Abstract
Although patients receiving extracorporeal life support (ECLS) as a bridge to transplantation have demonstrated worse outcomes than those without ECLS, we investigated the key factors in the improvement of their posttransplant outcome. From December 2003 to December 2018, 257 adult patients who underwent heart transplantation (HTx) at our institution were included. We identified 100 patients (38.9%) who underwent HTx during ECLS (ECLS group). The primary outcome was 30-day mortality after HTx. The median duration of ECLS was 10.0 days. The 30-day mortality rate was 3.9% (9.2% in peripheral ECLS, 2.9% in central ECLS, and 1.9% in non-ECLS). The use of ECLS was not an independent predictor of 30-day and 1-year mortality (p = 0.248 and p = 0.882, respectively). Independent predictors of 30-day mortality were found to be higher ejection fraction (p < 0.001), Sequential Organ Failure Assessment score (p < 0.001), and total bilirubin level (p = 0.005). In a subgroup analysis, cannulation type was not a predictor of 30-day mortality (p = 0.275). Early ECLS application to prevent organ failure and sophisticated management of acute heart failure may be important steps in achieving favorable survival after HTx.
1. Introduction
Venoarterial (VA) extracorporeal life support (ECLS) is a type of temporary mechanical circulatory support (MCS) for patients in cardiogenic shock [1]. ECLS can be initiated for either newly diagnosed acute cardiac failure or decompensated chronic heart failure in patients already awaiting heart transplantation (HTx) [2]. Although a durable left ventricular assist device (LVAD) is a choice of MCS for bridge to transplantation (BTT), it has several contraindications, such as intolerance for a vitamin K antagonist, poor right ventricular function, restrictive cardiomyopathy, severe intracardiac problems, and patient refusal to use long-term MCS. Furthermore, the health care system, availability of devices, patient’s insurance policy, sociocultural background, and organ transplantation system vary widely by region and country [3].
Although the outcomes of HTx performed directly after ECLS have been poor [4,5], careful patient management in an experienced center showed a favorable outcome [6]. Because the highest priority of HTx in ECLS has been established in most countries, the short waiting time may justify direct HTx from ECLS [7,8,9]. However, the factors that improve post-HTx survival with the liberal use of ECLS as a BTT are not well known. Hence, we reviewed our 15-year experience of HTx to determine the key factors that improve outcome.
2. Methods
2.1. Study Patients
A total of 262 adult patients underwent HTx at Samsung Medical Center from December 2003 to December 2018 (Figure 1). All patients were on the waiting list for HTx in the Korean Network for Organ Sharing (KONOS). We excluded patients who underwent repeated transplantation and those younger than 18 years. In addition, we excluded five patients who had undergone implantation of a durable LVAD before HTx. In the final cohort of 257 cases, 100 patients (38.9%), who were supported by VA ECLS or temporary LVAD while awaiting HTx, were assigned to the ECLS group. In 65 patients, peripheral cannulation was maintained until HTx, and these patients were assigned to the peripheral ECLS group. The other 35 patients who had central ECLS at the time of HTx were assigned to the central ECLS group.
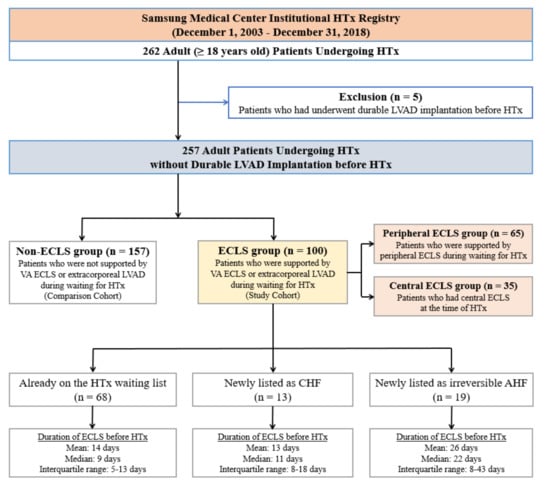
Figure 1.
Flow diagram of patient recruitment. HTx, heart transplantation; LVAD, left ventricular assist device; ECLS, extracorporeal life support; VA, venoarterial; CHF, chronic heart failure; AHF, acute heart failure.
2.2. Indication for ECLS Installation and Criteria for ECLS as a BTT
In patients who were already on the waiting list for HTx, the degree of organ failure, incidence of ventricular arrhythmia, and symptoms of low cardiac output and pulmonary edema were principally used in the decision to commence ECLS. In cases of deterioration of organ failure or symptoms related to heart failure, patients were closed monitored by both heart failure physicians and cardiac intensivists. The decision to commence ECLS was taken using a multidisciplinary team approach. Patients who could not be weaned from VA ECLS and met all following criteria were considered potential candidates of ECLS as a BTT: normal mentation; no irreversible organ failure; age < 70 years; absence of active infection; absence of severe pulmonary hypertension; no recent history of malignancy; and good social support. Final listing for HTx was made after discussion among the multidisciplinary team.
2.3. Management and Cannulation Strategies of ECLS
Durable LVAD was not covered by Korean National Insurance before October 2018. Therefore, most patients who failed to respond to medical therapy or intra-aortic balloon pump underwent ECLS as BTT. Patients at our institution with acute and chronic heart failure were observed by a multidisciplinary heart failure team, which was formally established in 2014. Since 2014, all patients have been provided care under the updated guidelines of modern critical care, including prevention and management of pain, agitation, delirium, immobilization, and sleep deprivation [10].
The establishment of peripheral VA ECLS using Seldinger’s technique at our institution has been described previously [1]. When the left ventricle was distended, atrial septal puncture was performed by interventionists in a catheterization laboratory (n = 17). We have not used intra-aortic balloon pumps as a means of left heart decompression [11,12]. In 18 patients (51.4%) in the central ECLS group, peripheral VA ECLS was initially implemented and later converted to central ECLS. Another 17 patients (48.6%) in the central ECLS group underwent central cannulation from the beginning of ECLS. In our institution, surgical left heart decompressive procedures are typically performed during central cannulation. The strategy of left heart decompression at our institution has been previously published [13]. Among these 35 patients in the central ECLS group, 10 patients (28.6%) had LVAD-type cannulation using an ECLS device. We avoided mechanical ventilation and immobilization as much as possible in accordance with the stability of the cannulation site and the patients’ general condition. This strategy enables patients who are receiving ECLS to wait for HTx with a minimal risk of ventilator-associated pneumonia and complications related to peripheral cannulation.
2.4. Endpoints and Follow-Up
The primary outcome of the study was 30-day mortality. All patients were monitored after surgery by HTx physicians at Samsung Medical Center. Baseline characteristics of clinical data were collected from medical records and databases. We acquired follow-up clinical data, including vital status, through a review of medical records and telephone interviews. To complete the data, including mortality, we confirmed information by the National Registry of Births and Deaths using the unique personal identification number for each patient.
2.5. Statistical Analysis
Baseline characteristics, anthropometric, and clinical characteristics of the study population are presented as either mean ± standard deviation, median with interquartile range (IQR), or frequency and proportion. Wilcoxon rank sum tests were used to compare skewed continuous variables. For categorical variables, the chi-square and Fisher’s exact tests were used to compare variables among the groups. The Cox proportional hazards regression model for univariable and multivariable analyses were used to determine independent predictors of 30-day mortality. Multivariable analyses were performed using a stepwise variable selection method, in which all variables with a p value of less than 0.15 were included in the univariable analyses. To estimate the survival curves during the follow-up period, we used the Kaplan–Meier method, and survival rates were compared among groups using the log rank test.
We adjusted for differences in baseline characteristics using weighted Cox proportional hazards regression models with inverse probability of treatment weighting (IPTW) to reduce potential confounding factors [14]. Variables for adjustment are summarized in Table A1. The standardized difference was calculated from the mean and prevalence for continuous and dichotomous variables, respectively, and the results are shown in Table 1.

Table 1.
Baseline characteristics of all patients.
All tests were two-tailed. p values of less than 0.05 were used to denote statistical significance. Statistical analysis was performed with the R programming language, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
2.6. Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2019-09-109). Informed consent from the study participants was waived due to the retrospective nature of this study.
3. Results
3.1. Baseline Characteristics
The median age of all patients was 53 years (range, 18–78 years), and 79 patients (30.7%) were female. The median duration of ECLS before HTx was 10.0 days (IQR, 7–17 days). In the ECLS group, 68 ECLS patients were on the waiting list for HTx at the time of VA ECLS implantation. In the other 13 ECLS patients, decompensated chronic heart failure was diagnosed after hospitalization. Another 19 ECLS patients were diagnosed with irreversible acute heart failure (Figure 1). Table 1 summarizes the baseline characteristics of the patients in the ECLS and non-ECLS groups and we found significant differences in several characteristics between the two groups.
3.2. Perioperative Outcomes and Predictors of 30-Day Mortality
Although cardiopulmonary bypass time and total ischemic time were similar in the two groups, postoperative complications were more frequent in the ECLS group than in the non-ECLS group. Table 2 summarizes the operative and postoperative data.

Table 2.
Operative and postoperative characteristics of all patients.
The 30-day mortality rate in the overall cohort was 3.9% (n = 10). After univariable adjustment including IPTW, 30-day mortality rates did not differ significantly between the two groups (7.0% in ECLS and 1.9% in non-ECLS; p = 0.248). The 1-year mortality rate in the overall cohort was 12.8% (n = 33). After univariable adjustment including IPTW, 1-year mortality rates did not differ significantly between the two groups (16.0% in ECLS and 10.8% in non-ECLS; p = 0.882). After multivariable adjustment including IPTW, Kaplan–Meier curve did not show a significant difference in mortality up to one year after HTx (Figure 2; log rank p = 0.991).
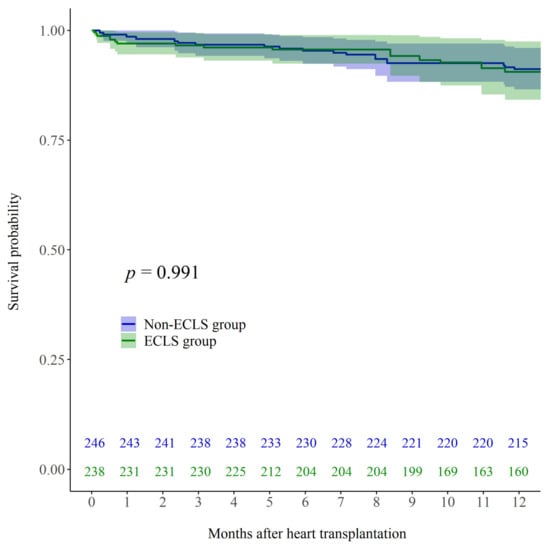
Figure 2.
Kaplan–Meier post-HTx survival curves for patients who received ECLS while waiting for HTx (ECLS; green line) and those who did not receive ECLS before HTx (Non-ECLS; blue line) after multivariable adjustment including IPTW. HTx, heart transplantation; ECLS, extracorporeal life support; IPTW, inverse probability of treatment weighting.
Table 3 summarizes the results of the Cox proportional hazards regression model for univariable and multivariable analyses of 30-day mortality after IPTW. Univariable analysis including IPTW indicated that the use of ECLS was not a predictor of 30-day mortality (p = 0.248; hazard ratio (HR) 2.132; 95% confidence interval (CI) 0.590–7.704). Multivariable analysis including IPTW indicated that left ventricular ejection fraction (LVEF; p < 0.001; HR 1.064; 95% CI 1.025–1.103), Sequential Organ Failure Assessment (SOFA) score (p < 0.001; HR 1.349; 95% CI 1.184–1.538), and the total bilirubin level (p = 0.005; HR 1.055; 95% CI 1.016−1.095) were independent predictors of 30-day mortality.

Table 3.
Independent predictors of 30-day mortality after IPTW.
3.3. Subgroup Analysis of the ECLS Group According to Cannulation Type
Table 4 summarizes the baseline characteristics and postoperative data of patients in the peripheral and central ECLS groups. The durations of ECLS and ventilator support before HTx were longer in the central ECLS group compared to the peripheral ECLS group.

Table 4.
Subgroup analysis of the ECLS group according to the cannulation type.
No significant differences in 30-day outcomes were observed between the two groups (9.2% mortality in the peripheral ECLS group and 2.9% mortality in the central ECLS group; p = 0.275). Further, one-year outcomes did not differ significantly between the two groups (18.5% mortality in the peripheral ECLS group and 11.4% mortality in the central ECLS group; p = 0.379).
Kaplan–Meier analysis demonstrated no significant differences in mortality up to one year after HTx among the peripheral ECLS, central ECLS, and non-ECLS groups (Figure A1; log rank p = 0.179).
4. Discussion
Although the best option for BTT is durable LVAD, there are a few circumstances in which ECLS can be favored. First, in some countries, a durable LVAD is still not available or is too restrictive [15]. For example, the approval process by the National Health Insurance in Korea takes a few weeks. Second, when a patient has severe right ventricular dysfunction or restrictive cardiomyopathy, the implantation of durable LVAD is not a viable option [16,17]. Furthermore, in most national organ-sharing systems, highest priority for HTx is given to patients on temporary MCS such as ECLS. In such transplantation systems, the waiting time on ECLS may be within the safe range, namely, less than a few weeks, if a high level of ICU and ECLS care is provided.
Although post-HTx complications were more common in the ECLS group than in the non-ECLS group, there was no statistically significant difference in 30-day mortality between the two groups even after adjustment by IPTW. High LVEF, high SOFA score, and high total bilirubin level were found to be independent risk factors. Thus, timing of ECLS insertion is important, and ECLS should be deployed before severe multiorgan failure, including hepatic failure. The finding that high LVEF was an independent predictor of 30-day mortality was interesting. We believe that patients with relatively high LVEF have developed acute heart failure due to acute myocardial infarction, myocarditis, hypertrophic cardiomyopathy at burn-out stage, or restrictive cardiomyopathy. These conditions, diseases, and sequelae may not be favorable to HTx. Therefore, we suggest special attention be paid to patients with irreversible acute heart failure.
The most important step to improve outcomes of HTx is timely ECLS initiation. The second step would be applying sophisticated ECLS and up-to-date intensive care to prevent both ECLS and intensive care-related complications. For patients who are waiting for HTx while on ECLS, the final step could be the liberal use of central cannulation. In some relatively stable patients who require a prolonged waiting time for HTx, primary central ECLS can be performed. In other patients who already had peripheral ECLS, cannulation can be stitched to a central type after careful discussion and hemodynamic stabilization. Central cannulation and circuit configurations vary according to the patient’s right ventricular and pulmonary function. In general, we consider central conversion on the 14th day of peripheral cannulation. Figure 3 shows our recent strategy.
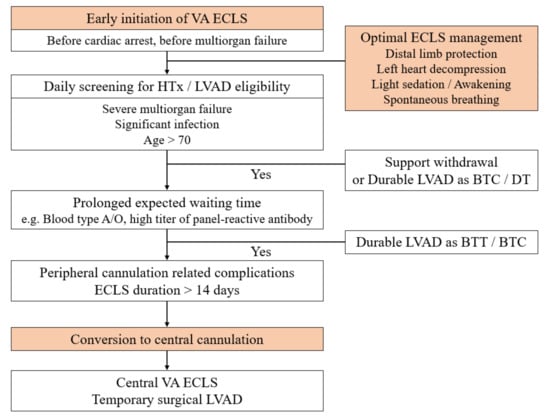
Figure 3.
The recent strategy of ECLS as BTT in our institution. ECLS, extracorporeal life support; BTT, bridge to transplantation; VA, venoarterial; HTx, heart transplantation; LVAD, left ventricular assist device; BTC, bridge to candidacy; DT, destination therapy.
Moonsamy et al. also reported that temporary circulatory support (TCS)-VAD had a survival advantage over ECLS and was similar to durable LVAD as a BTT in the United Network of Organ Sharing (UNOS) database [7]. In their study, there is no information about cannulation type or ECLS duration. In our study, the definition of ECLS includes TCS-VAD described in Moonsamy’s study, because 35.0% of our ECLS patients had nonperipheral cannulation, including central VA ECLS and temporary VAD with/without membrane oxygenator TCS-VAD. In other words, in an emergency setting, temporary VAD implantation cannot be placed as conveniently and quickly as ECLS. We believe that some patients in the TCS-VAD group probably had been converted from peripheral ECLS, like our patients. Therefore, we believe that various cannulation options should be offered to patients, including either direct central cannulation or peripheral ECLS first. To reduce peripheral cannulation-related complications, the timely conversion to central cannulation is critical.
Coutance et al. also compared ECLS-bridged HTx and non-ECLS-bridged HTx in their large retrospective study [6]. Although they emphasized strong ECLS care, including the prevention of ECLS complications and the application of light sedation, their selection criteria for HTx in ECLS patients seems to be quite strict. In contrast to our procedure, they performed HTx only for patients without other organ failure. Because the study by Coutance et al. included only relatively low-risk patients [6], it is difficult to determine how we can improve the outcome of HTx. Table 5 presents a comparison of previous studies (Coutance et al. and Moonsamy et al.) with the current study [6,7]. Each paper showed various HTx waiting periods, and to improve HTx outcomes, it will be important to implement the optimal settings for BTT based on the circumstances of each country.

Table 5.
Comparison of recent and current studies.
We used an approach to prevent the occurrence of ECLS-related complications. Most patients had a preventive distal limb perfusion catheter. For example, in the ECLS group, the rate of limb ischemia was 5.0% (Table 2). During the same period as our study, the rate of limb ischemia in all patients with VA ECLS at our institution was 5.69%. Taking into consideration that the rate of limb ischemia has been reported to be as high as 70% [18], our outcome is good. Intensivists have maintained the most up-to-date ICU care, such as light sedation, minimally invasive ventilation, and aggressive mobilization. High-quality ECLS and ICU care were also emphasized in the article by Coutance et al. [6]. Although post-HTx complications were more common in the ECLS group than in the non-ECLS group, they did not affect post-HTx survival.
Study Limitations
As this study was retrospective and involved 257 patients at a single tertiary center, its statistical power may be limited. The duration of ECLS before HTx in the ECLS group was short (median: 10 days, mean: 16.0 ± 18.9 days). However, the duration was longer than in previous studies [6,19]. We did not include patients who were listed for HTx but did not receive transplantation. Thus, the study may have some selection bias.
Further, data regarding transfusion rates were not included in our database. Accordingly, patients in the ECLS group may have received greater blood transfusion volumes than those in the non-ECLS group, and some perioperative complications are known to be associated with rate of blood transfusion.
As implantable LVADs were not covered by Korean National Insurance until September 2018, the present study population did not have access to a LVAD [20]. In the era of implantable LVAD, ECLS is still used as a BTT strategy in South Korea, many European countries, many Asian countries, and North America. The number of patients using ECLS as BTT can vary according to national transplant systems and insurance policies. We believe that our clinical experience may be of value to many clinicians.
5. Conclusions
After adjustment including IPTW, the use of ECLS before HTx was not a risk factor of 30-day mortality. Degree of organ failure, particularly hepatic failure and acute heart failure, were independent predictors of 30-day mortality. Early ECLS application to prevent organ failure and sophisticated management of acute heart failure may be important steps in achieving favorable survival after HTx.
Author Contributions
Conceptualization, J.H.L., J.-O.C., and Y.H.C.; Methodology, J.H.L., N.C., Y.J.K., J.H.Y., S.H.S., and Y.H.C.; Formal analysis, J.H.L., N.C., J.-O.C., and Y.H.C.; Writing—original draft preparation, J.H.L. and Y.J.K.; Writing—review and editing, K.S., W.S.K., D.K., J.H.Y., E.-S.J., S.H.S., J.-O.C., and Y.H.C.; Supervision, J.-O.C. and Y.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: KMDF_PR_20200901_0159, 9991006829).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2019-09-109).
Informed Consent Statement
Informed consent from the study participants was waived due to the retrospective nature of this study.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors thank Kyunga Kim and Na-Young Hwang (Statistics and Data Center at Samsung Medical Center) for their help with the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BTC: bridge to candidacy; BTT, bridge to transplantation; CI, confidence interval; CPR, cardiopulmonary resuscitation; ECLS, extracorporeal life support; HR, hazard ratio; HTx, heart transplantation; ICU, intensive care unit; IPTW, inverse probability of treatment weighting; IQR, interquartile range; KONOS, Korean Network for Organ Sharing; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; SOFA, Sequential Organ Failure Assessment; TCS, temporary circulatory support; UNOS, United Network of Organ Sharing; VA, venoarterial.
Appendix A

Table A1.
Variables for adjustment.
Table A1.
Variables for adjustment.
| Variables |
|---|
| Age of recipient |
| Female recipients |
| Age of donor |
| Female donors |
| Gender mismatch |
| Diabetes |
| Hypertension |
| Body mass index |
| Previous cardiac surgery |
| Previous PCI or CABG |
| Stroke |
| Chronic renal insufficiency |
| Dialysis |
| CPR |
| LVEF |
| DCMP |
| ICMP |
| SOFA score |
| Total bilirubin |
PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; CPR, cardiopulmonary resuscitation; LVEF, left ventricular ejection fraction; DCMP, dilated cardiomyopathy; ICMP, ischemic cardiomyopathy; SOFA, Sequential Organ Failure Assessment; HTx, heart transplantation; ECLS, extracorporeal life support.
Appendix B
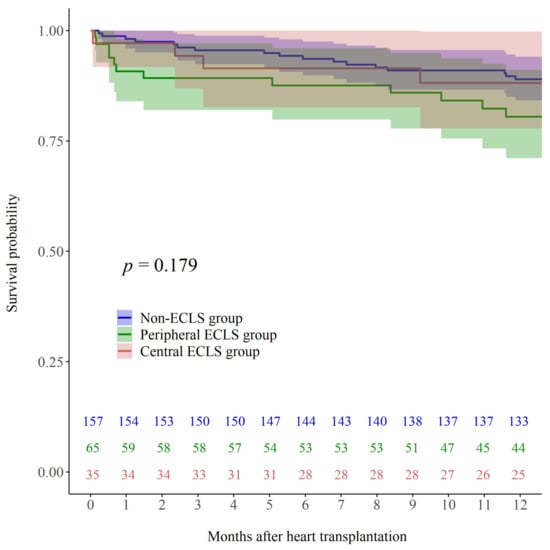
Figure A1.
Kaplan–Meier post-HTx survival curves for patients who were supported by peripheral ECLS while waiting for HTx (Peripheral ECLS; green line), those who had central ECLS at the time of HTx (Central ECLS; red line), and those who did not receive ECLS before HTx (Non-ECLS; blue line).
References
- Cho, Y.H.; Yang, J.H.; Sung, K.; Jeong, D.S.; Park, P.W.; Kim, W.S.; Lee, Y.T.; Jeon, E.S. Extracorporeal life support as a bridge to heart transplantation: Importance of organ failure in recipient selection. ASAIO J. 2015, 61, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Tarzia, V.; Bortolussi, G.; Bianco, R.; Buratto, E.; Bejko, J.; Carrozzini, M.; De Franceschi, M.; Gregori, D.; Fichera, D.; Zanella, F.; et al. Extracorporeal life support in cardiogenic shock: Impact of acute versus chronic etiology on outcome. J. Thorac. Cardiovasc. Surg. 2015, 150, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sivathasan, C.; Lim, C.P.; Kerk, K.L.; Sim, D.K.; Mehra, M.R. Mechanical circulatory support and heart transplantation in the Asia Pacific region. J. Heart Lung Transpl. 2017, 36, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. New Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Holley, C.T.; Harvey, L.; John, R. Left ventricular assist devices as a bridge to cardiac transplantation. J. Thorac. Dis. 2014, 6, 1110–1119. [Google Scholar] [PubMed]
- Coutance, G.; Jacob, N.; Demondion, P.; Nguyen, L.S.; Bouglé, A.; Bréchot, N.; Varnous, S.; Leprince, P.; Combes, A.; Lebreton, G. Favorable outcomes of a direct heart transplantation strategy in selected patients on extracorporeal membrane oxygenation support. Crit. Care Med. 2020, 48, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Moonsamy, P.; Axtell, A.L.; Ibrahim, N.E.; Funamoto, M.; Tolis, G.; Lewis, G.D.; D’Alessandro, D.A.; Villavicencio, M.A. Survival after heart transplantation in patients bridged with mechanical circulatory support. J. Am. Coll. Cardiol. 2020, 75, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Takeda, K.; Kurlansky, P.A.; Naka, Y.; Takayama, H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J. Thorac. Cardiovasc. Surg. 2018, 155, 1607–1618.e6. [Google Scholar] [CrossRef] [PubMed]
- Parhar, K.K.; Fedak, P.W.M. Bridging to heart transplant with extracorporeal membrane oxygenation: Good or VAD? J. Thorac. Cardiovasc. Surg. 2018, 155, 1619–1620. [Google Scholar] [CrossRef] [PubMed]
- Delvin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar]
- Choi, M.S.; Sung, K.; Cho, Y.H. Clinical pearls of venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Korean Circ. J. 2019, 49, 657–677. [Google Scholar] [CrossRef] [PubMed]
- Na, S.J.; Chung, C.R.; Jeon, K.; Park, C.M.; Suh, G.Y.; Ahn, J.H.; Carriere, K.C.; Song, Y.B.; Choi, J.O.; Hahn, J.Y.; et al. Association between presence of a cardiac intersivist and mortality in an adult cardiac care unit. J. Am. Coll. Cardiol. 2016, 68, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Na, S.J.; Yang, J.H.; Yang, J.H.; Sung, K.; Choi, J.O.; Hahn, J.Y.; Jeon, E.S.; Cho, Y.H. Left heart decompression at venoarterial extracorporeal membrane oxygenation initiation in cardiogenic shock: Prophylactic versus therapeutic strategy. J. Thorac. Dis. 2019, 11, 3746–3756. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.M.; Hernán, M.Á.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Sivathasan, C.; Hayward, C.; Jansz, P.; Sibal, A.K.; Chen, C.; Cally, H.K.L.; Balakrishnam, K.R.; Cho, Y.H.; Nordin, M.N.; Barril, J.B.; et al. Durable mechanical circulatory support across the Asia-Pacific region. J. Heart Lung Transpl. 2020, 39, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Dipchand, A.I.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Yusen, R.D.; Stehlik, J. The registry of the International Society for Heart and Lung Transplantation: Thirty-third adult heart transplantation report—2016; focus theme: Primary diagnostic indications for transplant. J. Heart Lung Transpl. 2016, 35, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transpl. 2017, 36, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Bonicolini, E.; Martucci, G.; Simons, J.; Raffa, G.M.; Spina, C.; Coco, V.L.; Arcadipane, A.; Pilato, M.; Lorusso, R. Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: A narrative review of incidence, prevention, monitoring, and treatment. Crit. Care 2019, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Rousse, N.; Juthier, F.; Pincon, C.; Hysi, I.; Banfi, C.; Robin, E.; Fayad, G.; Jegou, B.; Prat, A.; Vincentelli, A. ECMO as a bridge to decision: Recovery, VAD, or heart transplantation? Int. J. Cardiol. 2015, 187, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, I.; Lee, H.; Sung, K.; Lee, Y.T.; Kim, D.; Yang, J.H.; Choi, J.O.; Jeon, E.S.; Cho, Y.H. Use of durable left ventricular assist devices for high-risk patients: Korean experience before insurance coverage. J. Thorac. Dis. 2020, 12, 7236–7244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).