Interstitial Lung Abnormalities Detected by CT in Asbestos-Exposed Subjects Are More Likely Associated to Age
Abstract
1. Introduction
2. Material and Methods
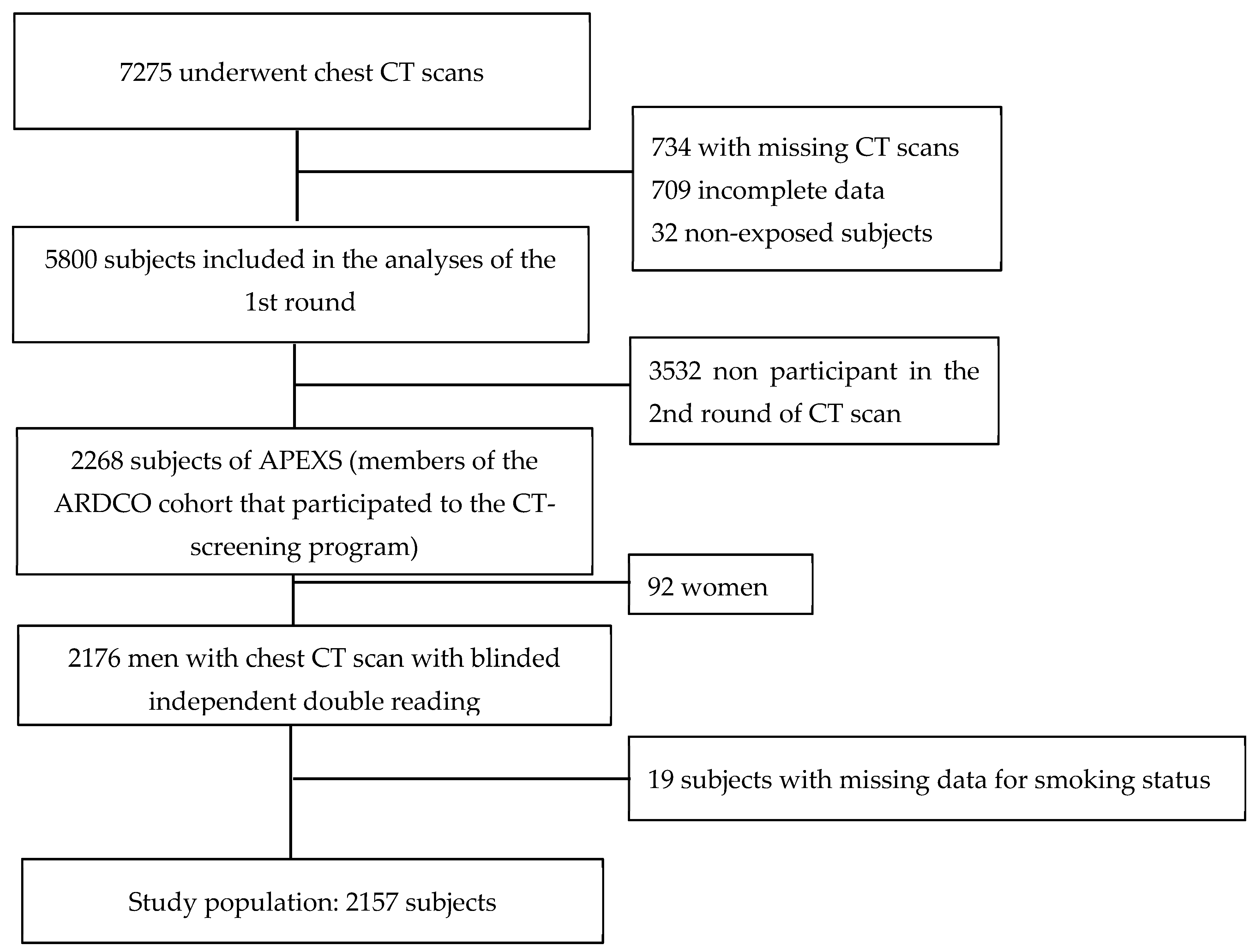
2.1. Study Population
2.2. Asbestos Exposure and Smoking Status
2.3. CT Scanning
2.4. Statistical Analysis
3. Results
3.1. Subjects’ Demographic Data, Smoking Data, Asbestos Exposure and Frequency of Interstitial Abnormalities at CT
3.2. Association between Possible and Definite CT Patterns of UIP and Age, Smoking Status and the Level of Exposure to Asbestos
3.3. Association between CT Severity of Emphysema and Age, Smoking Status and the Level of Exposure to Asbestos
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paris, C.; Thierry, S.; Brochard, P.; Letourneux, M.; Schorlé, E.; Stoufflet, A.; Ameille, J.; Conso, F.; Pairon, J.C.; Members, T.N.A. Pleural plaques and asbestosis: Dose- and time-response relationships based on HRCT data. Eur. Respir. J. 2009, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Gamsu, G.; Ray, C. High-resolution CT of benign asbestos-related diseases: Clinical and radiographic correlation. Am. J. Roentgenol. 1988, 151, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Gamsu, G.; Aberle, D.R. CT findings in pulmonary asbestosis. Am. J. Roentgenol. 1995, 165, 486–487. [Google Scholar] [CrossRef]
- Staples, C.A.; Gamsu, G.; Ray, C.S.; Webb, W.R. High Resolution Computed Tomography and Lung Function in Asbestos-exposed Workers with Normal Chest Radiographs. Am. Rev. Respir. Dis. 1989, 139, 1502–1508. [Google Scholar] [CrossRef]
- Algranti, E.; Mendonça, E.; DeCapitani, E.; Freitas, J.; Silva, H.; Bussacos, M. Non-malignant asbestos-related diseases in Brazilian asbestos-cement workers. Am. J. Ind. Med. 2001, 40, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Benichou, J.; Raffaelli, C.; Genevois, A.; Fournier, L.; Menard, G.; Broessel, N.; Ameille, J.; Brochard, P.; Gillon, J.-C.; et al. Factors associated with early-stage pulmonary fibrosis as determined by high-resolution computed tomography among persons occupationally exposed to asbestos. Scand. J. Work. Environ. Health 2004, 30, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Vierikko, T.; Järvenpää, R.; Toivio, P.; Uitti, J.; Oksa, P.; Lindholm, T.; Vehmas, T. Clinical and HRCT screening of heavily asbestos-exposed workers. Int. Arch. Occup. Environ. Health 2010, 83, 47–54. [Google Scholar] [CrossRef]
- Lederer, D.J.; Enright, P.L.; Kawut, S.M.; Hoffman, E.A.; Hunninghake, G.; Van Beek, E.J.R.; Austin, J.H.M.; Jiang, R.; Lovasi, G.S.; Barr, R.G. Cigarette smoking is associated with subclinical parenchymal lung disease: The Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am. J. Respir. Crit. Care Med. 2009, 180, 407–414. [Google Scholar] [CrossRef]
- Araki, T.; Putman, R.K.; Hatabu, H.; Gao, W.; Dupuis, J.; Latourelle, J.C.; Nishino, M.; Zazueta, O.E.; Kurugol, S.; Ross, J.C.; et al. Development and Progression of Interstitial Lung Abnormalities in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2016, 194, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Putman, R.K.; Hatabu, H.; Araki, T.; Gudmundsson, G.; Gao, W.; Nishino, M.; Okajima, Y.; Dupuis, J.; Latourelle, J.C.; Cho, M.H.; et al. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA 2016, 315, 672–681. [Google Scholar] [CrossRef]
- Copley, S.J.; Wells, A.U.; Hawtin, K.E.; Gibson, D.J.; Hodson, J.M.; Jacques, A.E.T.; Hansell, D.M. Lung Morphology in the Elderly: Comparative CT Study of Subjects over 75 Years Old versus Those under 55 Years Old. Radiology 2009, 251, 566–573. [Google Scholar] [CrossRef]
- Washko, G.R.; Hunninghake, G.M.; Fernandez, I.E.; Nishino, M.; Okajima, Y.; Yamashiro, T.; Ross, J.C.; Estépar, R.S.J.; Lynch, D.A.; Brehm, J.; et al. Lung Volumes and Emphysema in Smokers with Interstitial Lung Abnormalities. New Engl. J. Med. 2011, 364, 897–906. [Google Scholar] [CrossRef]
- Sverzellati, N.; Guerci, L.; Randi, G.; Calabro, E.; La Vecchia, C.; Marchianò, A.; Pesci, A.; Zompatori, M.; Pastorino, U. Interstitial lung diseases in a lung cancer screening trial. Eur. Respir. J. 2011, 38, 392–400. [Google Scholar] [CrossRef]
- Hunninghake, G.M.; Hatabu, H.; Okajima, Y.; Gao, W.; Dupuis, J.; Latourelle, J.; Nishino, M.; Araki, T.; Zazueta, O.E.; Kurugol, S.; et al. MUC5B Promoter Polymorphism and Interstitial Lung Abnormalities. New Engl. J. Med. 2013, 368, 2192–2200. [Google Scholar] [CrossRef]
- Putman, R.K.; Gudmundsson, G.; Axelsson, G.T.; Hida, T.; Honda, O.; Araki, T.; Yanagawa, M.; Nishino, M.; Miller, E.R.; Eiriksdottir, G.; et al. Imaging Patterns Are Associated with Interstitial Lung Abnormality Progression and Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ameille, J.; Letourneux, M.; Paris, C.; Brochard, P.; Stoufflet, A.; Schorlé, E.; Gislard, A.; Laurent, F.; Conso, F.; Pairon, J.-C. Does Asbestos Exposure Cause Airway Obstruction, in the Absence of Confirmed Asbestosis? Am. J. Respir. Crit. Care Med. 2010, 182, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Clin, B.; Luc, A.; Morlais, F.; Paris, C.; Ameille, J.; Brochard, P.; De Girolamo, J.; Gislard, A.; Laurent, F.; Letourneux, M.; et al. Pulmonary nodules detected by thoracic computed tomography scan after exposure to asbestos: Diagnostic significance. Int. J. Tuberc. Lung Dis. 2011, 15, 1707–1714. [Google Scholar] [CrossRef][Green Version]
- Pairon, J.-C.; Laurent, F.; Rinaldo, M.; Clin, B.; Andujar, P.; Ameille, J.; Brochard, P.; Chammings, S.; Ferretti, G.; Salle, F.G.; et al. Pleural Plaques and the Risk of Pleural Mesothelioma. J. Natl. Cancer Inst. 2013, 105, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Royston, P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat. Med. 2000, 19, 1831–1847. [Google Scholar] [CrossRef]
- Vehmas, T.; Kivisaari, L.; Huuskonen, M.S.; Jaakkola, M.S. Scoring CT/HRCT findings among asbestos-exposed workers: Effects of patient’s age, body mass index and common laboratory test results. Eur. Radiol. 2004, 15, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M. Thin-Section CT of the Lungs: The Hinterland of Normal. Radiology 2010, 256, 695–711. [Google Scholar] [CrossRef]
- Jin, G.Y.; Lynch, D.; Chawla, A.; Garg, K.; Tammemagi, M.C.; Sahin, H.; Misumi, S.; Kwon, K.S. Interstitial lung abnormalities in a CT lung cancer screening population: Prevalence and progression rate. Radiology 2013, 268, 563–571. [Google Scholar] [CrossRef]
- Copley, S.J.; Wells, A.U.; Sivakumaran, P.; Rubens, M.B.; Lee, Y.C.G.; Desai, S.; Macdonald, S.L.S.; Thompson, R.I.; Colby, T.V.; Nicholson, A.G.; et al. Asbestosis and Idiopathic Pulmonary Fibrosis: Comparison of Thin-Section CT Features. Radiology 2003, 229, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Laurent, F.; Paris, C.; Ferretti, G.; Beigelman-Aubry, C.; Montaudon, M.; Latrabe, V.; Jankowski, A.; Badachi, Y.; Clin, B.; Gislard, A.; et al. Inter-reader agreement in HRCT detection of pleural plaques and asbestosis in participants with previous occupational exposure to asbestos. Occup. Environ. Med. 2014, 71, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, K.B.; Samet, J.M.; Stidley, C.A.; Colby, T.V.; Waldron, J.A. Cigarette smoking: A risk factor for idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1997, 155, 242–248. [Google Scholar] [CrossRef]
- Huuskonen, O.; Kivisaari, L.; Zitting, A.; Kaleva, S.; Vehmas, T. Emphysema findings associated with heavy asbestos-exposure in high resolution computed tomography of finnish construction workers. J. Occup. Health 2004, 46, 266–271. [Google Scholar] [CrossRef] [PubMed]
| n | % | |
|---|---|---|
| Pleural plaques | ||
| No | 1604 | 74.4 |
| Yes | 553 | 25.6 |
| Interstitial abnormalities | ||
| Absent or gravity-dependent opacities | 1794 | 83.2 |
| Minor interstitial abnormalities | 226 | 10.5 |
| Interstitial pattern inconsistent with UIP | 82 | 3.8 |
| UIP pattern or possible UIP pattern | 55 | 2.5 |
| Emphysema | ||
| None | 1716 | 79.6 |
| Minor (less than 25% of lung volume) | 281 | 13.0 |
| Moderate (25% to 50%) | 104 | 4.8 |
| Severe (more than 50% of lung volume) | 56 | 2.6 |
| Absent or Gravity-Dependent Opacities | Minor Interstitial Abnormalities | Interstitial Abnormalities Inconsistent with UIP | UIP Pattern or Possible UIP Pattern | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Age (years) | ||||||||
| Mean (SD) | 69.4 (5.4) | 71.5 (5.0) | 72.4 (6.7) | 72.3 (4.9) | ||||
| Smoking status | ||||||||
| Non-smoker | 519 | 28.9 | 69 | 30.5 | 22 | 26.8 | 9 | 16.4 |
| Ex-smoker | 1163 | 64.8 | 150 | 66.4 | 56 | 68.3 | 43 | 78.2 |
| Smoker | 112 | 6.2 | 7 | 3.1 | 4 | 4.9 | 3 | 5.5 |
| Maximum Level of Exposure Based on Labor History | ||||||||
| Low + Low intermediate | 518 | 28.9 | 50 | 22.1 | 20 | 24.4 | 17 | 30.9 |
| High intermediate | 843 | 47.0 | 127 | 56.2 | 43 | 52.4 | 17 | 30.9 |
| High | 433 | 24.1 | 49 | 21.7 | 19 | 23.2 | 21 | 38.2 |
| CEI to Asbestos (Unit of Exposure x Years) | ||||||||
| (0–3.3) | 352 | 19.6 | 37 | 16.4 | 12 | 14.6 | 11 | 20.0 |
| (3.3–13.6) | 353 | 19.7 | 35 | 15.5 | 12 | 14.6 | 10 | 18.2 |
| (13.6–32) | 362 | 20.2 | 53 | 23.5 | 22 | 26.8 | 9 | 16.4 |
| (32–64) | 373 | 20.8 | 59 | 26.1 | 20 | 24.4 | 12 | 21.8 |
| (64 and more) | 354 | 19.7 | 42 | 18.6 | 16 | 19.5 | 13 | 23.6 |
| Mean (SD) | 60.6 (99.1) | 58.0 (90.7) | 63.0 (97.3) | 72.4 (110.4) | ||||
| Time Since First Exposure (Years) | ||||||||
| <40 | 120 | 6.7 | 5 | 2.2 | 5 | 6.1 | 3 | 5.5 |
| (40–50) | 623 | 34.7 | 59 | 26.1 | 17 | 20.7 | 14 | 25.5 |
| >50 | 1051 | 58.6 | 162 | 71.7 | 60 | 73.2 | 38 | 69.1 |
| Mean (SD) | 50.2 (7.0) | 52.7 (6.14) | 52.6 (7.2) | 52.4 (7.3) | ||||
| Duration of Exposure to Asbestos (years) | ||||||||
| <10 | 84 | 4.7 | 7 | 3.1 | 2 | 2.4 | 4 | 7.3 |
| (10–20) | 161 | 9.0 | 15 | 6.6 | 6 | 7.3 | 5 | 9.1 |
| (20–30) | 298 | 16.6 | 39 | 17.3 | 7 | 8.5 | 7 | 12.7 |
| (30–40) | 829 | 46.2 | 107 | 47.3 | 46 | 56.1 | 25 | 45.5 |
| >40 | 422 | 23.5 | 58 | 25.7 | 21 | 25.6 | 14 | 25.5 |
| Mean (SD) | 31.9 (10.1) | 32.9 (9.1) | 33.9 (8.8) | 31.7 (11.3) | ||||
| Univariate Model | Multivariate Models | ||
|---|---|---|---|
| OR (IC95%) | OR (IC95%) | OR (IC95%) | |
| Duration of exposure (year) | 0.99 (0.97–1.02) | - | - |
| Time since the first exposure (year) | 1.04 (1.00–1.08) | 1.00 (0.96–1.04) | 1.00 (0.96–1.04) |
| Maximum level of exposure Low + Low intermediate (n = 605) Intermediate high (n = 1030) High (n = 522) | 1 0.58 (0.29–1.15) 1.45 (0.76–2.78) | - | 1 0.58 (0.29–1.15) 1.39 (0.71–2.69) |
| CEI to asbestos (100 units of exposure x years) | 1.12 (0.88–1.42) | 1.09 (0.85–1.40) | - |
| Age at the time of CT examination (year) | 1.08 (1.03–1.13) | 1.08 (1.03–1.13) | 1.08 (1.03–1.13) |
| Smoking status Non-smoker (n = 619) Ex-smoker (n = 1412) Smoker (n = 126) | 1 2.13 (1.03–4.39) 1.65 (0.44–6.19) | 1 2.03 (0.98–4.21) 1.97 (0.52–7.47) | 1 1.92 (0.92–4.00) 1.86 (0.49–7.06) |
| No Emphysema | Minimal Emphysema | Moderate Emphysema (25% to 50%) | Severe Emphysema (More than 50%) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Age (years) | ||||||||
| Mean (SD) | 69.8 (5.4) | 70.1 (5.6) | 69.2 (5.1) | 69.7 (6.2) | ||||
| Smoking Status | ||||||||
| Non smoker | 558 | 32.5 | 51 | 18.1 | 6 | 5.8 | 4 | 7.1 |
| Ex-smoker | 1080 | 62.9 | 208 | 74.0 | 82 | 78.8 | 42 | 75.0 |
| Smoker | 78 | 4.5 | 22 | 7.8 | 16 | 15.4 | 10 | 17.9 |
| Maximum Level of Exposure | ||||||||
| Low + Low intermediate | 500 | 29.1 | 72 | 25.6 | 25 | 24 | 8 | 14.3 |
| High intermediate | 816 | 47.6 | 135 | 48.0 | 55 | 52.9 | 24 | 42.9 |
| High | 400 | 23.3 | 74 | 26.3 | 24 | 23.1 | 24 | 42.9 |
| CEI to Asbestos (Unit of Exposure x Years) | ||||||||
| (0–3.3) | 334 | 19.5 | 54 | 19.2 | 16 | 15.4 | 8 | 14.3 |
| (3.3–13.6) | 337 | 19.6 | 43 | 15.3 | 22 | 21.2 | 8 | 14.3 |
| (13.6–32) | 341 | 19.9 | 61 | 21.7 | 26 | 25.0 | 18 | 32.1 |
| (32–64) | 373 | 21.7 | 64 | 22.8 | 21 | 20.2 | 6 | 10.7 |
| (64 and more) | 331 | 19.3 | 59 | 21.0 | 19 | 18.3 | 16 | 28.6 |
| Mean (SD) | 60.0 (98.3) | 64.2 (100.5) | 60.0 (98.7) | 68.1 (94.6) | ||||
| Time Since First Exposure (Years) | ||||||||
| <40 | 104 | 6.1 | 18 | 6.4 | 4 | 3.8 | 7 | 12.5 |
| (40–50) | 573 | 33.4 | 85 | 30.2 | 36 | 34.6 | 19 | 33.9 |
| >50 | 1039 | 60.5 | 178 | 63.3 | 64 | 61.5 | 30 | 53.6 |
| Mean (SD) | 50.6 (7.1) | 51.0 (6.6) | 50.8 (6.5) | 49.8 (7.9) | ||||
| Duration (Years) | ||||||||
| <10 | 75 | 4.4 | 11 | 3.9 | 7 | 6.7 | 4 | 7.1 |
| (10–20) | 139 | 8.1 | 29 | 10.3 | 10 | 9.6 | 9 | 16.1 |
| (20–30) | 278 | 16.2 | 43 | 15.3 | 20 | 19.2 | 10 | 17.9 |
| (30–40) | 814 | 47.4 | 135 | 48.0 | 38 | 36.5 | 20 | 35.7 |
| >40 | 410 | 23.9 | 63 | 22.4 | 29 | 27.9 | 13 | 23.2 |
| Mean (SD) | 32.2 (9.9) | 32.0 (9.6) | 31.1 (10.9) | 29.2 (11.9) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, F.; Benlala, I.; Dournes, G.; Gramond, C.; Thaon, I.; Clin, B.; Brochard, P.; Gislard, A.; Andujar, P.; Chammings, S.; et al. Interstitial Lung Abnormalities Detected by CT in Asbestos-Exposed Subjects Are More Likely Associated to Age. J. Clin. Med. 2021, 10, 3130. https://doi.org/10.3390/jcm10143130
Laurent F, Benlala I, Dournes G, Gramond C, Thaon I, Clin B, Brochard P, Gislard A, Andujar P, Chammings S, et al. Interstitial Lung Abnormalities Detected by CT in Asbestos-Exposed Subjects Are More Likely Associated to Age. Journal of Clinical Medicine. 2021; 10(14):3130. https://doi.org/10.3390/jcm10143130
Chicago/Turabian StyleLaurent, François, Ilyes Benlala, Gael Dournes, Celine Gramond, Isabelle Thaon, Bénédicte Clin, Patrick Brochard, Antoine Gislard, Pascal Andujar, Soizick Chammings, and et al. 2021. "Interstitial Lung Abnormalities Detected by CT in Asbestos-Exposed Subjects Are More Likely Associated to Age" Journal of Clinical Medicine 10, no. 14: 3130. https://doi.org/10.3390/jcm10143130
APA StyleLaurent, F., Benlala, I., Dournes, G., Gramond, C., Thaon, I., Clin, B., Brochard, P., Gislard, A., Andujar, P., Chammings, S., Gallet, J., Lacourt, A., Delva, F., Paris, C., Ferretti, G., & Pairon, J.-C. (2021). Interstitial Lung Abnormalities Detected by CT in Asbestos-Exposed Subjects Are More Likely Associated to Age. Journal of Clinical Medicine, 10(14), 3130. https://doi.org/10.3390/jcm10143130







