Long-Term Imaging Follow-Up in DIPNECH: Multicenter Experience
Abstract
:1. Introduction
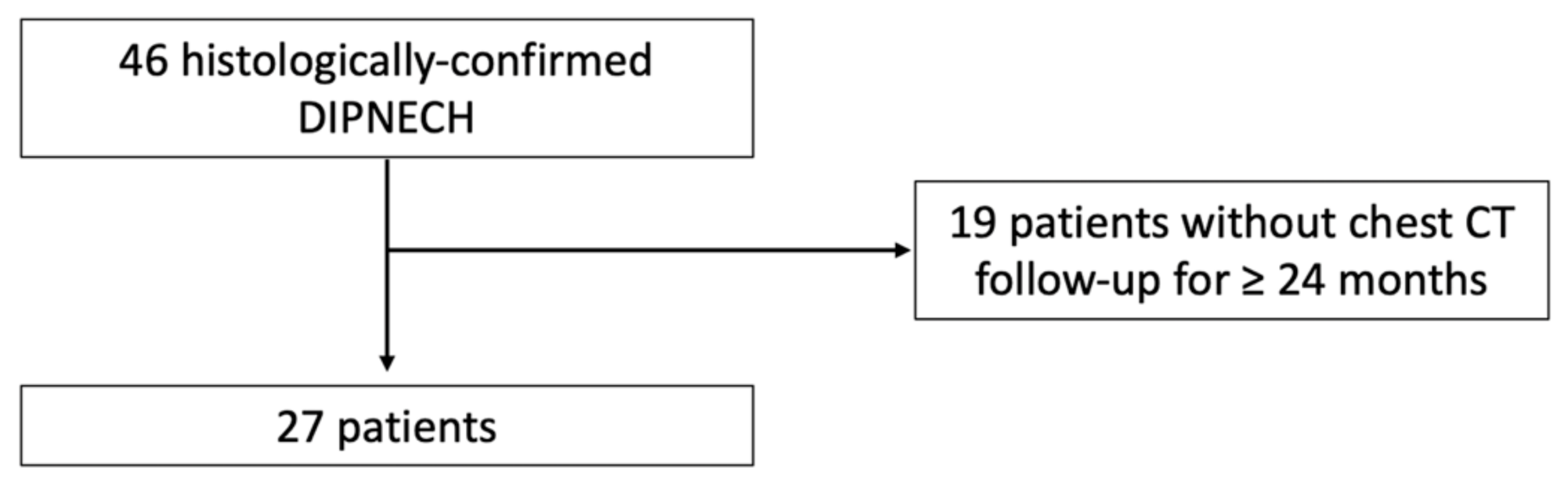
2. Materials and Methods
2.1. Study Population
- either diffuse pulmonary neuroendocrine cells hyperplasia on a surgical biopsy or lung resection specimen;
- or presence of multiple pulmonary nodules on CT with one proven carcinoid lung tumor;
- either symptomatic chronic obstructive airway disease in the absence of other etiology;
- or diffuse mosaic perfusion on CT in the absence of other etiology.
2.2. Image Analysis
2.3. Clinical Data
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics at Baseline
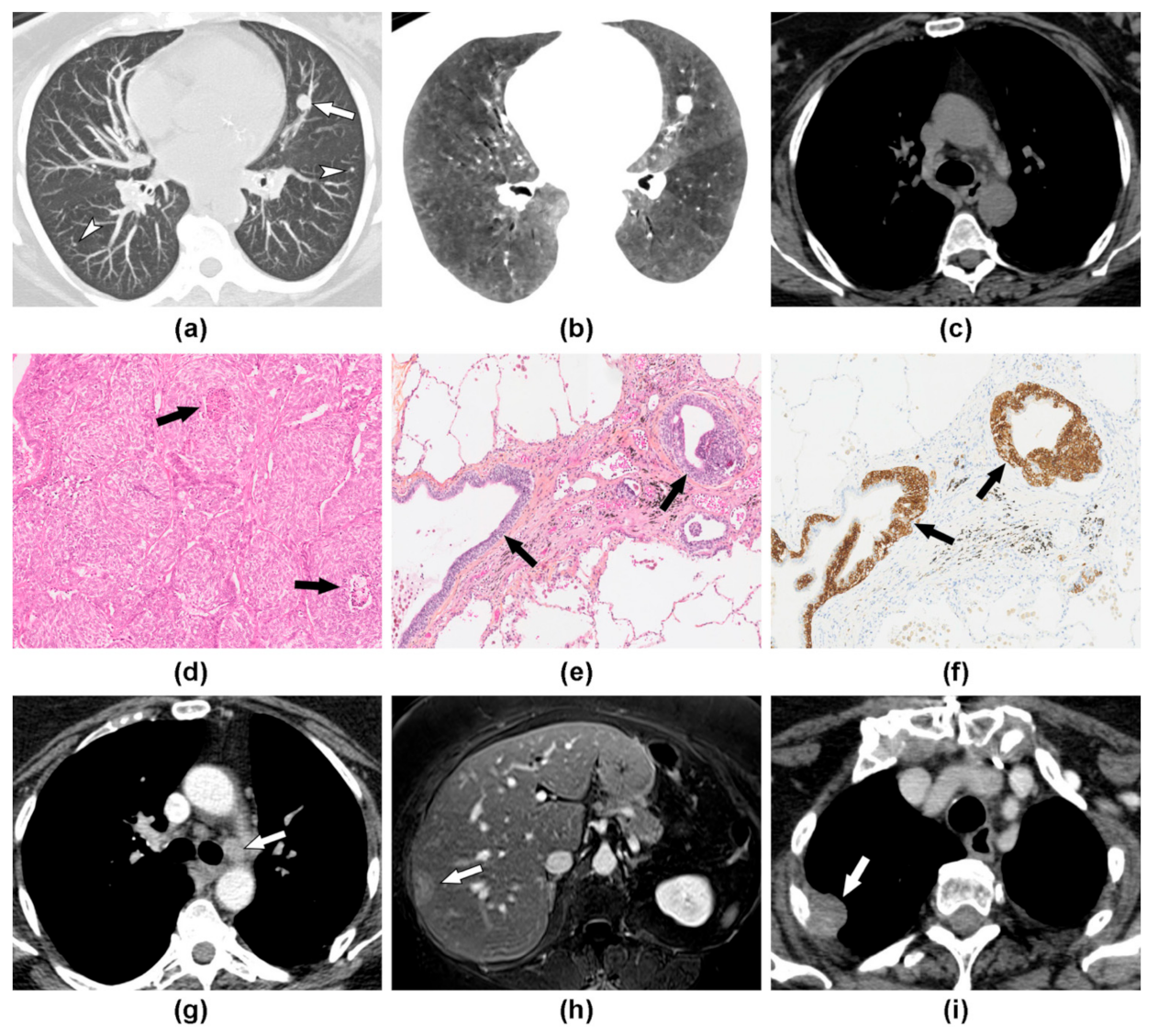
3.2. Imaging Findings at Baseline
3.3. Disease Evolution and Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer, Ed.; World Health Organization Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2015; ISBN 978-92-832-2436-5. [Google Scholar]
- Nassar, A.A.; Jaroszewski, D.E.; Helmers, R.A.; Colby, T.V.; Patel, B.M.; Mookadam, F. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: A Systematic Overview. Am. J. Respir. Crit. Care Med. 2011, 184, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, S.M.; Miller, Y.E.; Waldron, J.A.; Bogin, R.M.; Sunday, M.E.; Staton, G.W.; Beam, W.R.; King, T.E. Brief Report: Idiopathic Diffuse Hyperplasia of Pulmonary Neuroendocrine Cells and Airways Disease. N. Engl. J. Med. 1992, 327, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Bongiovanni, M.; Cavallo, A.; Filosso, P.L.; Giobbe, R.; Mancuso, M.; Molinatti, M.; Oliaro, A. The Significance of Associated Pre-Invasive Lesions in Patients Resected for Primary Lung Neoplasms. Eur. J. Cardio Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2004, 26, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorshtein, A.; Gross, D.J.; Barak, D.; Strenov, Y.; Refaeli, Y.; Shimon, I.; Grozinsky-Glasberg, S. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia and the Associated Lung Neuroendocrine Tumors: Clinical Experience with a Rare Entity. Cancer 2012, 118, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Gosney, J.R.; Hansell, D.M.; Wells, A.U.; du Bois, R.M.; Burke, M.M.; Sheppard, M.N.; Nicholson, A.G. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: An under-Recognised Spectrum of Disease. Thorax 2007, 62, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, G.; Cavazza, A.; Spagnolo, P.; Sverzellati, N.; Longo, L.; Jukna, A.; Montanari, G.; Carbonelli, C.; Vincenzi, G.; Bogina, G.; et al. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia Syndrome. Eur. Respir. J. 2016, 47, 1829–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogg, J.C. Pathophysiology of Airflow Limitation in Chronic Obstructive Pulmonary Disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef]
- Burgel, P.-R. The Role of Small Airways in Obstructive Airway Diseases. Eur. Respir. Rev. 2011, 20, 023–033. [Google Scholar] [CrossRef] [Green Version]
- Brody, A.S. Early Morphologic Changes in the Lungs of Asymptomatic Infants and Young Children with Cystic Fibrosis. J. Pediatr. 2004, 144, 145–146. [Google Scholar] [CrossRef]
- Mall, M.A.; Stahl, M.; Graeber, S.Y.; Sommerburg, O.; Kauczor, H.-U.; Wielpütz, M.O. Early Detection and Sensitive Monitoring of CF Lung Disease: Prospects of Improved and Safer Imaging: Early Detection and Sensitive Monitoring of CF. Pediatr. Pulmonol. 2016, 51, S49–S60. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.M.; Vummidi, D.; Benditt, J.O.; Godwin, J.D.; Pipavath, S. What Is DIPNECH? Clin. Imaging 2012, 36, 647–649. [Google Scholar] [CrossRef]
- Flint, K.; Ye, C.; Henry, T.L. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH) with Liver Metastases. BMJ Case Rep. 2019, 12, e228536. [Google Scholar] [CrossRef]
- Chassagnon, G.; Favelle, O.; Marchand-Adam, S.; De Muret, A.; Revel, M.P. DIPNECH: When to Suggest This Diagnosis on CT. Clin. Radiol. 2015, 70, 317–325. [Google Scholar] [CrossRef]
- Carr, L.L.; Chung, J.H.; Achcar, R.D.; Lesic, Z.; Rho, J.Y.; Yagihashi, K.; Tate, R.M.; Swigris, J.J.; Kern, J.A. The Clinical Course of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia. Chest 2015, 147, 415–422. [Google Scholar] [CrossRef]
- Marchevsky, A.M.; Wirtschafter, E.; Walts, A.E. The Spectrum of Changes in Adults with Multifocal Pulmonary Neuroendocrine Proliferations: What Is the Minimum Set of Pathologic Criteria to Diagnose DIPNECH? Hum. Pathol. 2015, 46, 176–181. [Google Scholar] [CrossRef]
- Aubry, M.-C.; Thomas, C.F.; Jett, J.R.; Swensen, S.J.; Myers, J.L. Significance of Multiple Carcinoid Tumors and Tumorlets in Surgical Lung Specimens. Chest 2007, 131, 1635–1643. [Google Scholar] [CrossRef]
- Myint, Z.W.; McCormick, J.; Chauhan, A.; Behrens, E.; Anthony, L.B. Management of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: Review and a Single Center Experience. Lung 2018, 196, 577–581. [Google Scholar] [CrossRef]
- Mengoli, M.C.; Rossi, G.; Cavazza, A.; Franco, R.; Marino, F.Z.; Migaldi, M.; Gnetti, L.; Silini, E.M.; Ampollini, L.; Tiseo, M.; et al. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH) Syndrome and Carcinoid Tumors with/without NECH: A Clinicopathologic, Radiologic, and Immunomolecular Comparison Study. Am. J. Surg. Pathol. 2018, 1. [Google Scholar] [CrossRef]
- Trisolini, R.; Valentini, I.; Tinelli, C.; Ferrari, M.; Guiducci, G.M.; Parri, S.N.F.; Dalpiaz, G.; Cancellieri, A. DIPNECH: Association between Histopathology and Clinical Presentation. Lung 2016, 194, 243–247. [Google Scholar] [CrossRef]
- Lee, J.S.; Brown, K.K.; Cool, C.; Lynch, D.A. Diffuse Pulmonary Neuroendocrine Cell Hyperplasia: Radiologic and Clinical Features. J. Comput. Assist. Tomogr. 2002, 26, 180–184. [Google Scholar] [CrossRef]
- Ryu, J.H.; Myers, J.L.; Swensen, S.J. Bronchiolar Disorders. Am. J. Respir. Crit. Care Med. 2003, 168, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Abbott, G.F.; Rosado-de-Christenson, M.L.; Rossi, S.E.; Suster, S. Imaging of Small Airways Disease. J. Thorac. Imaging 2009, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Almquist, D.R.; Sonbol, M.B.; Kosiorek, H.; Halfdanarson, T.; Ross, H.J.; Jaroszewski, D. Clinical Characteristics of DIPNECH: A Retrospective Analysis. Chest 2020, S0012369220321942. [Google Scholar] [CrossRef]
- Cusumano, G.; Fournel, L.; Strano, S.; Damotte, D.; Charpentier, M.C.; Galia, A.; Terminella, A.; Nicolosi, M.; Regnard, J.F.; Alifano, M. Surgical Resection for Pulmonary Carcinoid: Long-Term Results of Multicentric Study—The Importance of Pathological N Status, More than We Thought. Lung 2017, 195, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary Neuroendocrine (Carcinoid) Tumors: European Neuroendocrine Tumor Society Expert Consensus and Recommendations for Best Practice for Typical and Atypical Pulmonary Carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef] [PubMed]

| All (n = 27) | Lymphatic or Distant Metastasis (n = 3) | No Lymphatic or Distant Metastasis (n = 24) | p Value * | |
|---|---|---|---|---|
| Female | 25/27 (93) | 3/3 (100%) | 22/24 (92%) | 1 |
| Age * (years) | 63 (59–72) | 62 (61–67) | 64 (59–72) | 0.969 |
| ≥1 atypical carcinoid | 6/27 (22%) | 3/3 (100%) | 3/24 (13%) | 0.005 |
| Circumstances of the Diagnosis: | 0.162 | |||
| Symptomatic patient | 15/24 (62%) | 1/3 (33%) | 14/21 (67%) | |
| Incidental finding | 5/24 (21%) | 1/3 (33%) | 4/21 (19%) | |
| Staging of unrelated neoplasm | 4/24 (17%) | 1/3 (33%) | 3/21 (14%) | |
| Respiratory Symptoms: | 18/22 (82%) | 2/3 (67%) | 16/19 (84%) | 0.470 |
| Cough | 12/22 (55%) | 1/3 (33%) | 11/19 (58%) | 0.571 |
| Dyspnea | 10/22 (45%) | 0/3 (0%) | 10/19 (53%) | 0.221 |
| Other respiratory symptoms | 8/22 (36%) | 1/3 (33%) | 7/19 (37%) | 1 |
| Non-smoker | 11/20 (55%) | 2/2 (100%) | 9/18 (50%) | 0.479 |
| Baseline PFT: | ||||
| Obstructive syndrome | 9/18 (50%) | 2/2 (100%) | 7/16 (44%) | 0.471 |
| FEV1 (% pred) | 82 (72–89) | 79 (78–80) | 84 (63–90) | 0.574 |
| All (n = 27) | Lymphatic or Distant Metastasis (n = 3) | No Lymphatic or Distant Metastasis (n = 24) | p Value * | |
|---|---|---|---|---|
| Baseline CT Scan | ||||
| Mosaic perfusion | 27/27 (100%) | 3/3 (100%) | 24/24 (100%) | 1 |
| Pulmonary nodules | 27/27 (100%) | 3/3 (100%) | 24/24 (100%) | 1 |
| Pulmonary nodules < 5 mm | 26/27 (96%) | 3/3 (100%) | 23/24 (96%) | 1 |
| ≥10 nodules | 20/27 (74%) | 2/3 (67%) | 18/24 (75%) | 1 |
| Pulmonary nodules ≥ 5 mm | 24/27 (89%) | 3/3 (100%) | 21/24 (88%) | 1 |
| ≥10 nodules | 3/27 (11%) | 0/3 (0%) | 3/24 (13%) | 1 |
| Size of the largest nodule, mm | 9 (8–13) | 10 (9–11) | 9 (8–17) | 0.611 |
| ≥1 centrally located nodule | 3/27 (11%) | 0/3 (0%) | 3/24 (13%) | 1 |
| ≥1 calcified nodule | 3/27 (11%) | 0/3 (0%) | 3/24 (13%) | 1 |
| Subpleural atelectasis | 18/27 (67%) | 2/3 (67%) | 16/24 (67%) | 1 |
| Mucoid impaction | 10/27 (37%) | 1/3 (33%) | 9/24 (38%) | 1 |
| Bronchial thickening | 9/27 (33%) | 1/3 (33%) | 8/24 (33%) | 1 |
| Bronchiectasis | 5/27 (19%) | 1/3 (33%) | 4/24 (17%) | 0.474 |
| Pulmonary cysts | 1/27 (4%) | 0/3 (0%) | 1/24 (4%) | 1 |
| Lymph node enlargement | 0/27 (0%) | 0/3 (0%) | 0/24 (0%) | 1 |
| Distant metastasis | 0/27 (0%) | 0/3 (0%) | 0/24 (0%) | 1 |
| Interval time between baseline and follow-up chest CT (months) | 63 (31–80) | 101 (62–110) | 62 (32–78) | 0.563 |
| Disease Evolution on CHESt CT | ||||
| Increase in mosaic perfusion | 5/27 (19%) | 0/3 (0%) | 5/24 (21%) | 1 |
| Increase in nodule size | 18/27 (67%) | 3/3 (100%) | 15/24 (63%) | 0.529 |
| Increase in number of nodules | 17/27 (63%) | 3/3 (100%) | 14/24 (58%) | 0.274 |
| Lymph node enlargement | 3/27 (11%) | 3/3 (100%) | 0/24 (0%) | <0.001 |
| Distant metastasis | 2/27 (7%) | 2/3 (67%) | 0/24 (0%) | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.; Bommart, S.; Marchand-Adam, S.; Lederlin, M.; Fournel, L.; Charpentier, M.-C.; Groussin, L.; Wislez, M.; Revel, M.-P.; Chassagnon, G. Long-Term Imaging Follow-Up in DIPNECH: Multicenter Experience. J. Clin. Med. 2021, 10, 2950. https://doi.org/10.3390/jcm10132950
Chung C, Bommart S, Marchand-Adam S, Lederlin M, Fournel L, Charpentier M-C, Groussin L, Wislez M, Revel M-P, Chassagnon G. Long-Term Imaging Follow-Up in DIPNECH: Multicenter Experience. Journal of Clinical Medicine. 2021; 10(13):2950. https://doi.org/10.3390/jcm10132950
Chicago/Turabian StyleChung, Cécile, Sébastien Bommart, Sylvain Marchand-Adam, Mathieu Lederlin, Ludovic Fournel, Marie-Christine Charpentier, Lionel Groussin, Marie Wislez, Marie-Pierre Revel, and Guillaume Chassagnon. 2021. "Long-Term Imaging Follow-Up in DIPNECH: Multicenter Experience" Journal of Clinical Medicine 10, no. 13: 2950. https://doi.org/10.3390/jcm10132950
APA StyleChung, C., Bommart, S., Marchand-Adam, S., Lederlin, M., Fournel, L., Charpentier, M.-C., Groussin, L., Wislez, M., Revel, M.-P., & Chassagnon, G. (2021). Long-Term Imaging Follow-Up in DIPNECH: Multicenter Experience. Journal of Clinical Medicine, 10(13), 2950. https://doi.org/10.3390/jcm10132950






