Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Clinical Characteristics and Pregnancy Outcomes
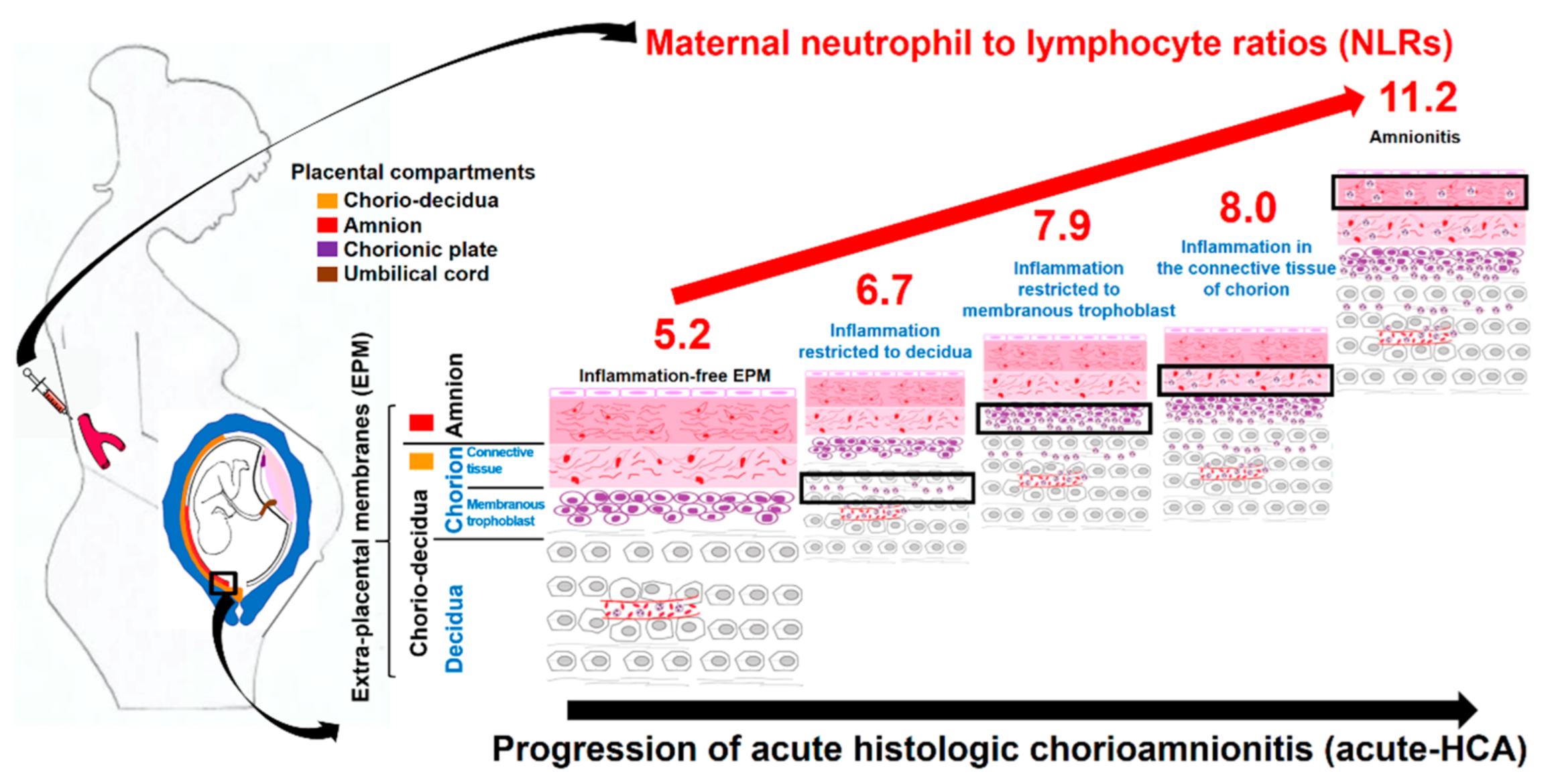
2.3. Diagnosis of Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
2.4. Maternal Neutrophil to Lymphocyte Ratio (NLR)
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Pregnancy Outcomes According to the Progression of Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
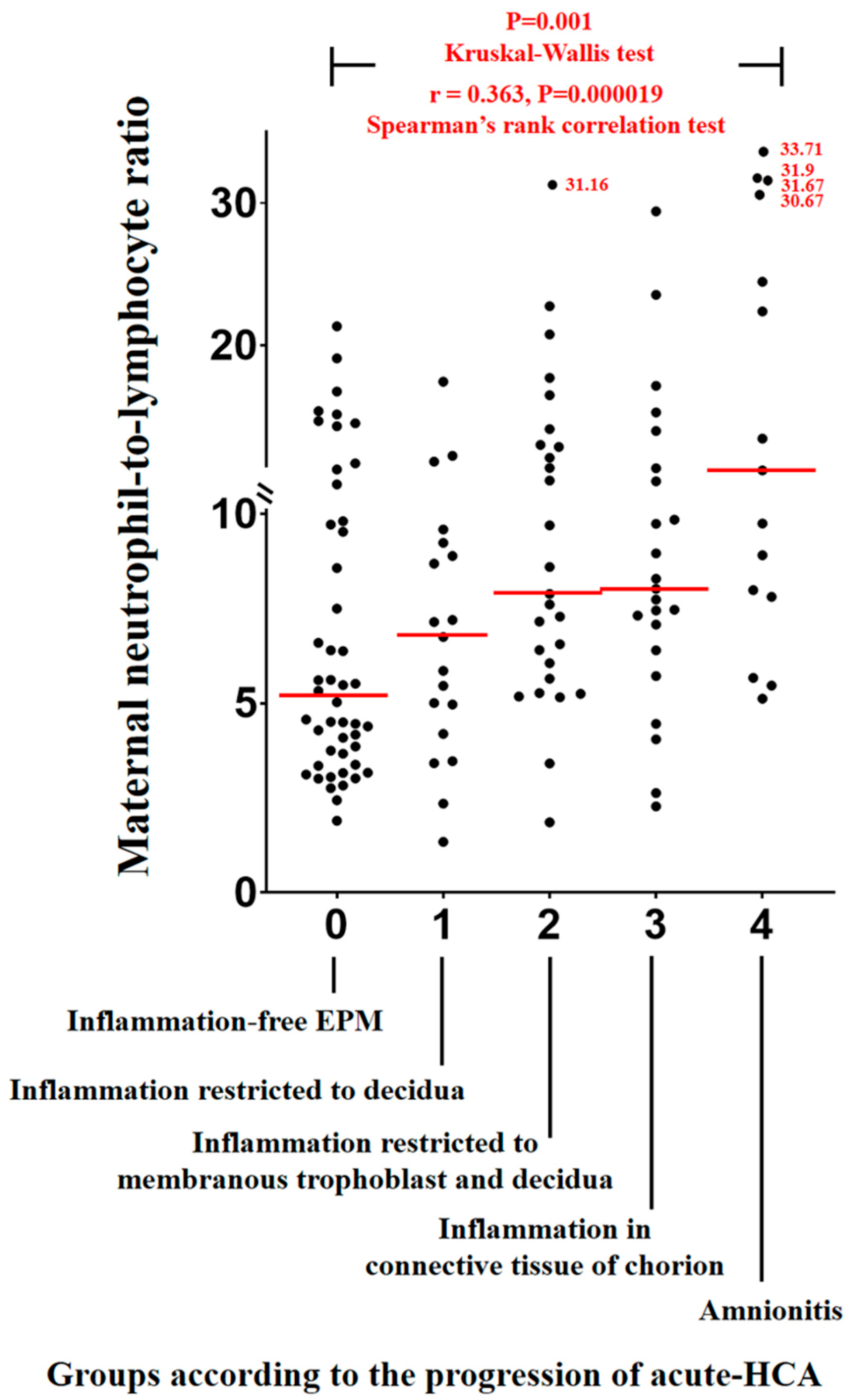
3.2. Maternal Neutrophil to Lymphocyte Ratios (NLRs) According to the Progression of Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
3.3. Diagnostic Indices, Predictive Values, and Likelihood Ratios of Increased Maternal Neutrophil to Lymphocyte Ratio (NLR) for the Identification of Amnionitis
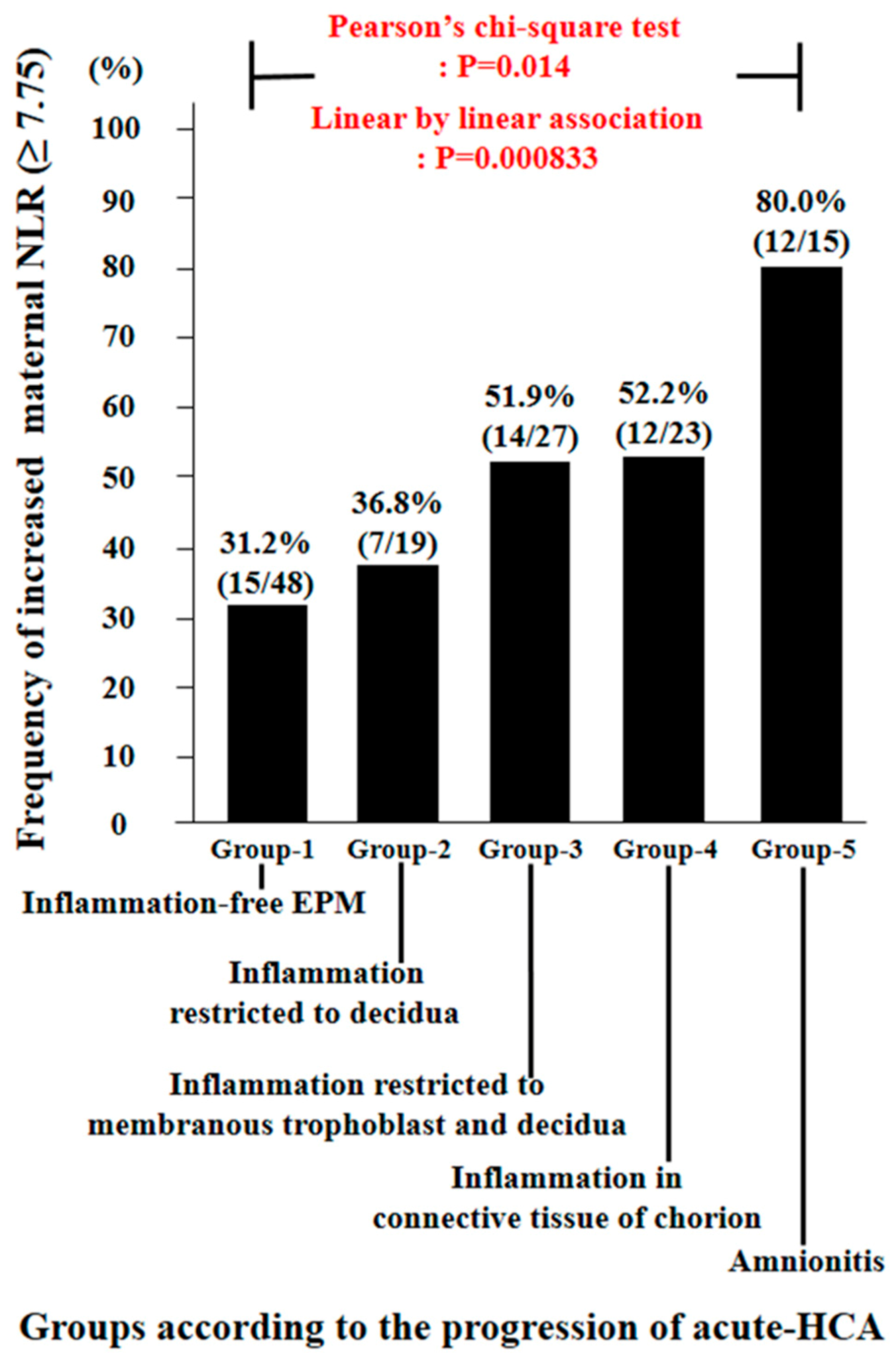
3.4. The Frequency of Increased Maternal Neutrophil to Lymphocyte Ratio (NLR) According to the Progression of Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
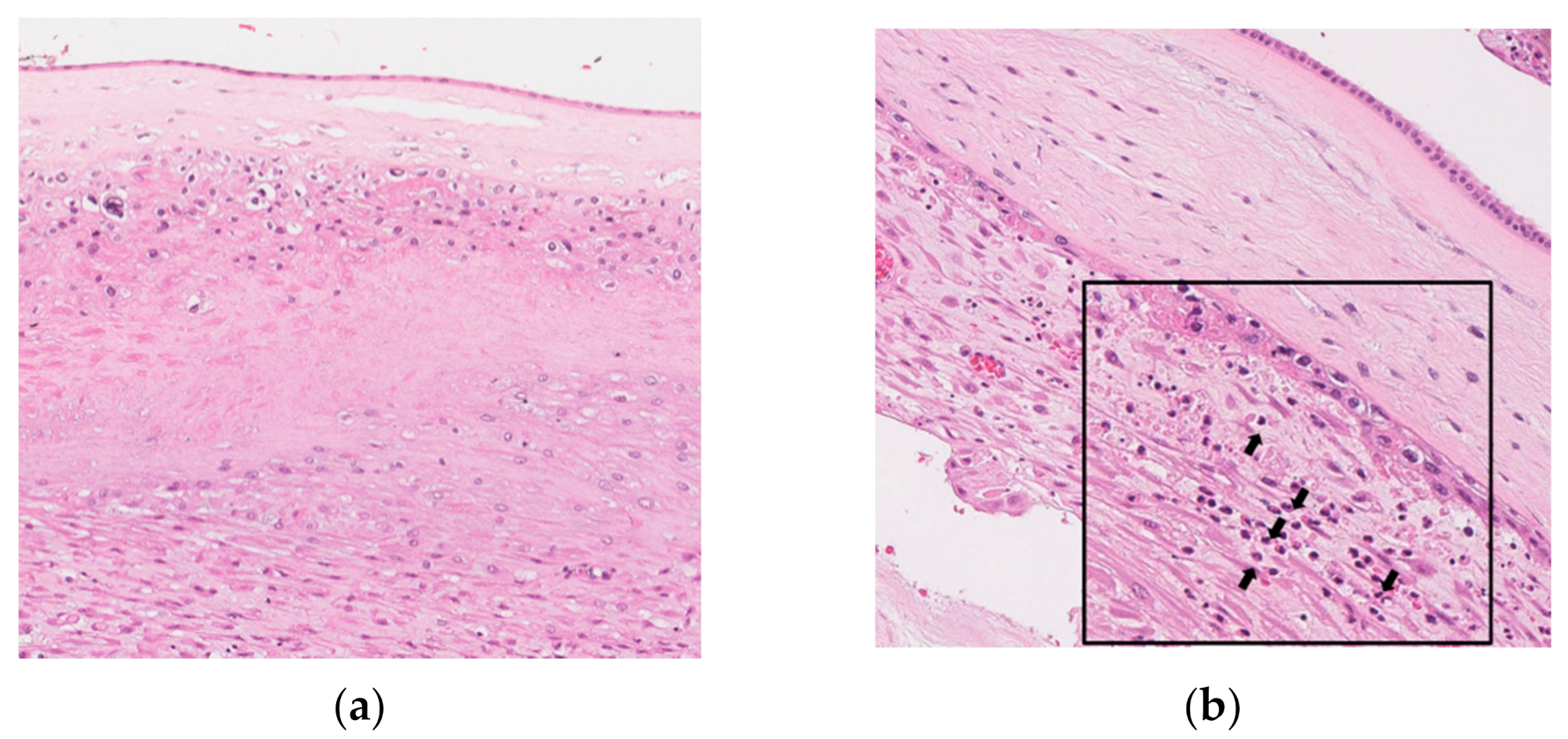
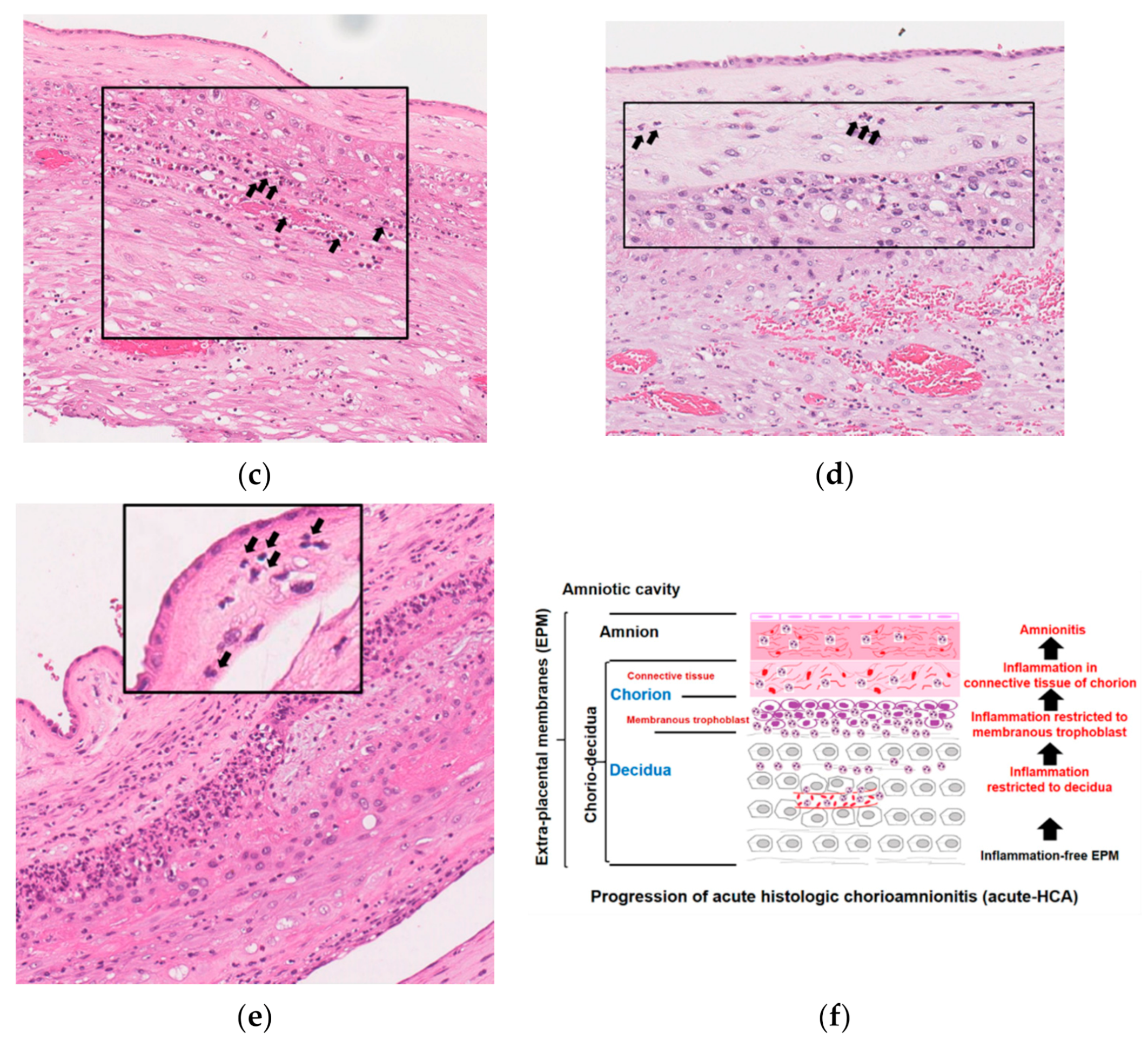
3.5. Histopathology and Schema of the Progression of Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
4. Discussion
4.1. Principal Findings of This Study
4.2. Limitations of Previous Studies Reporting the Relationship between Maternal Inflammatory Blood Markers and Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
4.3. The Usefulness of Neutrophil to Lymphocyte Ratio (NLR) as a Maternal Inflammatory Blood Marker during Pregnancy
4.4. Biologic Plausibility about Increased Maternal Inflammatory Blood Markers According to the Progression of Acute Histologic Chorioamnionitis (Acute-HCA) in Extra-Placental Membranes (EPM)
4.5. Major Strengths and Limitation of This Study
4.6. Significance of This Study
4.7. Unanswered Questions and Proposals for Future Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine Infection and Preterm Delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazor, M. Infection and Preterm Labor. Clin. Obstet. Gynecol. 1988, 31, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Romero, R.; Ghezzi, F.; Yoon, B.H.; Mazor, M.; Berry, S.M. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 1998, 179, 194–202. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Redline, R.W.; Faye-Petersen, O.; Heller, D.; Qureshi, F.; Savell, V.; Vogler, C. Amniotic Infection Syndrome: Nosology and Reproducibility of Placental Reaction Patterns. Pediatr. Dev. Pathol. 2003, 6, 435–448. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Hyunyoon, B.; Kwanjun, J.; Hoonpark, K.; Chulsyn, H.; Gomez, R.; Romero, R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet. Gynecol. 1996, 88, 1034–1040. [Google Scholar] [CrossRef]
- Yoon, B.; Yang, S.; Jun, J.; Park, K.; Kim, C.; Romero, R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: A comparison with amniotic fluid white blood cell count. Obstet. Gynecol. 1996, 87, 231–237. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Bin Moon, J.; Shim, S.-S.; Kim, M.; Kim, G.; Jun, J.K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef]
- Shim, S.-S.; Romero, R.; Hong, J.-S.; Park, C.-W.; Jun, J.K.; Kim, B.I.; Yoon, B.H. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2004, 191, 1339–1345. [Google Scholar] [CrossRef]
- Oh, J.-W.; Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K. Inflammation in the connective-tissue of chorion, but not inflammation restricted to the trophoblast-layer of chorion and the decidua, is associated with the development of amnionitis and more intense acute-histologic chorioamnionitis in the context of choriodeciduitis. Placenta 2017, 57, 327–328. [Google Scholar] [CrossRef]
- Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K.; Romero, R.; Yoon, B.H. The Involvement of Human Amnion in Histologic Chorioamnionitis is an Indicator that a Fetal and an Intra-Amniotic Inflammatory Response is more Likely and Severe: Clinical Implications. Placenta 2009, 30, 56–61. [Google Scholar] [CrossRef]
- Kidokoro, K.; Furuhashi, M.; Kuno, N.; Ishikawa, K. Amniotic fluid neutrophil elastase and lactate dehydrogenase: Association with histologic chorioamnionitis. Acta Obstet. Gynecol. Scand. 2006, 85, 669–674. [Google Scholar] [CrossRef]
- Erdemir, G.; Kultursay, N.; Calkavur, S.; Zekioğlu, O.; Koroglu, O.A.; Cakmak, B.; Yalaz, M.; Akisu, M.; Sagol, S. Histological Chorioamnionitis: Effects on Premature Delivery and Neonatal Prognosis. Pediatr. Neonatol. 2013, 54, 267–274. [Google Scholar] [CrossRef]
- Park, C.-W.; Yoon, B.H.; Park, J.S.; Jun, J.K. An elevated maternal serum C-reactive protein in the context of intra-amniotic inflammation is an indicator that the development of amnionitis, an intense fetal and AF inflammatory response are likely in patients with preterm labor: Clinical implications. J. Matern. Neonatal Med. 2013, 26, 847–853. [Google Scholar] [CrossRef]
- Kim, M.-A.; Lee, Y.S.; Seo, K. Assessment of Predictive Markers for Placental Inflammatory Response in Preterm Births. PLoS ONE 2014, 9, e107880. [Google Scholar] [CrossRef]
- Martinez-Portilla, R.J.; Hawkins-Villarreal, A.; Alvarez-Ponce, P.; Chinolla-Arellano, Z.L.; Moreno-Espinosa, A.L.; Sandoval-Mejia, A.L.; Moreno-Uribe, N. Maternal Serum Interleukin-6: A Non-Invasive Predictor of Histological Chorioamnionitis in Women with Preterm-Prelabor Rupture of Membranes. Fetal Diagn. Ther. 2018, 45, 168–175. [Google Scholar] [CrossRef]
- Howman, R.A.; Charles, A.; Jacques, A.; Doherty, D.A.; Simmer, K.; Strunk, T.; Richmond, P.; Cole, C.H.; Burgner, D.P. Inflammatory and Haematological Markers in the Maternal, Umbilical Cord and Infant Circulation in Histological Chorioamnionitis. PLoS ONE 2012, 7, e51836. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, A.; Zhang, W.; Chen, M.; Wang, Y.; Wang, Y.; Zhou, Q. Related Factors and Adverse Neonatal Outcomes in Women with Preterm Premature Rupture of Membranes Complicated by Histologic Chorioamnionitis. Med. Sci. Monit. 2015, 21, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Park, K.H.; Lee, S.M. Non-Invasive Prediction of Histologic Chorioamnionitis in Women with Preterm Premature Rupture of Membranes. Yonsei Med. J. 2016, 57, 461–468. [Google Scholar] [CrossRef]
- Park, J.W.; Park, K.H.; Kook, S.Y.; Jung, Y.M.; Kim, Y.M. Immune biomarkers in maternal plasma to identify histologic chorioamnionitis in women with preterm labor. Arch. Gynecol. Obstet. 2019, 299, 725–732. [Google Scholar] [CrossRef]
- Hackney, D.N.; MacPherson, T.; Dunigan, J.T.; Simhan, H.N. First-trimester maternal plasma concentrations of C-reactive protein in low-risk patients and the subsequent development of chorioamnionitis. Am. J. Perinatol. 2008, 25, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Shen, C.-M.; Wu, Y.-Y.; Yuh, Y.-S.; Kua, K.-E. Subclinical Histologic Chorioamnionitis and Related Clinical and Laboratory Parameters in Preterm Deliveries. Pediatr. Neonatol. 2009, 50, 217–221. [Google Scholar] [CrossRef]
- Yamada, T.; Matsubara, S.; Minakami, H.; Ohkuchi, A.; Hiratsuka, M.; Sato, I. Relation between viability of vaginal polymorphonuclear leukocytes and presence of listologic chorioamnionitis. Acta Obstet. et Gynecol. Scand. 2000, 79, 818–823. [Google Scholar] [CrossRef]
- Kurakazu, M.; Yotsumoto, F.; Arima, H.; Izuchi, D.; Urushiyama, D.; Miyata, K.; Kiyoshima, C.; Fukagawa, S.; Yoshikawa, K.; Kurakazu, M.; et al. The combination of maternal blood and amniotic fluid biomarkers improves the predictive accuracy of histologic chorioamnionitis. Placenta 2019, 80, 4–7. [Google Scholar] [CrossRef]
- Maeda, K.; Matsuzaki, N.; Fuke, S.; Mitsuda, N.; Shimoya, K.; Nakayama, M.; Suehara, N.; Aono, T.; Mitsuda, N. Value of the Maternal Interleukin 6 Level for Determination of Histologic Chorioamnionitis in Preterm Delivery. Gynecol. Obstet. Investig. 1997, 43, 225–231. [Google Scholar] [CrossRef]
- Kwak, D.-W.; Cho, H.Y.; Kwon, J.-Y.; Park, Y.-W.; Kim, Y.-H. Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes. J. Périnat. Med. 2015, 43, 409–415. [Google Scholar] [CrossRef]
- Steinborn, A. Serum intercellular adhesion molecule-1 levels and histologic chorioamnionitis. Obstet. Gynecol. 2000, 95, 671–676. [Google Scholar] [CrossRef]
- Oh, K.; Park, K.; Kim, S.-N.; Jeong, E.; Lee, S.; Yoon, H. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta 2011, 32, 732–736. [Google Scholar] [CrossRef]
- Makino, I.; Makino, Y.; Yoshihara, F.; Nishikimi, T.; Kawarabayashi, T.; Kangawa, K.; Shibata, K. Decreased mature adrenomedullin levels in feto-maternal tissues of pregnant women with histologic chorioamnionitis. Biochem. Biophys. Res. Commun. 2003, 301, 437–442. [Google Scholar] [CrossRef]
- Cho, H.Y.; Jung, I.; Kwon, J.-Y.; Kim, S.J.; Park, Y.W.; Kim, Y.-H. The Delta Neutrophil Index as a predictive marker of histological chorioamnionitis in patients with preterm premature rupture of membranes: A retrospective study. PLoS ONE 2017, 12, e0173382. [Google Scholar] [CrossRef] [PubMed]
- Broumand, F.; Naji, S.; Seivani, S. Predictive values of maternal serum levels of procalcitonin, ESR, CRP, and WBC in the diagnosis of chorioamnionitis in mothers with preterm premature rupture of membrane. Iranian J. Neonatol. 2018, 9, 50–60. [Google Scholar] [CrossRef]
- Shimoya, K.; Matsuzaki, N.; Taniguchi, T.; Okada, T.; Saji, F.; Murata, Y. Interleukin-8 level in maternal serum as a marker for screening of histological chorioamnionitis at term. Int. J. Gynecol. Obstet. 1997, 57, 153–159. [Google Scholar] [CrossRef]
- Ahmed, W.A.S.; Ahmed, M.R.; Mohamed, M.L.; Hamdy, M.A.; Kamel, Z.; Elnahas, K.M. Maternal serum interleukin-6 in the management of patients with preterm premature rupture of membranes. J. Matern. Neonatal Med. 2015, 29, 3162–3166. [Google Scholar] [CrossRef]
- Yoneda, S.; Shiozaki, A.; Ito, M.; Yoneda, N.; Inada, K.; Yonezawa, R.; Kigawa, M.; Saito, S. Accurate Prediction of the Stage of Histological Chorioamnionitis before Delivery by Amniotic Fluid IL-8 Level. Am. J. Reprod. Immunol. 2015, 73, 568–576. [Google Scholar] [CrossRef]
- Gulati, S.; Bhatnagar, S.; Raghunandan, C.; Bhattacharjee, J. Interleukin-6 as a Predictor of Subclinical Chorioamnionitis in Preterm Premature Rupture of Membranes. Am. J. Reprod. Immunol. 2012, 67, 235–240. [Google Scholar] [CrossRef]
- Caloone, J.; Rabilloud, M.; Boutitie, F.; Traverse-Glehen, A.; Allias-Montmayeur, F.; Denis, L.; Boisson-Gaudin, C.; Hot, I.J.; Guerre, P.; Cortet, M.; et al. Accuracy of several maternal seric markers for predicting histological chorioamnionitis after preterm premature rupture of membranes: A prospective and multicentric study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 133–140. [Google Scholar] [CrossRef]
- Cobo, T.; Kacerovsky, M.; Palacio, M.; Hornychova, H.; Hougaard, D.M.; Skogstrand, K.; Jacobsson, B. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: Analyses of multiple proteins in the amniotic fluid. J. Matern. Neonatal Med. 2012, 25, 1995–2001. [Google Scholar] [CrossRef]
- Gulati, S.; Agrawal, S.; Raghunandan, C.; Bhattacharya, J.; Saili, A.; Agarwal, S.; Sharma, D. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: A prospective cohort study. J. Matern. Neonatal Med. 2012, 25, 1428–1432. [Google Scholar] [CrossRef]
- Škrablin, S.; Lovrić, H.; Banović, V.; Kralik, S.; Dijaković, A.; Kalafatic, D. Maternal plasma interleukin-6, interleukin-1β and C-reactive protein as indicators of tocolysis failure and neonatal outcome after preterm delivery. J. Matern. Neonatal Med. 2007, 20, 335–341. [Google Scholar] [CrossRef]
- Ohyama, M.; Itani, Y.; Yamanaka, M.; Goto, A.; Kato, K.; Ijiri, R.; Tanaka, Y. Re-evaluation of chorioamnionitis and funisitis with a special reference to subacute chorioamnionitis. Hum. Pathol. 2002, 33, 183–190. [Google Scholar] [CrossRef]
- Li, Z.; Huijun, Z.; Jianfang, Z.; Jianwen, Z. The value of the soluable intercellular adhesion molecule-1 levels in matermal serum for determination of occult chorioamnionitis in premature rupture of membranes. Acta Acad. Med. Wuhan 2004, 24, 154–157. [Google Scholar] [CrossRef]
- Blanc, W.A. Pathology of the placenta, membranes, and umbilical cord in bacterial, fungal, and viral infections in man. Monogr. Pathol. 1981, 22, 67–132. [Google Scholar]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.-H.; Syu, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Blanc, W.A. Amniotic infection syndrome; pathogenesis, morphology, and significance in circumnatal mortality. Clin. Obstet. Gynecol. 1959, 2, 705–734. [Google Scholar] [CrossRef]
- Salafia, C.M.; Weigl, C.; Silberman, L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet. Gynecol. 1989, 73, 383–389. [Google Scholar] [CrossRef]
- Abd-Elazeem, M.I.; Mohamed, R.A. Neutrophil-lymphocyte and platelet-lymphocyte ratios in rheumatoid arthritis patients: Relation to disease activity. Egypt. Rheumatol. 2018, 40, 227–231. [Google Scholar] [CrossRef]
- Fu, H.; Qin, B.; Hu, Z.; Ma, N.; Yang, M.; Wei, T.; Tang, Q.; Huang, Y.; Huang, F.; Liang, Y.; et al. Neutrophil- and Platelet-to-Lymphocyte Ratios are Correlated with Disease Activity in Rheumatoid Arthritis. Clin. Lab. 2015, 61, 269–273. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Yang, X.; Chen, L.; Yang, Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharmacol. 2016, 36, 94–99. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Zhang, W.; Ying, H.; Xu, Y.; Zhang, J.; Min, Q.; Chen, J. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are 2 new inflammatory markers associated with pulmonary involvement and disease activity in patients with dermatomyositis. Clin. Chim. Acta 2017, 465, 11–16. [Google Scholar] [CrossRef]
- Qin, B.; Ma, N.; Tang, Q.; Wei, T.; Yang, M.; Fu, H.; Hu, Z.; Liang, Y.; Yang, Z.; Zhong, R. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod. Rheumatol. 2016, 26, 372–376. [Google Scholar] [CrossRef]
- Mercan, R.; Bitik, B.; Tufan, A.; Bozbulut, U.B.; Atas, N.; Ozturk, M.A.; Haznedaroglu, S.; Goker, B. The Association between Neutrophil/Lymphocyte Ratio and Disease Activity in Rheumatoid Arthritis and Ankylosing Spondylitis. J. Clin. Lab. Anal. 2016, 30, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Balkarli, A.; Kucuk, A.; Babur, H.; Erbasan, F. Neutrophil/lymphocyte ratio and mean platelet volume in Behçet’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3045–3050. [Google Scholar] [PubMed]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Shin, T.G.; Jo, I.J.; Jeon, K.; Suh, G.Y.; Lee, T.R.; Yoon, H.; Cha, W.C.; Sim, M.S. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am. J. Emerg. Med. 2017, 35, 234–239. [Google Scholar] [CrossRef]
- Liu, X.; Shen, Y.; Wang, H.; Ge, Q.; Fei, A.; Pan, S. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Patients with Sepsis: A Prospective Observational Study. Mediat. Inflamm. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Ni, J.; Wang, H.; Li, Y.; Shu, Y.; Liu, Y. Neutrophil to lymphocyte ratio (NLR) as a prognostic marker for in-hospital mortality of patients with sepsis: A secondary analysis based on a single-center, retrospective, cohort study. Medicine 2019, 98, e18029. [Google Scholar] [CrossRef]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12, S16–S21. [Google Scholar] [CrossRef]
- Can, E.; Hamilcikan, Ş.; Can, C. The Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio for Detecting Early-onset Neonatal Sepsis. J. Pediatr. Hematol. 2018, 40, e229–e232. [Google Scholar] [CrossRef]
- Ozdemir, A. Predictive value of serum neutrophil-to-lymphocyte ratio in bronchopulmonary dysplasia: A retrospective observational study. Ann. Med. Res. 2018, 25, 512. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.-H.; Kim, A.; Yang, H.-R.; Jung, E.-Y.; Cho, S.-H. Maternal and Placental Risk Factors for Developing Necrotizing Enterocolitis in Very Preterm Infants. Pediatr. Neonatol. 2017, 58, 57–62. [Google Scholar] [CrossRef]
- Gezer, C.; Ekin, A.; Ertas, I.E.; Ozeren, M.; Solmaz, U.; Mat, E.; Taner, C.E. High first-trimester neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are indicators for early diagnosis of preeclampsia. Ginekol. Polska 2016, 87, 431–435. [Google Scholar] [CrossRef]
- Kurtoglu, E.; Kökçü, A.; Celik, H.; Tosun, M.; Malatyalioglu, E. May ratio of neutrophil to lymphocyte be useful in predicting the risk of developing preeclampsia? A pilot study. J. Matern. Neonatal Med. 2015, 28, 97–99. [Google Scholar] [CrossRef]
- Serin, S.; Avcı, F.; Ercan, O.; Köstü, B.; Bakacak, M.; Kıran, H. Is neutrophil/lymphocyte ratio a useful marker to predict the severity of pre-eclampsia? Pregnancy Hypertens. 2016, 6, 22–25. [Google Scholar] [CrossRef]
- Redman, C.W.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef]
- Canzoneri, B.J.; Lewis, D.F.; Groome, L.; Wang, Y. Increased Neutrophil Numbers Account for Leukocytosis in Women with Preeclampsia. Am. J. Perinatol. 2009, 26, 729–732. [Google Scholar] [CrossRef]
- Karim, S.A.; Khurshid, M.; Rizvi, J.H.; Jafarey, S.N.; Rizwana, I. Platelets and leucocyte counts in pregnancy. J. Pak. Med. Assoc. 1992, 42, 86–87. [Google Scholar]
- Balloch, A.J.; Cauchi, M.N. Reference ranges for haematology parameters in pregnancy derived from patient populations. Int. J. Lab. Hematol. 2008, 15, 7–14. [Google Scholar] [CrossRef]
- Belo, L.; Santos-Silva, A.; Rocha, S.; Caslake, M.; Cooney, J.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 46–51. [Google Scholar] [CrossRef]
- Lockitch, G. Handbook of Diagnostic Biochemistry and Hematology in Normal Pregnancy; CRC Press: Boca Baton, FL, USA, 1993. [Google Scholar]
- Sanci, M.; Töz, E.; Ince, O.; Özcan, A.; Polater, K.; Inan, A.H.; Beyan, E.; Akkaya, E. Reference values for maternal total and differential leukocyte counts in different trimesters of pregnancy and the initial postpartum period in western Turkey. J. Obstet. Gynaecol. 2017, 37, 571–575. [Google Scholar] [CrossRef]
- Presicce, P.; Park, C.-W.; Senthamaraikannan, P.; Bhattacharyya, S.; Jackson, C.; Kong, F.; Rueda, C.M.; DeFranco, E.; Miller, L.A.; Hildeman, D.A.; et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018, 3, 98306. [Google Scholar] [CrossRef]
- Smith, C.W.; Marlin, S.D.; Rothlein, R.; Toman, C.; Anderson, D.C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Investig. 1989, 83, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.K.; Ma, S.; Kim, M.B.; Rao, R.M.; Hartman, C.U.; Froio, R.M.; Yang, L.; Jones, T.; Liu, Y.; Nusrat, A.; et al. Coordinated Redistribution of Leukocyte LFA-1 and Endothelial Cell ICAM-1 Accompany Neutrophil Transmigration. J. Exp. Med. 2004, 200, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
| Group-0 † | Group-1 † | Group-2 † | Group-3 † | Group-4 † | p Value a | |
|---|---|---|---|---|---|---|
| 36.4% (48/132) | 14.4% (19/132) | 20.5% (27/132) | 17.4% (23/132) | 11.4% (15/132) | ||
| Maternal age, year (mean ± SD) | 32.8 ± 4.6 | 32.9 ± 3.4 | 32.5 ± 3.9 | 34.7 ± 3.6 | 33.2 ± 4.6 | NS (0.302) |
| Nulliparity | 50.0% (24/48) | 47.4% (9/19) | 37.0% (10/27) | 34.8% (8/23) | 33.3% (5/15) | NS (0.610) |
| Either clinical history of antenatal vaginal bleeding or evidence of placenta previa | 18.8% (9/48) | 15.8% (3/19) | 0% (0/27) | 4.3% (1/23) | 13.3% (2/15) | NS (0.107) |
| Preterm-PROM as a cause of PTB | 50.0% (24/48) | 31.6% (6/19) | 59.3% (16/27) | 69.6% (16/23) | 46.7% (7/15) | NS (0.145) |
| Male Newborn | 58.3% (28/48) | 57.9% (11/19) | 70.4% (19/27) | 34.8% (8/23) | 66.7% (10/15) | NS (0.125) |
| Cesarean delivery | 41.7% (20/48) | 31.6% (6/19) | 18.5% (5/27) | 34.8% (8/23) | 33.3% (5/15) | NS (0.378) |
| Median GA at delivery, weeks (range) | 31.6 (21.6–33.9) | 30.3 (23.4–33.7) | 30.3 (20.6–33.4) | 28.0 (22.0–31.9) b | 26.6 (21.3–31.4) c, d | <0.001 |
| Birth weight, g (mean ± SD) | 1572 ± 567 | 1478 ± 547 | 1444 ± 636 | 1129 ± 330 e | 1057 ± 419 e | 0.003 |
| 1 min Apgar score of <7 | 77.1% (37/48) | 63.2% (12/19) | 66.7% (18/27) | 91.3% (21/23) | 80.0% (12/15) | NS (0.193) |
| 5 min Apgar score of <7 | 31.2% (15/48) | 26.3% (5/19) | 22.2% (6/27) | 43.5% (10/23) | 60.0% (9/15) | NS (0.101) |
| Meconium staining | 8.3% (4/48) | 0% (0/19) | 0% (0/27) | 17.4% (4/23) | 6.7% (1/15) | NS (0.108) |
| Antenatal use of corticosteroids | 81.2% (39/48) | 68.4% (13/19) | 77.8% (21/27) | 82.6% (19/23) | 86.7% (13/15) | NS (0.702) |
| Antenatal use of antibiotics | 70.8% (34/48) | 73.7% (14/19) | 81.5% (22/27) | 100% (23/23) f | 93.3% (14/15) | 0.029 |
| Antenatal use of tocolytics | 66.7% (32/48) | 78.9% (15/19) | 81.5% (22/27) | 91.3% (21/23) | 86.7% (13/15) | NS (0.145) |
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Positive LR (95% CI) | Negative LR (95% CI) | |
|---|---|---|---|---|---|---|
| NLR ≥ 7.75 | 80.0% (12/15) | 59.0% (69/117) | 20.0% (12/60) | 95.8% (69/72) | 2.9487 (1.0597–8.2047) | 0.5128 (0.3674–0.7158) |
| Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Increased maternal NLR (≥7.75) | 5.559 | 1.255–24.621 | 0.024 |
| Gestational age at delivery (on a daily basis) | 0.716 | 0.562–0.912 | 0.007 |
| Parity (≥1) | 2.209 | 0.567–8.602 | NS (0.253) |
| Preterm-PROM as a cause of PTB | 1.258 | 0.311–5.091 | NS (0.748) |
| Vaginal delivery | 0.945 | 0.217–4.116 | NS (0.940) |
| Antenatal corticosteroids use | 9.474 | 0.989–90.794 | NS (0.051) |
| Antenatal antibiotics use | 0.980 | 0.076–12.607 | NS (0.987) |
| Antenatal tocolytics use | 1.367 | 0.193–9.665 | NS (0.754) |
| Meconium staining | 0.752 | 0.071–7.938 | NS (0.813) |
| Male sex of newborn | 1.765 | 0.486–6.406 | NS (0.388) |
| Either clinical history of antenatal vaginal bleedingor the evidence of placenta previa | 2.674 | 0.296–24.131 | NS (0.381) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Park, C.-W.; Moon, K.-C.; Park, J.-S.; Jun, J.-K. Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis. J. Clin. Med. 2021, 10, 2673. https://doi.org/10.3390/jcm10122673
Lee J-H, Park C-W, Moon K-C, Park J-S, Jun J-K. Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis. Journal of Clinical Medicine. 2021; 10(12):2673. https://doi.org/10.3390/jcm10122673
Chicago/Turabian StyleLee, Joon-Hyung, Chan-Wook Park, Kyung-Chul Moon, Joong-Shin Park, and Jong-Kwan Jun. 2021. "Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis" Journal of Clinical Medicine 10, no. 12: 2673. https://doi.org/10.3390/jcm10122673
APA StyleLee, J.-H., Park, C.-W., Moon, K.-C., Park, J.-S., & Jun, J.-K. (2021). Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis. Journal of Clinical Medicine, 10(12), 2673. https://doi.org/10.3390/jcm10122673







