Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anthropometric and Clinical Measurements
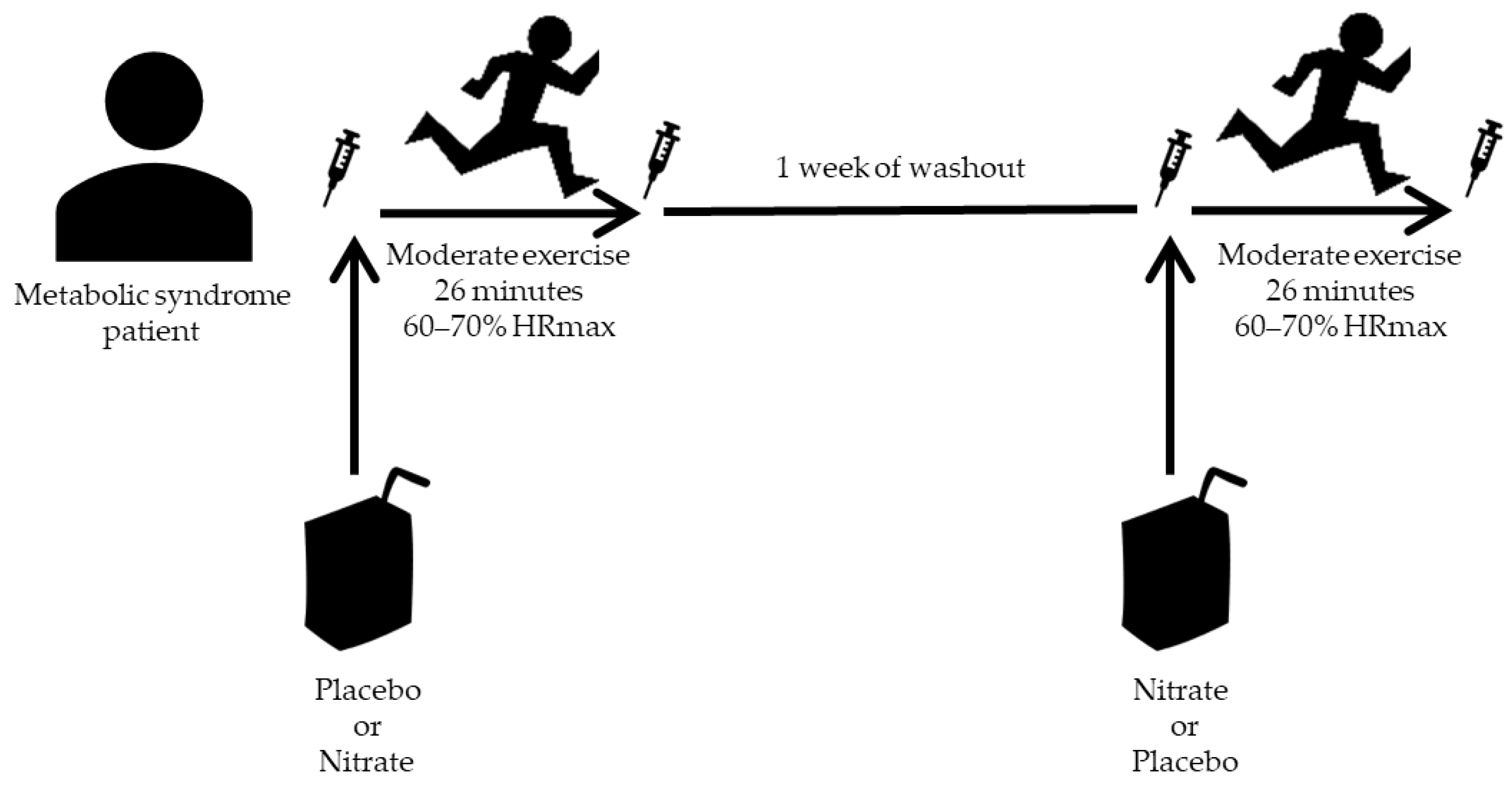
2.3. Experimental Procedure
2.4. Plasma and PBMCs Isolation
2.5. ‘Ex Vivo’ Stimulation of PBMCs with LPS and PMA
2.6. Oral Nitrate-Reducing Capability and Measurement of Nitrite and Nitrate Concentrations
2.7. Western Blot
2.8. PBMCs RNA Extraction and Real-Time PCR Assay
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffer, T.A.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Modulation of mitochondria and NADPH oxidase function by the nitrate-nitrite-NO pathway in metabolic disease with focus on type 2 diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165811. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Peng, R.; Yang, Z.; Peng, Y.Y.; Lu, N. Supplementation of dietary nitrate attenuated oxidative stress and endothelial dysfunction in diabetic vasculature through inhibition of NADPH oxidase. Nitric Oxide 2020, 96, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Ferreira, D.M.S.; Tarnawski, L.; McCann Haworth, S.; Xuechen, L.; Zhuge, Z.; Newton, P.T.; Massart, J.; Chagin, A.S.; Olofsson, P.S.; et al. Dietary nitrate attenuates high-fat diet-induced obesity via mechanisms involving higher adipocyte respiration and alterations in inflammatory status. Redox Biol. 2020, 28, 101387. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Capo, X.; Ferrer, M.D.; Olek, R.A.; Salaberry, E.; Suau, R.; Mari, B.; Llompart, I.; Tur, J.A.; Sureda, A.; Pons, A. Oral administration of sodium nitrate to metabolic syndrome patients attenuates mild inflammatory and oxidative responses to acute exercise. Antioxidants 2020, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, K.; Hickey, D.; Leveritt, M.; Fassett, R.; Ortiz de Zevallos Munoz, J.; Allen, J.D.; Briskey, D.; Parker, T.J.; Kerr, G.; Peake, J.M.; et al. Acute effects of nitrate-rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: A Randomized, placebo-controlled crossover study. Nutrients 2017, 9, 1270. [Google Scholar] [CrossRef]
- Raubenheimer, K.; Bondonno, C.; Blekkenhorst, L.; Wagner, K.H.; Peake, J.M.; Neubauer, O. Effects of dietary nitrate on inflammation and immune function, and implications for cardiovascular health. Nutr. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Habermeyer, M.; Roth, A.; Guth, S.; Diel, P.; Engel, K.H.; Epe, B.; Furst, P.; Heinz, V.; Humpf, H.U.; Joost, H.G.; et al. Nitrate and nitrite in the diet: How to assess their benefit and risk for human health. Mol. Nutr. Food Res. 2015, 59, 106–128. [Google Scholar] [CrossRef]
- WHO. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; WHO: Lyon, France, 2010. [Google Scholar]
- Busquets-Cortes, C.; Capo, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training and acute exercise modulates mitochondrial dynamics in football players’ blood mononuclear cells. Eur. J. Appl. Physiol. 2017, 117, 1977–1987. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Capo, X.; Martorell, M.; Busquets-Cortes, C.; Bouzas, C.; Carreres, S.; Mateos, D.; Sureda, A.; Tur, J.A.; Pons, A. Regular practice of moderate physical activity by older adults ameliorates their anti-inflammatory status. Nutrients 2018, 10, 1780. [Google Scholar] [CrossRef]
- Busquets-Cortes, C.; Capo, X.; Bibiloni, M.D.M.; Martorell, M.; Ferrer, M.D.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.A.; Pons, A.; et al. Peripheral blood mononuclear cells antioxidant adaptations to regular physical activity in elderly people. Nutrients 2018, 10, 1555. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, R.; Zeng, Y.; Tsao, R.; Mine, Y. Chinese sweet leaf tea (Rubus suavissimus) mitigates LPS-induced low-grade chronic inflammation and reduces the risk of metabolic disorders in a C57BL/6J mouse model. J. Agric. Food Chem. 2020, 68, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2020, 17, 100495. [Google Scholar] [CrossRef]
- Shao, B.; Munford, R.S.; Kitchens, R.; Varley, A.W. Hepatic uptake and deacylation of the LPS in bloodborne LPS-lipoprotein complexes. Innate Immun. 2012, 18, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M., Jr.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 signaling in obese NAFLD. Am. J. Phys. Gastrointest. Liver Phys. 2015, 309, G270–G278. [Google Scholar] [CrossRef] [PubMed]
- Mannisto, V.; Farkkila, M.; Pussinen, P.; Jula, A.; Mannisto, S.; Lundqvist, A.; Valsta, L.; Salomaa, V.; Perola, M.; Aberg, F. Serum lipopolysaccharides predict advanced liver disease in the general population. JHEP Rep. Innov. Hepatol. 2019, 1, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Capo, X.; Martorell, M.; Sureda, A.; Batle, J.M.; Tur, J.A.; Pons, A. Docosahexaenoic diet supplementation, exercise and temperature affect cytokine production by lipopolysaccharide-stimulated mononuclear cells. J. Physiol. Biochem. 2016, 72, 421–434. [Google Scholar] [CrossRef]
- Farinha, J.B.; Steckling, F.M.; Stefanello, S.T.; Cardoso, M.S.; Nunes, L.S.; Barcelos, R.P.; Duarte, T.; Kretzmann, N.A.; Mota, C.B.; Bresciani, G.; et al. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med. Open 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cui, G.; Chen, J.; Gao, H.; Wei, Y.; Uede, T.; Chen, Z.; Diao, H. Regular exercise enhances the immune response against microbial antigens through up-regulation of toll-like receptor signaling pathways. Cell. Physiol. Biochem. 2015, 37, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Araya, C.; Galindo, M.P. Size of sample in clinical investigation. Med. Clín. 2009, 133, 26–30. [Google Scholar]
- Iglesias Bonilla, P.; Mayoral Sanchez, E.; Lapetra Peralta, J.; Iborra Oquendo, M.; Villalba Alcala, F.; Cayuela Dominguez, A. Validation of two systems of self-measurement of blood pressure, the OMRON HEM-705 CP and OMRON M1 (HEM 422C2-E) models. Aten. Prim. 2002, 30, 22–28. [Google Scholar] [CrossRef]
- Capo, X.; Martorell, M.; Sureda, A.; Llompart, I.; Tur, J.A.; Pons, A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur. J. Nutr. 2015, 54, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Martorell, M.; Capo, X.; Bibiloni, M.M.; Sureda, A.; Mestre-Alfaro, A.; Batle, J.M.; Llompart, I.; Tur, J.A.; Pons, A. Docosahexaenoic acid supplementation promotes erythrocyte antioxidant defense and reduces protein nitrosative damage in male athletes. Lipids 2015, 50, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Liddle, L.; Monaghan, C.; Burleigh, M.C.; McIlvenna, L.C.; Muggeridge, D.J.; Easton, C. Changes in body posture alter plasma nitrite but not nitrate concentration in humans. Nitric Oxide 2018, 72, 59–65. [Google Scholar] [CrossRef]
- Sureda, A.; Martorell, M.; Bibiloni, M.D.M.; Bouzas, C.; Gallardo-Alfaro, L.; Mateos, D.; Capo, X.; Tur, J.A.; Pons, A. Effect of free fatty acids on inflammatory gene expression and hydrogen peroxide production by ex vivo blood mononuclear cells. Nutrients 2020, 12, 146. [Google Scholar] [CrossRef]
- Knight, E.; Petrella, R.J. Prescribing physical activity for healthy aging: Longitudinal follow-up and mixed method analysis of a primary care intervention. Physician Sportsmed. 2014, 42, 30–38. [Google Scholar] [CrossRef]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef]
- Thompson, P.D.; Buchner, D.; Pina, I.L.; Balady, G.J.; Williams, M.A.; Marcus, B.H.; Berra, K.; Blair, S.N.; Costa, F.; Franklin, B.; et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003, 107, 3109–3116. [Google Scholar] [CrossRef]
- Teri, L.; Gibbons, L.E.; McCurry, S.M.; Logsdon, R.G.; Buchner, D.M.; Barlow, W.E.; Kukull, W.A.; LaCroix, A.Z.; McCormick, W.; Larson, E.B. Exercise plus behavioral management in patients with Alzheimer disease: A randomized controlled trial. JAMA 2003, 290, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and beneficial role of ROS. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular reactive oxygen species (ROS) in cardiovascular pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Ludovico, P.; Burhans, W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014, 14, 33–39. [Google Scholar] [CrossRef]
- Cartoni, R.; Leger, B.; Hock, M.B.; Praz, M.; Crettenand, A.; Pich, S.; Ziltener, J.L.; Luthi, F.; Deriaz, O.; Zorzano, A.; et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J. Physiol. 2005, 567, 349–358. [Google Scholar] [CrossRef]
- Perry, C.G.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise training-induced regulation of mitochondrial quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Capo, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Zorzano, A.; Liesa, M.; Sebastian, D.; Segales, J.; Palacin, M. Mitochondrial fusion proteins: Dual regulators of morphology and metabolism. Semin. Cell Dev. Biol. 2010, 21, 566–574. [Google Scholar] [CrossRef] [PubMed]
- De Brito, O.M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610. [Google Scholar] [CrossRef]
- Sebastian, D.; Palacin, M.; Zorzano, A. Mitochondrial dynamics: Coupling mitochondrial fitness with healthy aging. Trends Mol. Med. 2017, 23, 201–215. [Google Scholar] [CrossRef]
- Tur, J.; Pereira-Lopes, S.; Vico, T.; Marin, E.A.; Munoz, J.P.; Hernandez-Alvarez, M.; Cardona, P.J.; Zorzano, A.; Lloberas, J.; Celada, A. Mitofusin 2 in macrophages links mitochondrial ROS production, cytokine release, phagocytosis, autophagy, and bactericidal activity. Cell Rep. 2020, 32, 108079. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch. Pharm. Res. 2009, 32, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlstrom, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Bescos, R.; Rodriguez, F.A.; Iglesias, X.; Ferrer, M.D.; Iborra, E.; Pons, A. Acute administration of inorganic nitrate reduces VO2peak in endurance athletes. Med. Sci. Sports Exerc. 2011, 43, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
- Shiva, S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Ingram, T.E.; Fraser, A.G.; Bleasdale, R.A.; Ellins, E.A.; Margulescu, A.D.; Halcox, J.P.; James, P.E. Low-dose sodium nitrite attenuates myocardial ischemia and vascular ischemia-reperfusion injury in human models. J. Am. Coll. Cardiol. 2013, 61, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zollbrecht, C.; Peleli, M.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Nitrite-mediated renal vasodilatation is increased during ischemic conditions via cGMP-independent signaling. Free Radic. Biol. Med. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Sack, M.N.; Greer, J.J.; Duranski, M.; Ringwood, L.A.; Burwell, L.; Wang, X.; MacArthur, P.H.; Shoja, A.; Raghavachari, N.; et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007, 204, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peleli, M.; Zollbrecht, C.; Giulietti, A.; Terrando, N.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Inorganic nitrite attenuates NADPH oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radic. Biol. Med. 2015, 83, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zollbrecht, C.; Persson, A.E.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Nitrite-mediated reduction of macrophage NADPH oxidase activity is dependent on xanthine oxidoreductase-derived nitric oxide but independent of S-nitrosation. Redox Biol. 2016, 10, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Qu, Y.; Wang, X.; An, W.; Li, Z.; Han, Z.; Qin, L. Nitrate partially inhibits lipopolysaccharide-induced inflammation by maintaining mitochondrial function. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Feng, Y.; Shu, C.; Yuan, R.; Bu, L.; Jia, M.; Pang, B. Dietary nitrate protects against skin flap ischemia-reperfusion injury in rats via modulation of antioxidative action and reduction of inflammatory responses. Front. Pharmacol. 2019, 10, 1605. [Google Scholar] [CrossRef]
- Jadert, C.; Petersson, J.; Massena, S.; Ahl, D.; Grapensparr, L.; Holm, L.; Lundberg, J.O.; Phillipson, M. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic. Biol. Med. 2012, 52, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortes, C.; Capo, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of millimolar steady-state hydrogen peroxide exposure on inflammatory and redox gene expression in immune cells from humans with metabolic syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Holden, N.S.; Squires, P.E.; Kaur, M.; Bland, R.; Jones, C.E.; Newton, R. Phorbol ester-stimulated NF-κB-dependent transcription: Roles for isoforms of novel protein kinase C. Cell. Signal. 2008, 20, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Sureda, A.; Mestre, A.; Tur, J.A.; Pons, A. The double edge of reactive oxygen species as damaging and signaling molecules in HL60 cell culture. Cell. Physiol. Biochem. 2010, 25, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Duquesnes, N.; Lezoualc’h, F.; Crozatier, B. PKC-delta and PKC-epsilon: Foes of the same family or strangers? J. Mol. Cell. Cardiol. 2011, 51, 665–673. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 2004, 3, 401–416. [Google Scholar] [CrossRef]
- Mortensen, R.F.; Zhong, W. Regulation of phagocytic leukocyte activities by C-reactive protein. J. Leukoc. Biol. 2000, 67, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Papa, S.; Bolanos, J.; Bruckdorfer, R.; Carlsen, H.; Elliott, R.M.; Flier, J.; Griffiths, H.R.; Heales, S.; Holst, B.; et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Asp. Med. 2002, 23, 209–285. [Google Scholar] [CrossRef]
- Niwa, Y.; Ozaki, Y.; Kanoh, T.; Akamatsu, H.; Kurisaka, M. Role of cytokines, tyrosine kinase, and protein kinase C on production of superoxide and induction of scavenging enzymes in human leukocytes. Clin. Immunol. Immunopathol. 1996, 79, 303–313. [Google Scholar] [CrossRef]
- Capo, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxid. Med. Cell. Longev. 2015, 2015, 187849. [Google Scholar] [CrossRef] [PubMed]
| Anthropometrical Parameters | Mean ± SEM |
|---|---|
| Age (years) | 66.5 ± 2.3 |
| Weight (kg) | 80.2 ± 4.2 |
| Height (cm) | 166 ± 2.8 |
| Body Mass Index (BMI, kg/m2) | 29.3 ± 1.7 |
| Waist Circumference (cm) | 102 ± 4 |
| Systolic Blood Pressure (mmHg) | 141 ± 5.7 |
| Diastolic Blood Pressure (mmHg) | 81.3 ± 1.7 |
| Clinical Parameters | |
| Glucose (mg/dL) | 93.3 ± 2.8 |
| Triglycerides (mg/dL) | 115 ± 7.6 |
| Cholesterol (mg/dL) | 174 ± 14 |
| HDL-Cholesterol (mg/dL) | 44.3 ± 4.9 |
| LDL-Cholesterol (mg/dL) | 106 ± 11 |
| Placebo | Nitrate | Effect Size | |
|---|---|---|---|
| Test duration (min) | 26.0 ± 0.1 | 26.0 ± 0.1 | 0.18 |
| Speed (Km/h) | 4.0 ± 0.0 | 4.0 ± 0.0 | |
| Heart Rate (Beats/min) | 108 ± 11 | 104 ± 9 | 0.18 |
| VO2 (mL/min) | 1478 ± 89 | 1389 ± 83 * | 0.46 |
| VO2 (mL/Kg min) | 18.8 ± 1.5 | 17.7 ± 1.5 * | 0.33 |
| VCO2 (mL/min) | 1311 ± 66 | 1275 ± 80 | 0.22 |
| Energy Expenditure (Kcal/min) | 7.3 ± 0.4 | 6.9 ± 0.4 * | 0.42 |
| Total Energy Expenditure (Kcal) | 189 ± 10 | 179 ± 11 * | 0.43 |
| Energy Efficiency (Kcal/Km) | 109 ± 6.1 | 103 ± 6.2 * | 0.25 |
| Respiratory Quotient | 0.89 ± 0.03 | 0.92 ± 0.01 | 0.44 |
| Energy from lipids (%) | 38.6 ± 12 | 29.5 ± 2.7 | 0.49 |
| Oral Washing | Water | Nitrate | |
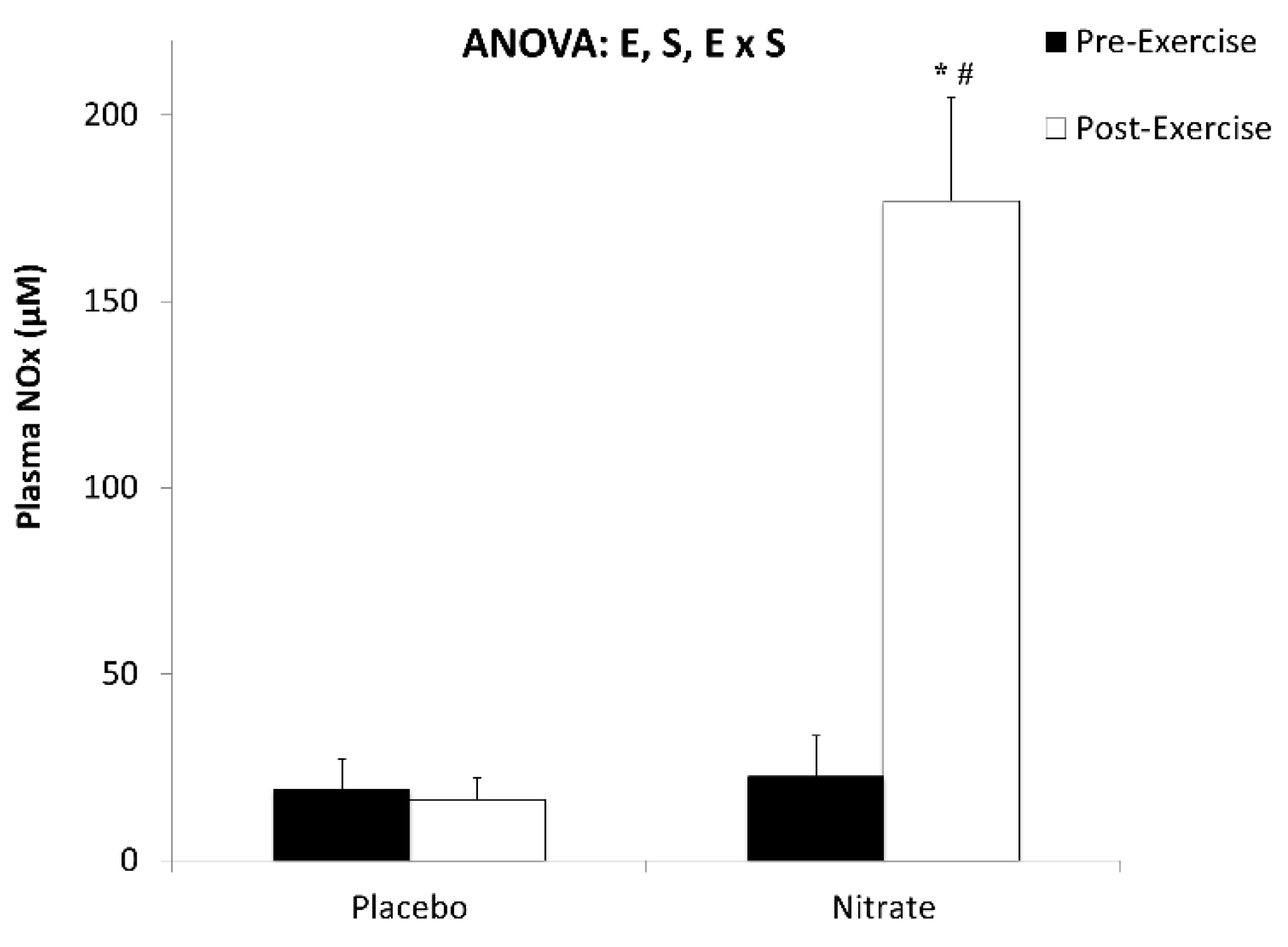
| Nitrite (nM) | 2718 ± 343 | 17,970 ± 3943 * | 2.22 |
| Nitrate (µM) | 17.5 ± 4.21 | 2488 ± 201 * | 7.10 |
| Gene (%) | Placebo | Nitrate Supplemented | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Pre-Exercise | Post-Exercise | Pre-Exercise | Post-Exercise | Two-Way ANOVA | |||
| E | S | E x S | |||||
| MitoND5 | 1.00 ± 0.15 | 1.51 ± 0.83 | 0.97 ± 0.22 | 1.32 ± 0.46 | |||
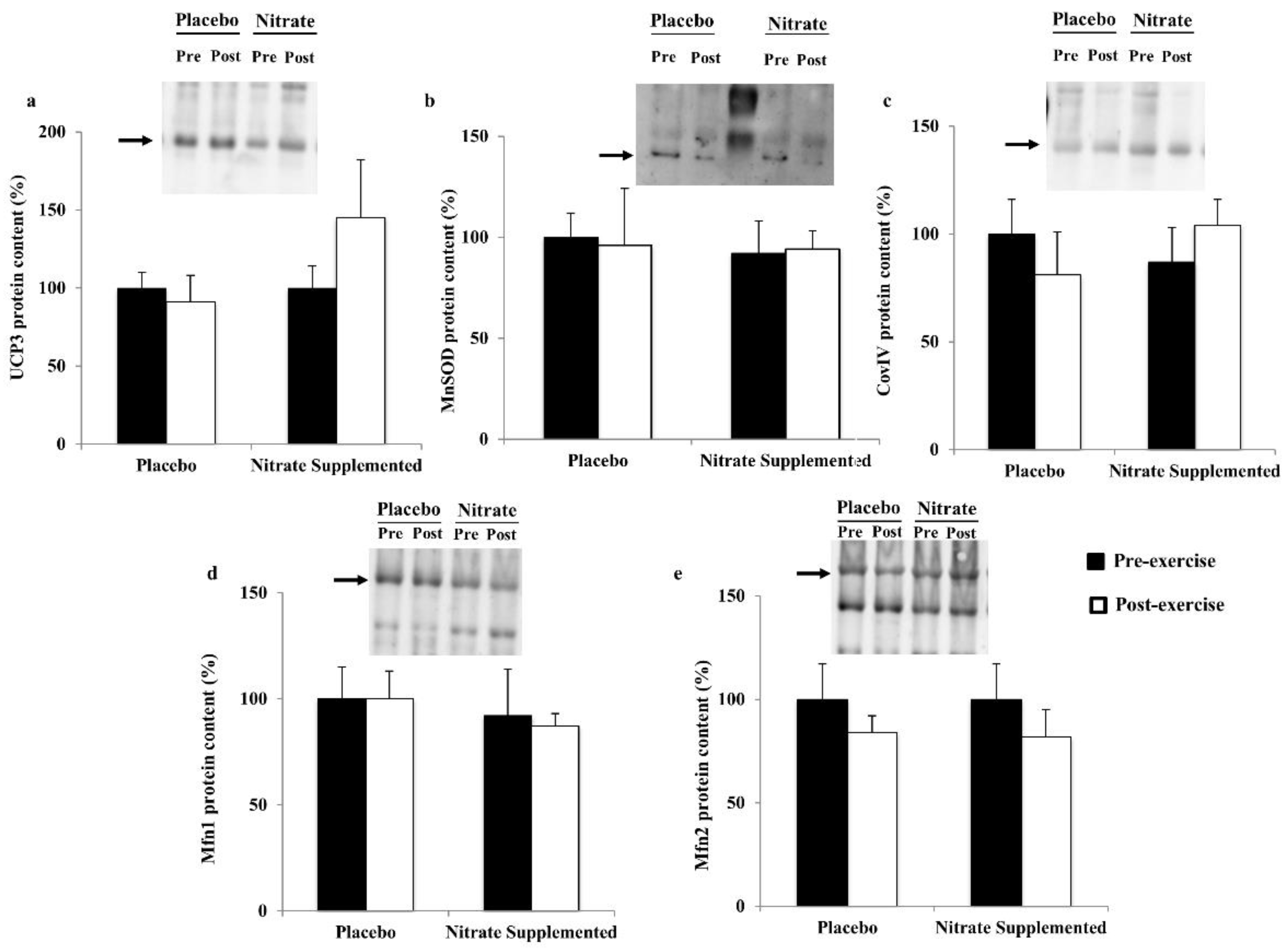
| CoxIV | 1.00 ± 0.14 | 0.37 ± 0.11 * | 0.90 ± 0.20 | 0.59 ± 0.14 | E | ||
| UCP3 | 1.00 ± 0.24 | 0.75 ± 0.35 | 0.60 ± 0.15 | 0.29 ± 0.09 | |||
| HO1 | 1.00 ± 0.18 | 0.55 ± 0.17 | 0.96 ± 0.22 | 0.71 ± 0.19 | |||
| MnSOD | 1.00 ± 0.33 | 0.49 ± 0.20 | 1.42 ± 0.54 | 3.72 ± 0.92 # | S | ||
| GPx | 1.00 ± 0.34 | 0.47 ± 0.20 | 1.14 ± 0.37 | 38.7 ± 9.6 *,# | E | S | E x S |
| CAT | 1.00 ± 0.59 | 0.46 ± 0.28 | 1.27 ± 0.44 | 34.7 ± 7.8 *,# | E | S | E x S |
| Mfn1 | 1.00 ± 0.26 | 1.14 ± 0.88 | 1.06 ± 0.52 | 0.18 ± 0.04 | E | ||
| Mfn2 | 1.00 ± 0.34 | 0.34 ± 0.11 | 1.07 ± 0.33 | 4.56 ± 1.04 *,# | E | S | E x S |
| Nrf2 | 1.00 ± 0.14 | 0.32 ± 0.09 | 1.16 ± 0.17 | 1.07 ± 0.37 | S | ||
| PGC1α | 1.00 ± 0.31 | 0.59 ± 0.35 | 1.22 ± 0.39 | 5.61 ± 1.34 # | S | E x S | |
| Tfam | 1.00 ± 0.19 | 0.24 ± 0.08 * | 1.00 ± 0.31 | 0.27 ± 0.09 * | E | ||
| Control | LPS | PMA | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| M | S | M x S | |||||
| GPx | Placebo | 1.00 ± 0.37 a | 3.03 ± 1.78 a | 6.53± 2.76 a | M | S | M x S |
| Nitrate | 1.00 ± 0.26 a | 5.30 ± 3.74 a | 48.8 ± 35.6 b | ||||
| CAT | Placebo | 1.00 ± 0.42 a | 4.93 ± 2.03 a,b | 23.1 ± 11.4 b | M x S | ||
| Nitrate | 1.00 ± 0.29 a,b | 18.9 ± 10.8 a,b | 1.80 ± 0.94 a,b | ||||
| IL6 | Placebo | 1.00 ± 0.39 | 4.51 ± 1.99 | 0.46 ± 0.25 | M | ||
| Nitrate | 1.00 ± 0.28 | 7.75 ± 4.63 | 0.16 ± 0.06 | ||||
| TNFα | Placebo | 1.00 ± 0.36 a | 4.28 ± 1.81 a,b | 14.6 ± 5.65 b | M x S | ||
| Nitrate | 1.00 ± 0.36 a,b | 7.40 ± 3.08 a,b | 0.78 ± 0.42 a,b | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, M.D.; Capó, X.; Reynés, C.; Quetglas, M.; Salaberry, E.; Tonolo, F.; Suau, R.; Marí, B.; Tur, J.A.; Sureda, A.; et al. Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients. J. Clin. Med. 2021, 10, 2618. https://doi.org/10.3390/jcm10122618
Ferrer MD, Capó X, Reynés C, Quetglas M, Salaberry E, Tonolo F, Suau R, Marí B, Tur JA, Sureda A, et al. Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients. Journal of Clinical Medicine. 2021; 10(12):2618. https://doi.org/10.3390/jcm10122618
Chicago/Turabian StyleFerrer, Miguel D., Xavier Capó, Clara Reynés, Magdalena Quetglas, Eduardo Salaberry, Federica Tonolo, Rafael Suau, Bartolomé Marí, Josep A. Tur, Antoni Sureda, and et al. 2021. "Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients" Journal of Clinical Medicine 10, no. 12: 2618. https://doi.org/10.3390/jcm10122618
APA StyleFerrer, M. D., Capó, X., Reynés, C., Quetglas, M., Salaberry, E., Tonolo, F., Suau, R., Marí, B., Tur, J. A., Sureda, A., & Pons, A. (2021). Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients. Journal of Clinical Medicine, 10(12), 2618. https://doi.org/10.3390/jcm10122618











