy aged: Effects determined by the hemisphere stimulated. Restor. Neurol. Neurosci. 2015, 33, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminski, E.; Steele, C.J.; Hoff, M.; Gundlach, C.; Rjosk, V.; Sehm, B.; Ragert, P. Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin. Neurophysiol. 2016, 127, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, H.; Hyde, J.; Hinder, M.R.; Kim, S.J.; McCormack, G.H.; Vickers, J.C.; Summers, J.J. Delayed plastic responses to anodal tDCS in older adults. Front. Aging Neurosci. 2014, 6, 115. [Google Scholar] [PubMed]
- Perceval, G.; Martin, A.K.; Copland, D.A.; Laine, M.; Meinzer, M. Multisession transcranial direct current stimulation facilitates verbal learning and memory consolidation in young and older adults. Brain Lang. 2020, 205, 104788. [Google Scholar] [CrossRef]
- Martin, A.K.; Meinzer, M.; Lindenberg, R.; Sieg, M.M.; Nachtigall, L.; Flöel, A. Effects of Transcranial Direct Current Stimulation on Neural Networks in Young and Older Adults. J. Cogn. Neurosci. 2017, 29, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
| Baharlouei et al., 2020 [25] | Kaminski et al., 2017 [26] | Ljubisavljevic et al., 2019 [27] | Manor et al., 2016 [28] | Manor et al., 2018 [29] | Oki et al., 2019 [30] | Yosephi et al., 2018 [31] | Zhou et al., 2015 [32] | Zhou et al., 2018 [33] | |
|---|---|---|---|---|---|---|---|---|---|
| Eligibility criteria | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Randomized allocation | 11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Concealed allocation | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Basal intergroup similarities | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Blinding of participants | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Blinding of therapists | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| Blinding of assessors | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Follow-up | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Intention-to-treat analysis | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Between-group statistical comparison | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Point measures and variability measures | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total Score | 9/10 | 6/10 | 8/10 | 8/10 | 9/10 | 7/10 | 8/10 | 8/10 | 8/10 |
| Study | Main Electrode | Electrode Size (cm2) | Intensity (mA) | Current Density (mA/cm2) | Location of Stimulation | Duration (min) and Mode | Number of Sessions (Washout Period) |
|---|---|---|---|---|---|---|---|
| Baharlouei et al., 2020 [25] | Anode | a: 27 c: 36 | 2 | a: 0.07 c: 0.05 | M1 | 20 (offline) | 2 (1 week) |
| Kaminski et al., 2017 [26] | Anode | a: 25 c: 50 | 1 | a: 0.04 c: 0.02 | M1 Bilateral | 20 (online) | 1 |
| Ljubisavljevic et al., 2019 [27] | Anode Bilateral | 35 | 1.5 | 0.04 | DLPFC | 30 (10 min online) | 2 (2 weeks) |
| Manor et al., 2016 [28] | Anode | 35 | 2 | 0.06 | DLPFC | 20 (offline) | 2 (1 week) |
| Manor et al., 2018 [29] | Anode | 35 | 2 | 0.06 | DLPFC | 20 (offline) | 10 (5 s/week) |
| Oki et al., 2019 [30] | Anode | 35 | 1.5 | 0.04 | M1 | 20; 17ʹ30ʺ(offline) | 3 (10 days) |
| Yosephi et al., 2018 [31] | Anode | 35 | 2 | 0.06 | M1 | 20 (online) | 6 (3 s/week) |
| Zhou et al., 2015 [32] | Anode | 35 | 2 | 0.06 | DLPFC | 20 (offline) | 2 (1 week) |
| Zhou et al., 2018 [33] | Anode | 35 | 2 | 0.06 | Sensorimotor cortex | 20 (offline) | 2 (1 week) |
| Study | Number of Participants | Age | Study Design | Outcome Measures | Measurement Time Point | Results (Versus Sham tDCS) |
|---|---|---|---|---|---|---|
| Baharlouei et al., 2020 [25] | 32 | 67.6 (6.3) | Double-blinded, sham-controlled, crossover study | CoP displacement, stride length, walking speed | Before and after | ↓ CoP, stride length and velocity |
| Kaminski et al., 2017 [26] | 30 | 67.7 (6.0) | Single-blinded, sham-controlled, randomized crossover study | Time in balance | Before and after | NS |
| Ljubisavljevic et al., 2019 [27] | 22 | 62.6 (3.2) | Double-blinded, sham-controlled, crossover study | Manual dexterity (GPT), Cognitive task (SSST), Dual-task, simple reaction time | Before, during, and after | ↓ Dual-task costs during bilateral tDCS |
| Manor et al., 2016 [28] | 37 | 61 (5.0) | Single-blinded, sham-controlled, randomized crossover study | Dual-task costs while standing, walking, and serial subtraction performance | Before and after | ↓ Dual-task costs while standing and walking |
| Manor et al., 2018 [29] | 19 | 80.5 (4.0) | Double-blinded, sham-controlled, randomized parallel study | MoCA, TUG, dual-task cost, walking speed, sway speed | Before, after, and 2 weeks later | ↑ MoCA, ↓ Dual task standing postural sway, ↓ Stride time dual-task |
| Oki et al., 2019 [30] | 11 | 85.8 (4.3) | Double-blinded, sham-controlled, randomized crossover study | Isometric maximal contractions, EMG activity | Before and after | NS |
| Yosephi et al., 2018 [31] | 65 | 66.1 (4.0) | Double-blinded, sham-controlled, randomized parallel study | Berg Balance Score (BBS), stability indices | Before and after | ↑ Postural stability indices and BBS scores |
| Zhou et al., 2015 [32] | 20 | 63 (3.6) | Double-blinded, sham-controlled, randomized crossover study | CoP fluctuations | Before and after | ↑ Complexity of standing postural sway |
| Zhou et al., 2018 [33] | 20 | 61 (4.0) | Double-blinded, sham-controlled, randomized crossover study | Standing vibratory threshold (SVT), TUG | Before and after | ↓SVT in both soles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino-Esteban, A.; Megía-García, Á.; Martín-Caro Álvarez, D.; Beltran-Alacreu, H.; Avendaño-Coy, J.; Gómez-Soriano, J.; Serrano-Muñoz, D. Can Transcranial Direct Current Stimulation Enhance Functionality in Older Adults? A Systematic Review. J. Clin. Med. 2021, 10, 2981. https://doi.org/10.3390/jcm10132981
Pino-Esteban A, Megía-García Á, Martín-Caro Álvarez D, Beltran-Alacreu H, Avendaño-Coy J, Gómez-Soriano J, Serrano-Muñoz D. Can Transcranial Direct Current Stimulation Enhance Functionality in Older Adults? A Systematic Review. Journal of Clinical Medicine. 2021; 10(13):2981. https://doi.org/10.3390/jcm10132981
Chicago/Turabian StylePino-Esteban, Andrés, Álvaro Megía-García, David Martín-Caro Álvarez, Hector Beltran-Alacreu, Juan Avendaño-Coy, Julio Gómez-Soriano, and Diego Serrano-Muñoz. 2021. "Can Transcranial Direct Current Stimulation Enhance Functionality in Older Adults? A Systematic Review" Journal of Clinical Medicine 10, no. 13: 2981. https://doi.org/10.3390/jcm10132981
APA StylePino-Esteban, A., Megía-García, Á., Martín-Caro Álvarez, D., Beltran-Alacreu, H., Avendaño-Coy, J., Gómez-Soriano, J., & Serrano-Muñoz, D. (2021). Can Transcranial Direct Current Stimulation Enhance Functionality in Older Adults? A Systematic Review. Journal of Clinical Medicine, 10(13), 2981. https://doi.org/10.3390/jcm10132981






