Robot-Assisted Maxillary Positioning in Orthognathic Surgery: A Feasibility and Accuracy Evaluation
Abstract
1. Introduction
2. Materials and Methods
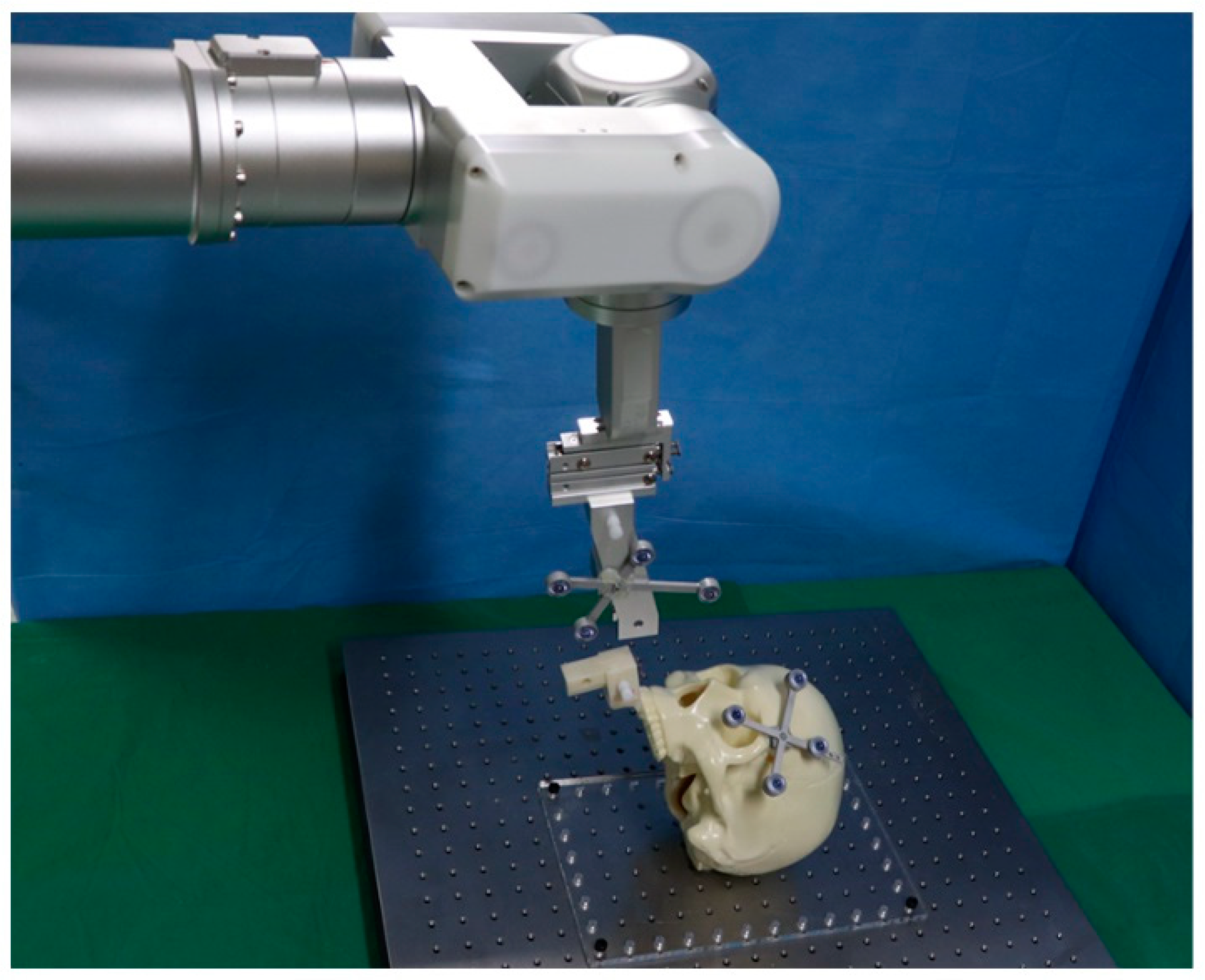
2.1. System Implementation
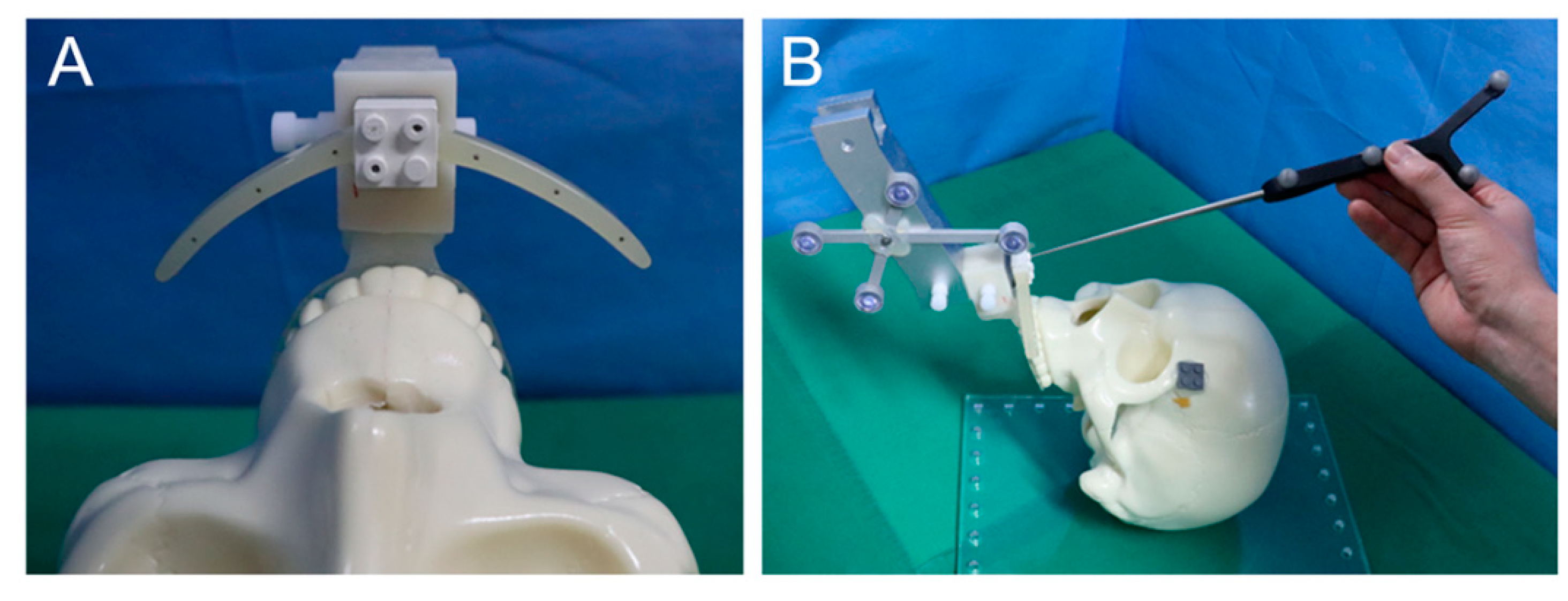
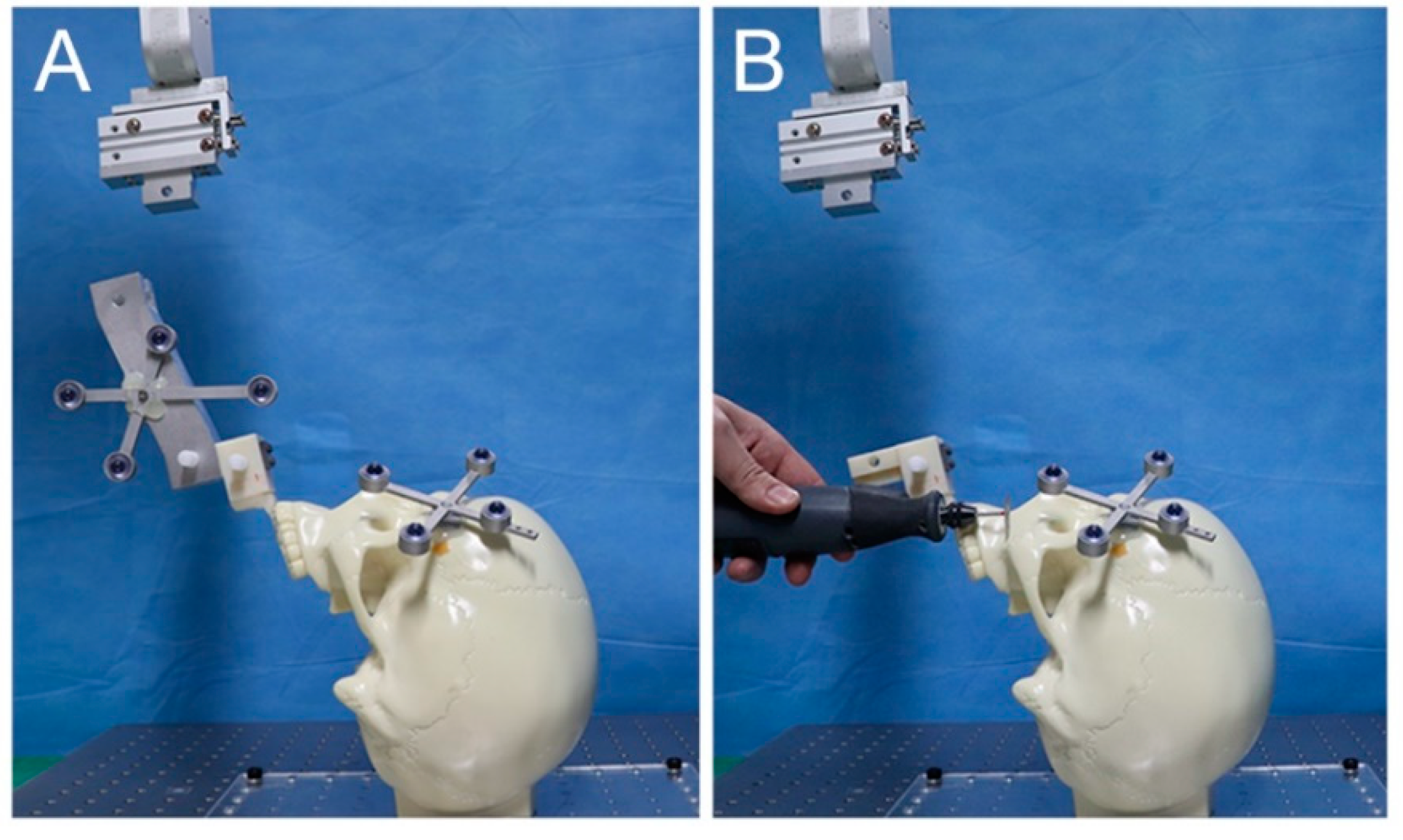
2.2. Experimental Procedures
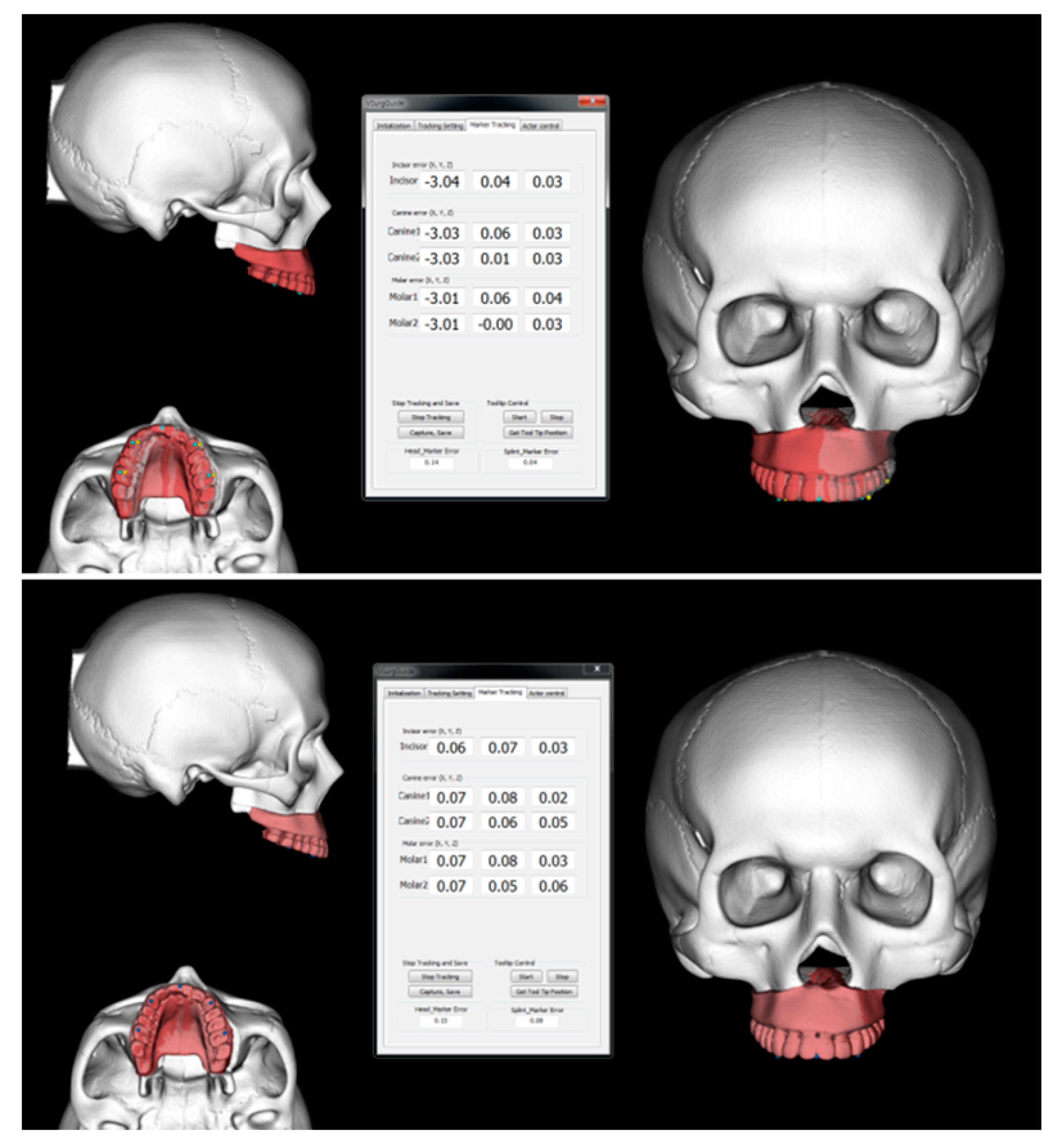
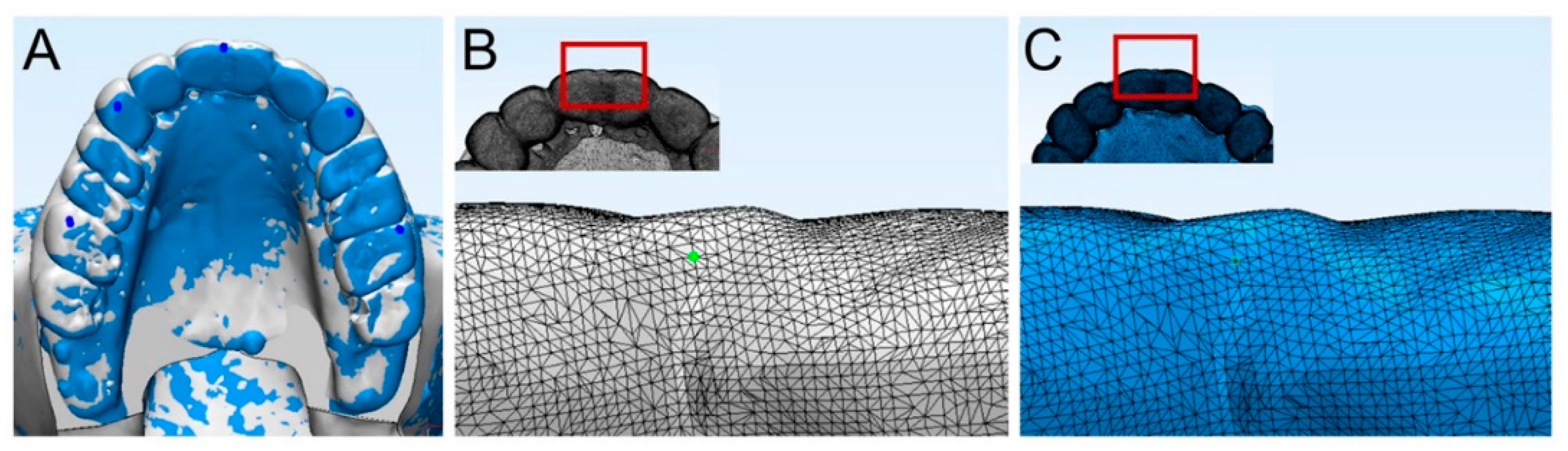
2.3. Accuracy Evaluation
2.4. Statistical Analysis
3. Results
3.1. Intraoperative Evaluation of the Discrepancy between the Planned and Actual Postoperative Maxillary Position Using Navigation
3.2. Postoperative Evaluation of the Discrepancy between the Planned and Actual Postoperative Maxillary Position Using CT Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, D.S.; Seo, J.S.; Choi, H.S. Skeletal stability after 2-jaw surgery via surgery-first approach in facial asymmetry patients using CBCT. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 11. [Google Scholar] [CrossRef]
- Jung, H.D.; Kim, S.Y.; Park, H.S.; Jung, Y.S. Orthognathic surgery and temporomandibular joint symptoms. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 14. [Google Scholar] [CrossRef]
- Ellis, E., 3rd. Bimaxillary surgery using an intermediate splint to position the maxilla. J. Oral Maxillofac. Surg. 1999, 57, 53–56. [Google Scholar] [CrossRef]
- Ellis, E., 3rd. Accuracy of model surgery: Evaluation of an old technique and introduction of a new one. J. Oral Maxillofac. Surg. 1990, 48, 1161–1167. [Google Scholar] [CrossRef]
- Kraeima, J.; Jansma, J.; Schepers, R.H. Splintless surgery: Does patient-specific CAD-CAM osteosynthesis improve accuracy of Le Fort I osteotomy? Br. J. Oral Maxillofac. Surg. 2016, 54, 1085–1089. [Google Scholar] [CrossRef]
- Azarmehr, I.; Stokbro, K.; Bell, R.B.; Thygesen, T. Surgical navigation: A systematic review of indications, treatments, and outcomes in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 2017, 75, 1987–2005. [Google Scholar] [CrossRef] [PubMed]
- Zinser, M.J.; Sailer, H.F.; Ritter, L.; Braumann, B.; Maegele, M.; Zoller, J.E. A paradigm shift in orthognathic surgery? A comparison of navigation, computer-aided designed/computer-aided manufactured splints, and “classic” intermaxillary splints to surgical transfer of virtual orthognathic planning. J. Oral Maxillofac. Surg. 2013, 71, 2151.e1–2151.e21. [Google Scholar] [CrossRef] [PubMed]
- Suojanen, J.; Leikola, J.; Stoor, P. The use of patient-specific implants in orthognathic surgery: A series of 32 maxillary osteotomy patients. J. Craniomaxillofac. Surg. 2016, 44, 1913–1916. [Google Scholar] [CrossRef]
- Heufelder, M.; Wilde, F.; Pietzka, S.; Mascha, F.; Winter, K.; Schramm, A.; Rana, M. Clinical accuracy of waferless maxillary positioning using customized surgical guides and patient specific osteosynthesis in bimaxillary orthognathic surgery. J. Craniomaxillofac. Surg. 2017, 45, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Ruckschloss, T.; Ristow, O.; Muller, M.; Kuhle, R.; Zingler, S.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Accuracy of patient-specific implants and additive-manufactured surgical splints in orthognathic surgery—A three-dimensional retrospective study. J. Craniomaxillofac. Surg. 2019, 47, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.B. Computer planning and intraoperative navigation in orthognathic surgery. J. Oral Maxillofac. Surg. 2011, 69, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Nova, I.; Kallus, S.; Ristow, O.; Freudlsperger, C.; Eisenmann, U.; Dickhaus, H.; Engel, M.; Hoffmann, J.; Seeberger, R. Can electromagnetic-navigated maxillary positioning replace occlusional splints in orthognathic surgery? A clinical pilot study. J. Craniomaxillofac. Surg. 2017, 45, 1593–1599. [Google Scholar] [CrossRef]
- Kim, D.S.; Woo, S.Y.; Yang, H.J.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Choi, S.C.; Hwang, S.J.; Yi, W.J. An integrated orthognathic surgery system for virtual planning and image-guided transfer without intermediate splint. J. Craniomaxillofac. Surg. 2014, 42, 2010–2017. [Google Scholar] [CrossRef]
- Wong, A.; Goonewardene, M.S.; Allan, B.P.; Mian, A.S.; Rea, A. Accuracy of maxillary repositioning surgery using CAD/CAM customized surgical guides and fixation plates. Int. J. Oral Maxillofac. Surg. 2021, 50, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Stokbro, K.; Bell, R.B.; Thygesen, T. Patient-Specific printed plates improve surgical accuracy in vitro. J. Oral Maxillofac. Surg. 2018, 76, 2647.e1–2647.e9. [Google Scholar] [CrossRef] [PubMed]
- Jakopec, M.; Harris, S.J.; Rodriguez y Baena, F.; Gomes, P.; Cobb, J.; Davies, B.L. The first clinical application of a “hands-on” robotic knee surgery system. Comput. Aided Surg. 2001, 6, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Li, L.J.; Shi, B.; Xu, C.W.; Luo, E. Robotic surgical systems in maxillofacial surgery: A review. Int. J. Oral Sci. 2017, 9, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.A. Medical robots: Current systems and research directions. J. Robot. 2012, 2012, 401613. [Google Scholar] [CrossRef]
- Cadiere, G.B.; Himpens, J.; Germay, O.; Izizaw, R.; Degueldre, M.; Vandromme, J.; Capelluto, E.; Bruyns, J. Feasibility of robotic laparoscopic surgery: 146 cases. World J. Surg. 2001, 25, 1467–1477. [Google Scholar] [CrossRef]
- Pugin, F.; Bucher, P.; Morel, P. History of robotic surgery: From AESOP(R) and ZEUS(R) to da Vinci(R). J. Visc. Surg. 2011, 148, e3–e8. [Google Scholar] [CrossRef]
- Wu, J.; Hui, W.; Chen, S.; Niu, J.; Lin, Y.; Luan, N.; Zhang, S.; Shen, S.G.F. Error analysis of robot-assisted orthognathic surgery. J. Craniofac. Surg. 2020, 31, 2324–2328. [Google Scholar] [CrossRef]
- Burgner, J.; Toma, M.; Vieira, V.; Eggers, G.; Raczkowsky, J.; Muhling, J.; Marmulla, R.; Worn, H. System for robot assisted orthognathic surgery. Int. J. Comput. Assist. Radiol. Surg. 2007, 2, S419–S421. [Google Scholar]
- Wang, X.; Song, R.; Liu, X.; Li, Q.; Cheng, T.; Xue, Y. System design for orthognathic aided robot. In Proceedings of the 5th annual IEEE International Conference on Cyber Technology in Automation, Control and Intelligent Systems, Shenyang, China, 6–10 June 2015; pp. 612–617. [Google Scholar]
- Han, J.J.; Woo, S.Y.; Yi, W.J.; Hwang, S.J. A robot arm and image-guided navigation assisted surgical system for maxillary repositioning in orthognathic surgery: A phantom skull-based trial. Appl. Sci. 2020, 10, 1549. [Google Scholar] [CrossRef]
- Schreurs, R.; Dubois, L.; Ho, J.; Klop, C.; Beenen, L.F.M.; Habets, P.; Becking, A.G.; Maal, T.J.J. Implant-Oriented navigation in orbital reconstruction part II: Preclinical cadaver study. Int. J. Oral Maxillofac. Surg. 2020, 49, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Bobek, S.L. Applications of navigation for orthognathic surgery. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yang, H.J.; Choi, M.H.; Woo, S.Y.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Choi, S.C.; Hwang, S.J.; Yi, W.J. Real-Time augmented model guidance for mandibular proximal segment repositioning in orthognathic surgery, using electromagnetic tracking. J. Craniomaxillofac. Surg. 2019, 47, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, S.; Badiali, G.; Lancellotti, L.; Babbi, L.; Bianchi, A.; Marchetti, C. Simulation-Guided navigation: A new approach to improve intraoperative three-dimensional reproducibility during orthognathic surgery. J. Craniofac. Surg. 2010, 21, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Van den Bempt, M.; Liebregts, J.; Maal, T.; Berge, S.; Xi, T. Toward a higher accuracy in orthognathic surgery by using intraoperative computer navigation, 3D surgical guides, and/or customized osteosynthesis plates: A systematic review. J. Craniomaxillofac. Surg. 2018, 46, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Suojanen, J.; Leikola, J.; Stoor, P. The use of patient-specific implants in orthognathic surgery: A series of 30 mandible sagittal split osteotomy patients. J. Craniomaxillofac. Surg. 2017, 45, 990–994. [Google Scholar] [CrossRef]
- Novelli, G.; Tonellini, G.; Mazzoleni, F.; Sozzi, D.; Bozzetti, A. Surgical navigation recording systems in orbitozygomatic traumatology. J. Craniofac. Surg. 2012, 23, 890–892. [Google Scholar] [CrossRef]


| Type of Movement | Direction and Amount of Movement | Number of Experiments |
|---|---|---|
| Bodily shift | Advancement by 3 mm and downward by 2 mm | 3 |
| Bodily shift to the right by 3 mm | 3 | |
| Rotation | Cant correction by 4 mm (#16: upward by 2 mm, #26: downward by 2 mm) | 3 |
| Posterior impaction (#16 and #26: upward by 3 mm) | 3 |
| Land- Marks | Medio-Lateral | Antero-Posterior | Supero-Inferior | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Fixation (mm) | After Fixation (mm) | p Value * | Before Fixation (mm) | After Fixation (mm) | p Value * | Before Fixation (mm) | After Fixation (mm) | p Value * | |
| #11 | 0.11 ± 0.14 | 0.14 ± 0.14 | 0.432 | 0.05 ± 0.05 | 0.17 ± 0.15 | 0.011 | 0.13 ± 0.15 | 0.22 ± 0.22 | 0.071 |
| #13 | 0.11 ± 0.13 | 0.15 ± 0.13 | 0.530 | 0.08 ± 0.05 | 0.23 ± 0.18 | 0.003 | 0.12 ± 0.10 | 0.23 ± 0.17 | 0.041 |
| #23 | 0.11 ± 0.13 | 0.15 ± 0.13 | 0.530 | 0.07 ± 0.05 | 0.12 ± 0.12 | 0.209 | 0.09 ± 0.11 | 0.18 ± 0.18 | 0.023 |
| #16 | 0.10 ± 0.11 | 0.18 ± 0.14 | 0.084 | 0.08 ± 0.06 | 0.24 ± 0.18 | 0.002 | 0.08 ± 0.05 | 0.19 ± 0.11 | 0.021 |
| #26 | 0.10 ± 0.10 | 0.18 ± 0.14 | 0.084 | 0.11 ± 0.11 | 0.14 ± 0.11 | 0.432 | 0.09 ± 0.10 | 0.18 ± 0.11 | 0.037 |
| Total | 0.10 ± 0.12 | 0.16 ± 0.13 | 0.020 | 0.08 ± 0.07 | 0.18 ± 0.15 | <0.001 | 0.10 ± 0.10 | 0.20 ± 0.16 | <0.001 |
| Land-Marks | Medio-Lateral | Antero-Posterior | Supero-Inferior | |||
|---|---|---|---|---|---|---|
| Mean ± SD (mm) | p Value * | Mean ± SD (mm) | p Value * | Mean ± SD (mm) | p Value * | |
| #11 | 0.22 ± 0.19 | 0.002 | 0.18 ± 0.12 | 0.328 | 0.16 ± 0.12 | 0.307 |
| #13 | 0.23 ± 0.19 | 0.002 | 0.19 ± 0.14 | 0.326 | 0.19 ± 0.10 | 0.530 |
| #23 | 0.20 ± 0.19 | 0.002 | 0.16 ± 0.09 | 0.388 | 0.15 ± 0.13 | 0.195 |
| #16 | 0.23 ± 0.20 | 0.002 | 0.21 ± 0.14 | 0.213 | 0.22 ± 0.11 | 0.656 |
| #26 | 0.23 ± 0.20 | 0.002 | 0.15 ± 0.08 | 0.724 | 0.17 ± 0.13 | 0.286 |
| Total | 0.22 ± 0.19 | <0.001 | 0.18 ± 0.12 | 0.303 | 0.18 ± 0.12 | 0.426 |
| Land-Marks | Medio-Lateral | Antero-Posterior | Supero-Inferior | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (mm) | Min (mm) | Max (mm) | Mean ± SD (mm) | Min (mm) | Max (mm) | Mean ± SD (mm) | Min (mm) | Max (mm) | |
| #11 | 0.39 ± 0.36 | 0.03 | 1.07 | 0.39 ± 0.22 | 0.16 | 0.87 | 0.38 ± 0.38 | 0.10 | 1.41 |
| #13 | 0.39 ± 0.37 | 0.01 | 0.99 | 0.47 ± 0.27 | 0.15 | 1.05 | 0.42 ± 0.34 | 0.02 | 1.18 |
| #23 | 0.40 ± 0.37 | 0.01 | 0.99 | 0.30 ± 0.23 | 0.05 | 0.83 | 0.31 ± 0.25 | 0.03 | 0.79 |
| #16 | 0.45 ± 0.35 | 0.07 | 0.96 | 0.48 ± 0.28 | 0.15 | 0.87 | 0.44 ± 0.35 | 0.00 | 1.15 |
| #26 | 0.46 ± 0.35 | 0.08 | 0.95 | 0.24 ± 0.20 | 0.05 | 0.73 | 0.34 ± 0.28 | 0.03 | 0.75 |
| Total | 0.42 ± 0.35 | 0.01 | 1.07 | 0.37 ± 0.25 | 0.05 | 1.05 | 0.38 ± 0.32 | 0.00 | 1.41 |
| Limits of Agreement (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Mean ± SD (mm) | 95% CI for Mean Discrepancy | Lower Limit | Upper Limit | |
| Signed difference | ||||
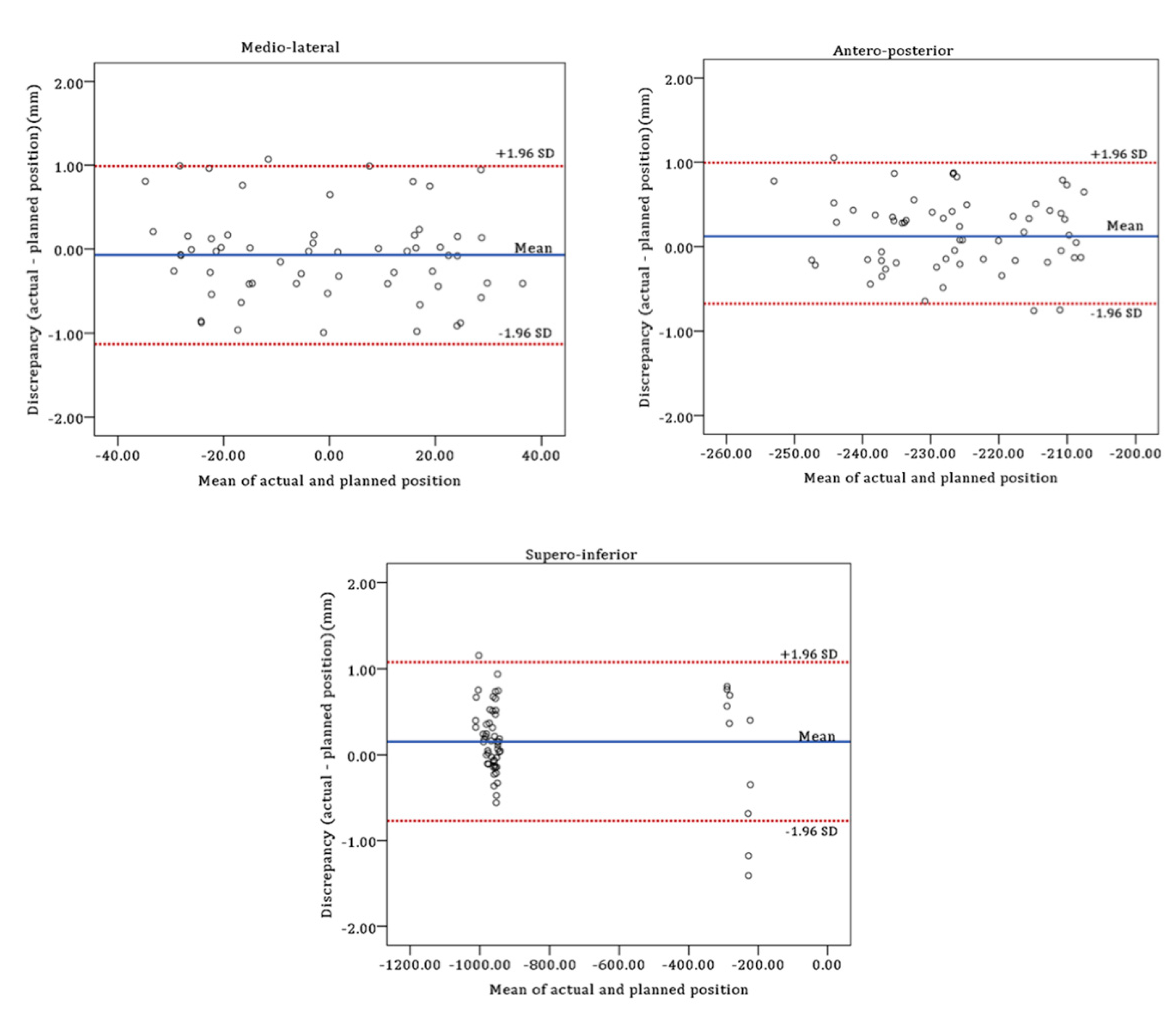
| Medio-lateral | −0.07 ± 0.54 | −0.21 to 0.07 | −1.13 (−1.37 to −0.89) | 0.99 (0.75 to 1.23) |
| Antero-posterior | 0.16 ± 0.43 | 0.05 to 0.27 | −0.68 (−0.87 to −0.49) | 0.99 (0.80 to 1.18) |
| Supero-inferior | 0.15 ± 0.47 | 0.03 to 0.28 | −0.77 (−0.98 to −0.56) | 1.08 (0.87 to 1.29) |
| Absolute difference | ||||
| Medio-lateral | 0.42 ± 0.35 | 0.33 to 0.51 | −0.27 (−0.42 to −0.11) | 1.10 (0.94 to 1.25) |
| Antero-posterior | 0.37 ± 0.25 | 0.31 to 0.44 | −0.12 (−0.24 to −0.01) | 0.87 (0.76 to 0.98) |
| Supero-inferior | 0.38 ± 0.32 | 0.30 to 0.46 | −0.24 (−0.38 to −0.10) | 1.00 (0.86 to 1.14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.J.; Woo, S.-Y.; Yi, W.-J.; Hwang, S.J. Robot-Assisted Maxillary Positioning in Orthognathic Surgery: A Feasibility and Accuracy Evaluation. J. Clin. Med. 2021, 10, 2596. https://doi.org/10.3390/jcm10122596
Han JJ, Woo S-Y, Yi W-J, Hwang SJ. Robot-Assisted Maxillary Positioning in Orthognathic Surgery: A Feasibility and Accuracy Evaluation. Journal of Clinical Medicine. 2021; 10(12):2596. https://doi.org/10.3390/jcm10122596
Chicago/Turabian StyleHan, Jeong Joon, Sang-Yoon Woo, Won-Jin Yi, and Soon Jung Hwang. 2021. "Robot-Assisted Maxillary Positioning in Orthognathic Surgery: A Feasibility and Accuracy Evaluation" Journal of Clinical Medicine 10, no. 12: 2596. https://doi.org/10.3390/jcm10122596
APA StyleHan, J. J., Woo, S.-Y., Yi, W.-J., & Hwang, S. J. (2021). Robot-Assisted Maxillary Positioning in Orthognathic Surgery: A Feasibility and Accuracy Evaluation. Journal of Clinical Medicine, 10(12), 2596. https://doi.org/10.3390/jcm10122596






