Risk Indicators Affecting the Survival of the Mandibular First Molar Adjacent to an Implant at the Mandibular Second Molar Site: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
- Patients who were more than 20 years old
- Patients who had no relevant systemic conditions or diseases
- Patients who had the mandibular first molars (MnM1s) adjacent to the single implants placed in the mandibular second molar sites (ImM2s)
- Patients who had the mandibular first molars (MnM1s) and the mandibular second molars (MnM2s) on the contralateral side
- Patients who had been followed up at least 2 years after prosthesis delivery of ImM2s
- Patients who had periapical radiographs after prosthesis delivery and before extraction of MnM1s or the last follow-up visits
- Patients who had an active infection or disease affecting bone metabolism and wound healing
- Patients who had regular use of steroids or other medications affecting bone turnover
2.2. Clinical Variables
2.3. Radiographic Measurements
2.4. Survival of Tooth Adjacent to Single Implant
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zembic, A.; Kim, S.; Zwahlen, M.; Kelly, J.R. Systematic review of the survival rate and incidence of biologic, technical, and esthetic complications of single implant abutments supporting fixed prostheses. Int. J. Oral. Maxillofac. Implant. 2014, 29, 99–116. [Google Scholar] [CrossRef]
- Van Velzen, F.J.; Ofec, R.; Schulten, E.A.; Ten Bruggenkate, C.M. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral. Implant. Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef]
- Jemt, T. Single-Implant Survival: More Than 30 Years of Clinical Experience. Int. J. Prosthodont. 2016, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Von Stein-Lausnitz, M.; Nickenig, H.J.; Wolfart, S.; Neumann, K.; Von Stein-Lausnitz, A.; Spies, B.C.; Beuer, F. Survival rates and complication behaviour of tooth implant-supported, fixed dental prostheses: A systematic review and meta-analysis. J. Dent. 2019, 88, 103167. [Google Scholar] [CrossRef]
- Katona, T.R.; Eckert, G.J. The mechanics of dental occlusion and disclusion. Clin. Biomech. 2017, 50, 84–91. [Google Scholar] [CrossRef]
- Yuan, J.C.; Sukotjo, C. Occlusion for implant-supported fixed dental prostheses in partially edentulous patients: A literature review and current concepts. J. Periodontal Implant. Sci. 2013, 43, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rangert, B.R.; Sullivan, R.M.; Jemt, T.M. Load factor control for implants in the posterior partially edentulous segment. Int. J. Oral. Maxillofac. Implant. 1997, 12, 360–370. [Google Scholar]
- Laney, W.R. Glossary of Oral and Maxillofacial Implants. Int. J. Oral. Maxillofac. Implant. 2017, 32, Gi-G200. [Google Scholar] [CrossRef] [PubMed]
- Koyano, K.; Esaki, D. Occlusion on oral implants: Current clinical guidelines. J. Oral. Rehabil. 2015, 42, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, T.J.; Misch, C.E.; Wang, H.L. Occlusal considerations in implant therapy: Clinical guidelines with biomechanical rationale. Clin. Oral Implant. Res. 2005, 16, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Craddock, H.; Youngson, C. Eruptive tooth movement—the current state of knowledge. Br. Dent. J. 2004, 197, 385–391. [Google Scholar] [CrossRef]
- Steedle, J.R.; Proffit, W.R. The pattern and control of eruptive tooth movements. Am. J. Orthod. 1985, 87, 56–66. [Google Scholar] [CrossRef]
- Wei, H.; Tomotake, Y.; Nagao, K.; Ichikawa, T. Implant prostheses and adjacent tooth migration: Preliminary retrospective survey using 3-dimensional occlusal analysis. Int. J. Prosthodont 2008, 21, 302–304. [Google Scholar] [PubMed]
- Newman, H.N. Attrition, eruption, and the periodontium. J. Dent. Res. 1999, 78, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kweon, H.H.-I.; Choi, S.-H.; Kim, Y.-T. Association between dental implants in the posterior region and traumatic occlusion in the adjacent premolars: A long-term follow-up clinical and radiographic analysis. J. Periodontal Implant Sci. 2016, 46, 396–404. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Xu, J.; Rong, Q.; Pan, S. Influence of occlusal contact and cusp inclination on the biomechanical character of a maxillary premolar: A finite element analysis. J. Prosthet. Dent. 2014, 112, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Torbjörner, A.; Fransson, B. Biomechanical aspects of prosthetic treatment of structurally compromised teeth. Int. J. Prosthodont 2004, 17, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, C.; Pink, C.; Daboul, A.; Samietz, S.; Volzke, H.; Schulz-Kornas, E.; Krey, K.F.; Holtfreter, B.; Kocher, T. Is Continuous Eruption Related to Periodontal Changes? A 16-Year Follow-up. J. Dent. Res. 2021, 22034521999363. [Google Scholar] [CrossRef]
- Oh, W.-S.; DeLong, R.; Anusavice, K.J. Factors affecting enamel and ceramic wear: A literature review. J. Prosthet. Dent. 2002, 87, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, M.; Kern, M. The influence of antagonistic surface roughness on the wear of human enamel and nanofilled composite resin artificial teeth. J. Prosthet. Dent. 2009, 101, 342–349. [Google Scholar] [CrossRef]
- Mair, L.H.; Stolarski, T.A.; Vowles, R.W.; Lloyd, C.H. Wear: Mechanisms, manifestations and measurement. Report of a workshop. J. Dent. 1996, 24, 141–148. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Glaros, A.G.; Kato, T.; Koyano, K.; Lavigne, G.J.; De Leeuw, R.; Manfredini, D.; Svensson, P.; Winocur, E. Bruxism defined and graded: An international consensus. J. Oral. Rehabil. 2013, 40, 2–4. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Hamp, S.E.; Nyman, S.; Lindhe, J. Periodontal treatment of multirooted teeth. Results after 5 years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Van der Zypen, E.; Stich, H.; Sutter, F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J. Maxillofac. Surg. 1981, 9, 15–25. [Google Scholar] [CrossRef]
- Mahalick, J.A.; Knap, F.J.; Weiter, E.J. Occusal wear in prosthodontics. J. Am. Dent. Assoc. 1971, 82, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Cavalleri, A.; Forjanic, M.; Zellweger, G.; Rousson, V. Wear of ceramic and antagonist--a systematic evaluation of influencing factors in vitro. Dent. Mater. 2008, 24, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Mundhe, K.; Jain, V.; Pruthi, G.; Shah, N. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J. Prosthet. Dent. 2015, 114, 358–363. [Google Scholar] [CrossRef]
- Metzler, K.T.; Woody, R.D.; Miller, A.W., 3rd; Miller, B.H. In vitro investigation of the wear of human enamel by dental porcelain. J. Prosthet. Dent. 1999, 81, 356–364. [Google Scholar] [CrossRef]
- Sulong, M.Z.; Aziz, R.A. Wear of materials used in dentistry: A review of the literature. J. Prosthet Dent. 1990, 63, 342–349. [Google Scholar] [CrossRef]
- Yip, K.H.; Smales, R.J.; Kaidonis, J.A. Differential wear of teeth and restorative materials: Clinical implications. Int. J. Prosthodont. 2004, 17, 350–356. [Google Scholar] [PubMed]
- Hacker, C.H.; Wagner, W.C.; Razzoog, M.E. An in vitro investigation of the wear of enamel on porcelain and gold in saliva. J. Prosthet. Dent. 1996, 75, 14–17. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.G.; Park, C.J.; Cho, L.R. Axial displacements in external and internal implant-abutment connection. Clin. Oral Implant. Res. 2014, 25, e83–e89. [Google Scholar] [CrossRef]
- Ko, K.H.; Huh, Y.H.; Park, C.J.; Cho, L.R. Axial displacement in cement-retained prostheses with different implant-abutment connections. Int. J. Oral. Maxillofac. Implant. 2019, 34, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Trulsson, M. Sensory-motor function of human periodontal mechanoreceptors. J. Oral. Rehabil. 2006, 33, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Van Steenberghe, D. From osseoperception to implant-mediated sensory-motor interactions and related clinical implications. J. Oral. Rehabil. 2006, 33, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.I.; Morrison, E.C.; Burt, B.A.; Caffesse, R.G.; Kavanagh, M.T. Natural history of periodontal disease in adults: Findings from the Tecumseh Periodontal Disease Study, 1959–1987. J. Dent. Res. 1990, 69, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Branschofsky, M.; Beikler, T.; Schafer, R.; Flemming, T.F.; Lang, H. Secondary trauma from occlusion and periodontitis. Quintessence Int. 2011, 42, 515–522. [Google Scholar]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; De Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S219–S229. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Y.; Yue, J.; Lu, W.; Zhou, W.; Zhou, X.; Ye, L.; Zheng, Q.; Zhang, L.; Huang, D. Occlusal trauma inhibits osteoblast differentiation and bone formation through IKK-NF-kappaB signaling. J. Periodontol. 2020, 91, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Sun, C.; Akcali, A.; Yeh, Y.C.; Tu, Y.K.; Donos, N. The effect of horizontal and vertical furcation involvement on molar survival: A retrospective study. J. Clin. Periodontol. 2018, 45, 373–381. [Google Scholar] [CrossRef] [PubMed]

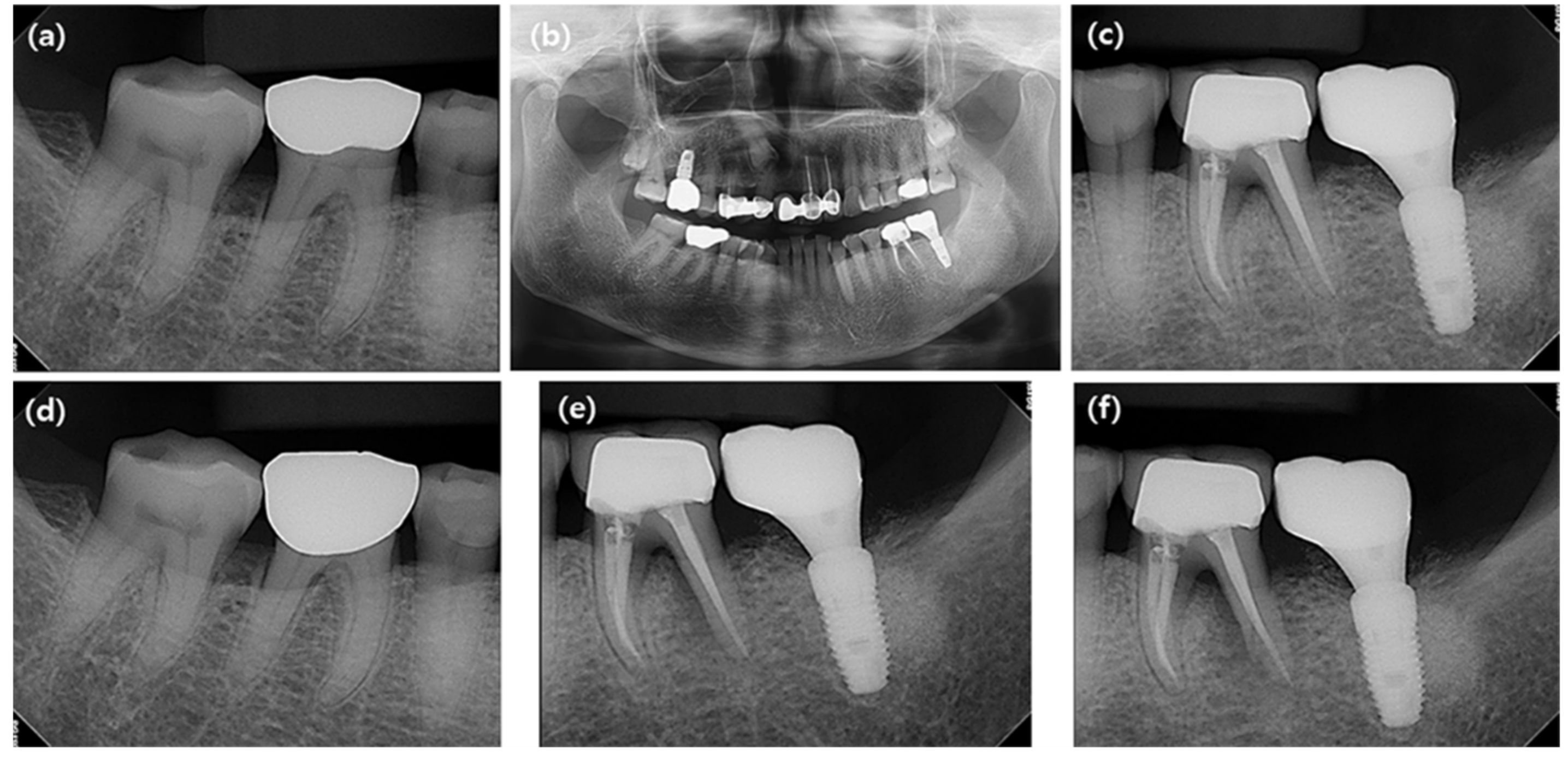

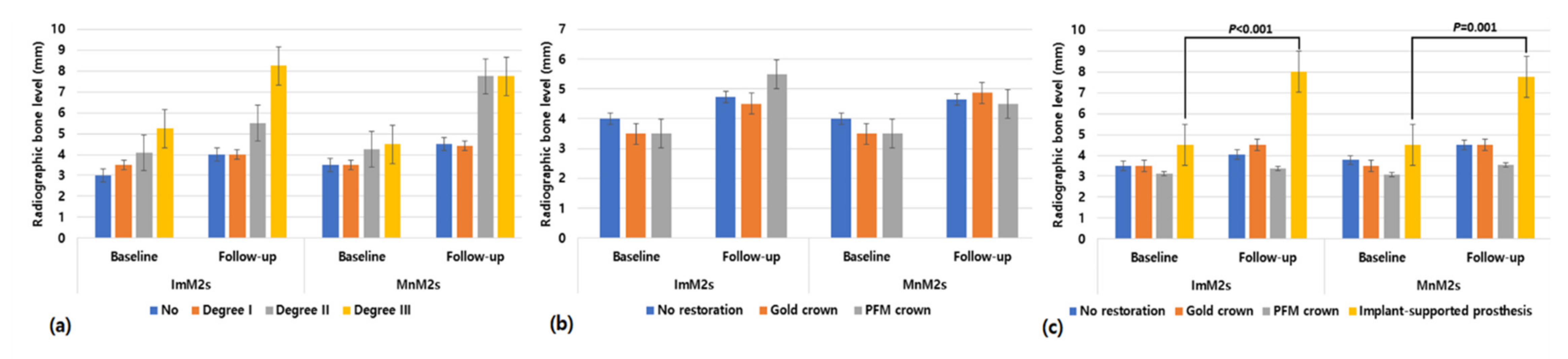
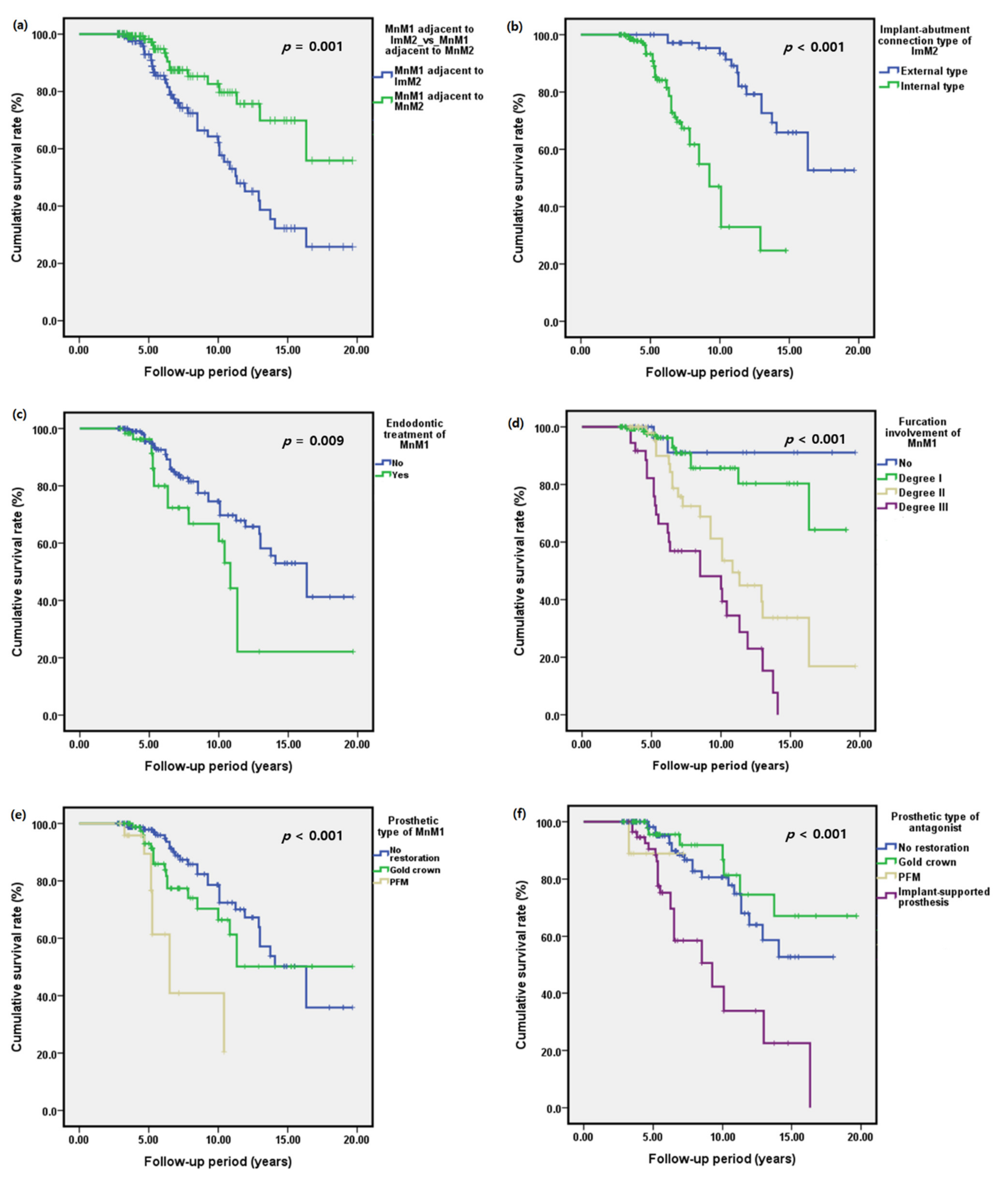
| Variables | N | ||
|---|---|---|---|
| Age (years) | <50 years | 44 (30.56%) | |
| ≥50 years | 100 (69.44%) | ||
| (Mean ± SD) | (54.49 ± 9.59) | ||
| Sex | Male | 89 (61.81%) | |
| Female | 55 (38.19%) | ||
| Smoking | Non-smoker | 106 (73.61%) | |
| Smoker | 38 (26.39%) | ||
| Bruxism | No | 110 (76.39%) | |
| Yes | 34 (23.61%) | ||
| Implant-abutment connection type | External type | 39 (27.08%) | |
| Internal type | 105 (72.92%) | ||
| Peri-implant condition of ImM2 | Healthy | 68 (47.22%) | |
| Peri-implant mucositis | 65 (45.14%) | ||
| Peri-implantitis | 11 (7.64%) | ||
| Distance between ImM2 and MnM1 | <3 mm | 0 (0%) | |
| ≥3 mm | 144 (100%) | ||
| Follow-up (years) | (Mean ± SD, range) | (6.94 ± 3.76, 2.75–19.67) | |
| MnM1 adjacent to ImM2 | MnM1 adjacent to MnM2 | ||
| Endodontic treatment | No | 114 (79.17%) | 118 (81.94%) |
| Yes | 30 (20.83%) | 26 (18.06%) | |
| Furcation involvement | No | 15 (10.42%) | 19 (13.19%) |
| Degree I | 74 (51.39%) | 85 (59.03%) | |
| Degree II | 33 (22.92%) | 26 (18.06%) | |
| Degree III | 22 (15.28%) | 14 (9.72%) | |
| Prosthetic type | no restoration | 84 (58.33%) | 87 (60.42%) |
| gold crown | 47 (32.64%) | 46 (31.94%) | |
| PFM | 13 (9.03%) | 11 (7.64%) | |
| Prosthetic type of antagonist | no restoration | 77 (53.47%) | 79 (54.86%) |
| gold crown | 35 (24.31%) | 31 (21.53%) | |
| PFM | 3 (2.08%) | 6 (4.17%) | |
| Implant-supported prosthesis | 29 (20.14%) | 28 (19.44%) | |
| MnM1s Adjacent to ImM2s | MnM1s Adjacent to MnM2 | |||
|---|---|---|---|---|
| Extraction | No | 103 | 128 | |
| Yes | 41 | 16 | ||
| Endodontic origin | 6 | 6 | ||
| Periodontal origin (with or without endodontic origin) | 31 | 10 | ||
| Root fracture | 4 | 0 | ||
| Variable | Univariate HR | 95% CI | p-Value | Multivariate HR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Age | <50 years | 1 | 1 | ||||
| ≥50 years | 1.64 | 0.89–3.02 | 0.115 | 1.64 | 0.89–3.03 | 0.116 | |
| Sex | Male | 1 | 1 | ||||
| Female | 0.76 | 0.43–1.36 | 0.358 | 0.57 | 0.25–1.31 | 0.187 | |
| Smoking | No | 1 | 1 | ||||
| Yes | 1.21 | 0.70–2.09 | 0.489 | 1.02 | 0.45–2.30 | 0.957 | |
| Bruxism | No | 1 | 1 | ||||
| Yes | 1.8 | 1.02–3.19 | 0.043 | 1.16 | 0.58–2.36 | 0.673 | |
| Mn. 2nd molar site | |||||||
| Implant supported crown | 1 | 1 | |||||
| Natural teeth | 0.39 | 0.22–0.69 | 0.001 | 0.42 | 0.23–0.77 | 0.005 | |
| Implant-abutment connection type | |||||||
| External type | 1 | 1 | |||||
| Internal type | 1.49 | 0.84–2.66 | 0.173 | 4.76 | 1.99–11.38 | <0.001 | |
| Implant health | |||||||
| Healthy | 1 | 0.419 | 1 | 0.029 | |||
| Peri-implant mucositis | 1.1 | 0.51–2.34 | 0.812 | 3.74 | 1.42–9.89 | 0.008 | |
| Peri-implantitis | 1.5 | 0.74–3.06 | 0.262 | 2.62 | 0.98–6.98 | 0.054 | |
| Endodontic treatment of MnM1 | |||||||
| No | 1 | 1 | |||||
| Yes | 2.12 | 1.18–3.82 | 0.012 | 1.4 | 0.50–3.93 | 0.518 | |
| Furcation involvement of MnM1 | |||||||
| 0 | 1 | <0.001 | 1 | <0.001 | |||
| Degree I | 1.93 | 0.43–8.71 | 0.395 | 1.82 | 0.37–8.92 | 0.463 | |
| Degree II | 6.52 | 1.52–28.01 | 0.012 | 5.23 | 1.11–24.63 | 0.036 | |
| Degree III | 14.03 | 3.28–60.08 | <0.001 | 13.23 | 2.71–64.59 | 0.001 | |
| Prosthetic type of MnM1 | |||||||
| No restoration | 1 | <0.001 | 1 | 0.004 | |||
| Gold crown | 1.45 | 0.81–2.59 | 0.207 | 2.79 | 1.18–6.61 | 0.019 | |
| PFM | 5.42 | 2.49–11.79 | <0.001 | 6.66 | 2.17–20.45 | 0.001 | |
| Antagonist prosthetic type of MnM1 | |||||||
| No restoration | 1 | <0.001 | 1 | <0.001 | |||
| Gold crown | 0.64 | 0.27–1.51 | 0.306 | 0.87 | 0.32–2.31 | 0.771 | |
| PFM | 2.39 | 0.32–18.08 | 0.397 | 0.24 | 0.02–2.62 | 0.242 | |
| Implant-supported prosthesis | 3.39 | 1.93–6.00 | <0.001 | 5.62 | 2.72–11.63 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.-B.; Kwon, K.-H.; Hwang, K.-G.; Han, J.-Y. Risk Indicators Affecting the Survival of the Mandibular First Molar Adjacent to an Implant at the Mandibular Second Molar Site: A Retrospective Study. J. Clin. Med. 2021, 10, 2543. https://doi.org/10.3390/jcm10122543
Park W-B, Kwon K-H, Hwang K-G, Han J-Y. Risk Indicators Affecting the Survival of the Mandibular First Molar Adjacent to an Implant at the Mandibular Second Molar Site: A Retrospective Study. Journal of Clinical Medicine. 2021; 10(12):2543. https://doi.org/10.3390/jcm10122543
Chicago/Turabian StylePark, Won-Bae, Koo-Hyun Kwon, Kyung-Gyun Hwang, and Ji-Young Han. 2021. "Risk Indicators Affecting the Survival of the Mandibular First Molar Adjacent to an Implant at the Mandibular Second Molar Site: A Retrospective Study" Journal of Clinical Medicine 10, no. 12: 2543. https://doi.org/10.3390/jcm10122543
APA StylePark, W.-B., Kwon, K.-H., Hwang, K.-G., & Han, J.-Y. (2021). Risk Indicators Affecting the Survival of the Mandibular First Molar Adjacent to an Implant at the Mandibular Second Molar Site: A Retrospective Study. Journal of Clinical Medicine, 10(12), 2543. https://doi.org/10.3390/jcm10122543






