Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size
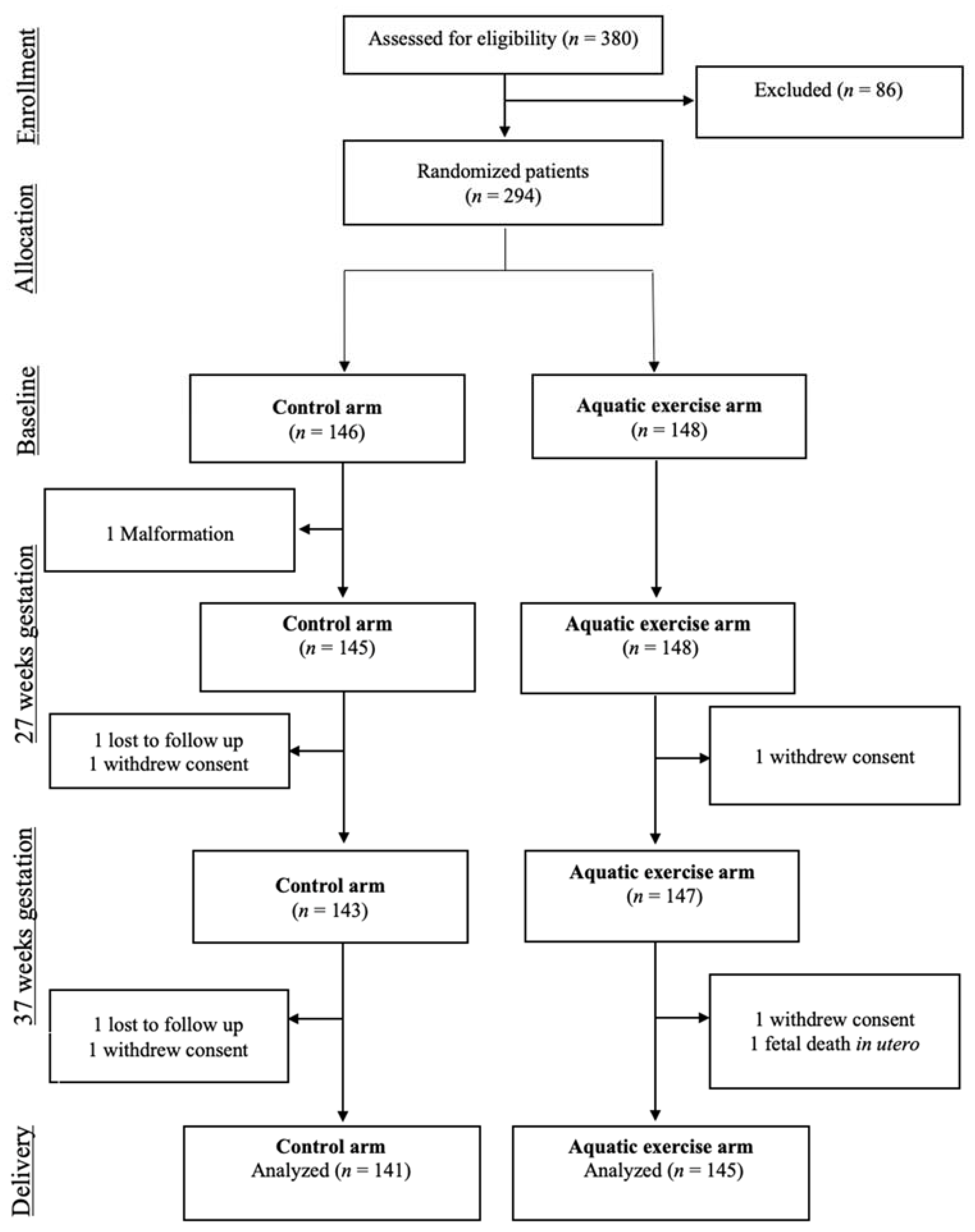
2.4. Randomization and Blinding
2.5. Intervention
2.6. Data Collection
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Stewart, D.E.; Robertson, P.; Dennis, C.; Grace, S.L.; Wallington, T. Postpartum Depression: Literature REVIEW of risk Factors and Interventions; University Health Network Women’s Health Program: Toronto, ON, USA, 2003. [Google Scholar]
- ACOG Committee Opinion No. 757: Screening for Perinatal Depression. Obstet. Gynecol. 2018, 132, e208–e212. [CrossRef]
- Fisher, J.; De Mello, M.C.; Patel, V.; Rahman, A.; Tran, T.; Holton, S.; Holmes, W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: A systematic review. Bull. World Heal. Organ. 2011, 90, 139–149H. [Google Scholar] [CrossRef]
- Gavin, N.I.; Gaynes, B.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal Depression. Obstet. Gynecol. 2005, 106, 1071–1083. [Google Scholar] [CrossRef]
- NICE. Antenatal and Postnatal Mental Health Overview; National Institute for Health and Care Excellence: London, UK, 2020. [Google Scholar]
- Hahn-Holbrook, J.; Cornwell-Hinrichs, T.; Anaya, I. Economic and Health Predictors of National Postpartum Depression Prevalence: A Systematic Review, Meta-analysis, and Meta-Regression of 291 Studies from 56 Countries. Front. Psychiatry 2018, 8, 248. [Google Scholar] [CrossRef]
- O’Hara, M.W.; Wisner, K.L. Perinatal mental illness: Definition, description and aetiology. Best Pr. Res. Clin. Obstet. Gynaecol. 2014, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muñoz, M.D.L.F.; Le, H.-N.; de la Cruz, I.V.; Crespo, M.E.O.; Méndez, N.I. Feasibility of screening and prevalence of prenatal depression in an obstetric setting in Spain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, J.; Martín-Santos, R.; Garcia-Esteve, L.; Carot, J.M.; Guillamat, R.; Gutiérrez-Zotes, A.; Gornemann, I.; Canellas, F.; Baca-García, E.; Jóver, M.; et al. Mood changes after delivery: Role of the serotonin transporter gene. Br. J. Psychiatry 2008, 193, 383–388. [Google Scholar] [CrossRef]
- Terrén, C.A.; Esteve, L.G.; Navarro, P.; Aguado, J.; Ojuel, J.; Tarragona, M.J. Prevalence of postpartum depression in Spanish mothers: Comparison of estimation by mean of the structured clinical interview for DSM-IV with the Edinburgh Postnatal Depression Scale. Med. Clínica 2003, 120, 326–329. [Google Scholar] [CrossRef]
- García-Esteve, L.; Giménez, A.T.; Gurrutxaga, M.L.I.; García, P.N.; Terrén, C.A.; Gelabert, E. Maternity, Migration, and Mental Health: Comparison Between Spanish and Latina Immigrant Mothers in Postpartum Depression and Health Behaviors. In Perinatal Depression among Spanish-Speaking and Latin American Women; Lara-Cinisomo, S., Wisner, K.L., Eds.; Springer: New York, NY, USA; pp. 15–37.
- Goodman, J.H.; Santangelo, G. Group treatment for postpartum depression: A systematic review. Arch. Women’s Ment. Health. 2011, 14, 277–293. [Google Scholar] [CrossRef]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of Persistent and Severe Postnatal Depression with Child Outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Wouk, K.; Stuebe, A.M.; Meltzer-Brody, S. Postpartum Mental Health and Breastfeeding Practices: An Analysis Using the 2010–2011 Pregnancy Risk Assessment Monitoring System. Matern. Child Health. J. 2017, 21, 636–647. [Google Scholar] [CrossRef]
- Grigoriadis, S.; Wilton, A.S.; Kurdyak, P.A.; Rhodes, A.E.; VonderPorten, E.H.; Levitt, A.; Cheung, A.; Vigod, S.N. Perinatal suicide in Ontario, Canada: A 15-year population-based study. Can. Med. Assoc. J. 2017, 189, E1085–E1092. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.M.; Khalifeh, H. Perinatal mental health: A review of progress and challenges. World Psychiatry 2020, 19, 313–327. [Google Scholar] [CrossRef]
- Ko, J.Y.; Farr, S.L.; Dietz, P.M.; Robbins, C.L. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005-2009. J. Women’s Health. 2012, 21, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Rockhill, K.M.; Tong, V.T.; Morrow, B.; Farr, S.L. Trends in Postpartum Depressive Symptoms — 27 States, 2004, 2008, and 2012. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 153–158. [Google Scholar] [CrossRef]
- Depression in Adults: Recognition and Management; Clinical Guidelines; National Institute for Health and Care Excellence: London, UK, 2009.
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013, CD004366. [Google Scholar] [CrossRef]
- Morres, I.D.; Hatzigeorgiadis, A.; Stathi, A.; Comoutos, N.; Arpin-Cribbie, C.; Krommidas, C.; Theodorakis, Y. Aerobic exercise for adult patients with major depressive disorder in mental health services: A systematic review and meta-analysis. Depression Anxiety 2019, 36, 39–53. [Google Scholar] [CrossRef]
- Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2020, 135, e178–e188. [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Artal, R. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br. J. Sports Med. 2003, 37, 6–12. [Google Scholar] [CrossRef]
- Rising, S.S.; Senterfitt, C. Repairing Health Care: Building Relationships Through Groups. Creative Nurs. 2009, 15, 178–182. [Google Scholar] [CrossRef]
- Dipietro, L.; Evenson, K.R.; Bloodgood, B.; Sprow, K.; Troiano, R.P.; Piercy, K.L.; Vaux-Bjerke, A.; Powell, K.E. Benefits of Physical Activity during Pregnancy and Postpartum: An Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1292–1302. [Google Scholar] [CrossRef]
- Daley, A.J.; Blamey, R.V.; Jolly, K.; Roalfe, A.K.; Turner, K.M.; Coleman, S.; McGuinness, M.; Jones, I.; Sharp, D.J.; MacArthur, C. A pragmatic randomized controlled trial to evaluate the effectiveness of a facilitated exercise intervention as a treatment for postnatal depression: The PAM-PeRS trial. Psychol. Med. 2015, 45, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Ruchat, S.-M.; Poitras, V.J.; Garcia, A.J.; Gray, C.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef]
- Pritchett, R.V.; Daley, A.J.; Jolly, K. Does aerobic exercise reduce postpartum depressive symptoms? a systematic review and meta-analysis. Br. J. Gen. Pr. 2017, 67, e684–e691. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, A.P.; Boulé, N.G.; Sivak, A.; Davenport, M.H. Effects of Exercise on Mild-to-Moderate Depressive Symptoms in the Postpartum Period. Obstet. Gynecol. 2017, 129, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; van der Waerden, J.; Melchior, M.; Bolze, C.; El-Khoury, F.; Pryor, L. Physical activity during pregnancy and postpartum depression: Systematic review and meta-analysis. J. Affect. Disord. 2019, 246, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Ruchat, S.-M.; Sobierajski, F.; Poitras, V.J.; Gray, C.; Yoo, C.; Skow, R.J.; Garcia, A.J.; Barrowman, N.; Meah, V.L.; et al. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 53, 99–107. [Google Scholar] [CrossRef]
- Poyatos-León, R.; García-Hermoso, A.; Sanabria-Martínez, G.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Martínez-Vizcaíno, V. Effects of exercise-based interventions on postpartum depression: A meta-analysis of randomized controlled trials. Birth 2017, 44, 200–208. [Google Scholar] [CrossRef]
- Kołomańska-Bogucka, D.; Mazur-Bialy, A.I. Physical Activity and the Occurrence of Postnatal Depression—A Systematic Review. Medicina 2019, 55, 560. [Google Scholar] [CrossRef]
- Vargas-Terrones, M.; Barakat, R.; Santacruz, B.; Buihgas, I.F.; Mottola, M.F. Physical exercise programme during pregnancy decreases perinatal depression risk: A randomised controlled trial. Br. J. Sports Med. 2018, 53, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cordero, M.J.; Sánchez-García, J.C.; Blanque, R.R.; López, A.M.S.; Mur-Villar, N. Moderate Physical Activity in an Aquatic Environment During Pregnancy (SWEP Study) and Its Influence in Preventing Postpartum Depression. J. Am. Psychiatr. Nurses Assoc. 2019, 25, 112–121. [Google Scholar] [CrossRef]
- Coll, C.D.V.N.; Domingues, M.R.; Stein, A.; Da Silva, B.G.C.; Bassani, D.G.; Hartwig, F.P.; Da Silva, I.C.M.; Da Silveira, M.F.; Da Silva, S.G.; Bertoldi, A.D. Efficacy of Regular Exercise During Pregnancy on the Prevention of Postpartum Depression: The PAMELA Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e186861. [Google Scholar] [CrossRef] [PubMed]
- Songøygard, K.M.; Stafne, S.N.; Evensen, K.A.I.; Salvesen, K.Å.; Vik, T.; Mørkved, S. Does exercise during pregnancy prevent postnatal depression? Acta Obstet. et Gynecol. Scand. 2011, 91, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A. Alterations in sleep during pregnancy and postpartum: A review of 30 years of research. Sleep Med. Rev. 1998, 2, 231–242. [Google Scholar] [CrossRef]
- Montgomery-Downs, H.E.; Insana, S.P.; Clegg-Kraynok, M.M.; Mancini, L.M. Normative longitudinal maternal sleep: The first 4 postpartum months. Am. J. Obstet. Gynecol. 2010, 203, 465.e1–465.e7. [Google Scholar] [CrossRef]
- Maracy, M.R.; Iranpour, S.; Kheirabadi, G.R.; Esmaillzadeh, A.; Heidari-Beni, M. Association between sleep quality and postpartum depression. J. Res. Med. Sci. 2016, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.; Dritsa, M.; Verreault, N.; Balaa, C.; Kudzman, J.; Khalifé, S. Sleep problems and depressed mood negatively impact health-related quality of life during pregnancy. Arch. Women’s Ment. Health 2010, 13, 249–257. [Google Scholar] [CrossRef]
- Chang, J.J.; Pien, G.W.; Duntley, S.P.; Macones, G.A. Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Med. Rev. 2010, 14, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dørheim, S.K.; Bondevik, G.T.; Eberhard-Gran, M.; Bjorvatn, B. Sleep and Depression in Postpartum Women: A Population-Based Study. Sleep 2009, 32, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Bos, S.C.; Soares, M.J.; Maia, B.R.; Pereira, A.T.; Valente, J.; Gomes, A.A.; Macedo, A.; Azevedo, M.H. Is insomnia in late pregnancy a risk factor for postpartum depression/depressive symptomatology? Psychiatry Res. 2011, 186, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Lan, S.-J.; Yen, Y.-Y.; Hsieh, Y.-P.; Kung, P.-T.; Lan, S.-H. Effects of Exercise on Sleep Quality in Pregnant Women: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Asian Nurs. Res. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Blanque, R.; Garcia, J.C.S.; López, A.M.S.; Expósito-Ruiz, M.; Aguilar-Cordero, M.J. Randomized Clinical Trial of an Aquatic Physical Exercise Program During Pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 2019, 48, 321–331. [Google Scholar] [CrossRef]
- Navas, A.; Artigues, C.; Leiva, A.; Portells, E.; Soler, A.; Cladera, A.; Ortas, S.; Alomar, M.; Gual, M.; Manzanares, C.; et al. Effectiveness and safety of moderate-intensity aerobic water exercise during pregnancy for reducing use of epidural analgesia during labor: Protocol for a randomized clinical trial. BMC Pregnancy Childbirth 2018, 18, 94. [Google Scholar] [CrossRef]
- Menard, M.K.; Kilpatrick, S.; Saade, G.; Hollier, L.M.; Joseph, G.F.; Barfield, W.; Callaghan, W.; Jennings, J.; Conry, J. Levels of maternal care. Am. J. Obstet. Gynecol. 2015, 212, 259–271. [Google Scholar] [CrossRef]
- Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011. Gac. Sanit. 2013, 27, 263–272. [Google Scholar] [CrossRef]
- Viñas, B.R.; Barba, L.R.; Ngo, J.; Serra-Majem, L. Validación en población catalana del cuestionario internacional de actividad física. Gac. Sanit. 2013, 27, 254–257. [Google Scholar] [CrossRef]
- Sember, V.; Meh, K.; Sorić, M.; Starc, G.; Rocha, P.; Jurak, G. Validity and Reliability of International Physical Activity Questionnaires for Adults across EU Countries: Systematic Review and Meta Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7161. [Google Scholar] [CrossRef]
- Allen, R.P.; Kosinski, M.; Hill-Zabala, C.E.; Calloway, M.O. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep). Sleep Med. 2009, 10, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: A description and its applications. European Quality of Life scale. Med. Clínica 1999, 112, 79–85. [Google Scholar]
- Vega-Dienstmaier, J.M.; Suárez, G.M.; Sánchez, M.C. Validation of a Spanish version of the Edinburgh Postnatal Depression Scale. Actas espanolas de Psiquiatr. 2002, 30, 106–111. [Google Scholar]
- Campbell, M.K.; Piaggio, G.; Elbourne, D.R.; Altman, D.G.; for the CONSORT Group. Consort 2010 statement: Extension to cluster randomised trials. BMJ 2012, 345, e5661. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2010, 30, 377–399. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ 2001, 79, 373–374. [Google Scholar]
- Arizabaleta, A.V.M.; Buitrago, L.O.; de Plata, A.C.A.; Escudero, M.M.; Ramírez-Vélez, R. Aerobic exercise during pregnancy improves health-related quality of life: A randomised trial. J. Physiother. 2010, 56, 253–258. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Montejo, R.; Luaces, M.; Zakynthinaki, M. Exercise during pregnancy improves maternal health perception: A randomized controlled trial. Am. J. Obstet. Gynecol. 2011, 204, 402.e1–402.e7. [Google Scholar] [CrossRef]
- Santiago, J.R.; Nolledo, M.S.; Kinzler, W.; Santiago, T.V. Sleep and Sleep Disorders in Pregnancy. Ann. Intern. Med. 2001, 134, 396–408. [Google Scholar] [CrossRef]
- Skouteris, H.; Germano, C.; Wertheim, E.H.; Paxton, S.J.; Milgrom, J. Sleep quality and depression during pregnancy: A prospective study. J. Sleep Res. 2008, 17, 217–220. [Google Scholar] [CrossRef]
- Wolfson, A.R.; Crowley, S.J.; Anwer, U.; Bassett, J.L. Changes in Sleep Patterns and Depressive Symptoms in First-Time Mothers: Last Trimester to 1-Year Postpartum. Behav. Sleep Med. 2003, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Perales, M.; Cordero, Y.; Bacchi, M.; Mottola, M.F. Influence of Land or Water Exercise in Pregnancy on Outcomes. Med. Sci. Sports Exerc. 2017, 49, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Malakooti, J.; Babapoor, J.; Mohammad-Alizadeh-Charandabi, S. The effect of a home-based exercise intervention on postnatal depression and fatigue: A randomized controlled trial. Int. J. Nurs. Pract. 2014, 21, 478–485. [Google Scholar] [CrossRef]
- Austin, M.-P.; Tully, L.; Parker, G. Examining the relationship between antenatal anxiety and postnatal depression. J. Affect. Disord. 2007, 101, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, C.A.; Gold, K.J.; Flynn, H.A.; Yoo, H.; Marcus, S.M.; Davis, M.M. Risk factors for depressive symptoms during pregnancy: A systematic review. Am. J. Obstet. Gynecol. 2010, 202, 5–14. [Google Scholar] [CrossRef]
- Ming, W.-K.; Ding, W.; Zhang, C.J.P.; Zhong, L.; Long, Y.; Li, Z.; Sun, C.; Wu, Y.; Chen, H.; Chen, H.; et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Magro-Malosso, E.R.; Saccone, G.; Di Tommaso, M.; Roman, A.; Berghella, V. Exercise during pregnancy and risk of gestational hypertensive disorders: A systematic review and meta-analysis. Acta Obstet. et Gynecol. Scand. 2017, 96, 921–931. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Alberton, C.L.; Bgeginski, R.; Pinto, S.S.; Nunes, G.N.; Andrade, L.S.; Brasil, B.; Domingues, M.R. Water-based exercises in pregnancy: Apparent weight in immersion and ground reaction force at third trimester. Clin. Biomech. 2019, 67, 148–152. [Google Scholar] [CrossRef]
- Sichani, L.E.; Bahadoran, P.; Fahami, F.; Esfahani, P.S. The investigation of effects of static immersion and calming in water on pregnant women’s stress participating in preparation classes for childbirth. J. Educ. Health Promot. 2019, 8, 238. [Google Scholar] [CrossRef]
- Soultanakis, H.N. Aquatic Exercise and Thermoregulation in Pregnancy. Clin. Obstet. Gynecol. 2016, 59, 576–590. [Google Scholar] [CrossRef] [PubMed]
| Group | ||
|---|---|---|
| Intervention (n = 148) | Control (n = 146) | |
| Age (years), mean ± SD | 31.1 ± 4.1 | 31.5 ± 4.2 |
| BMI (kg/m2), mean ± SD | 23.5 ± 3.2 | 23.4 ± 3.1 |
| Obstetric characteristics, n/N (%) | ||
| Nulligravid | 98/145 (67.6) | 98/141 (69.5) |
| Preterm | 1/145 (0.7) | 1/141 (0.7) |
| Spontaneous abortion | 39/145 (26.9) | 39/141 (27.6) |
| One previous child | 45/145 (21.0) | 38/141 (27.0) |
| Two or more previous children | 3/145 (2.1) | 4/141 (2.8) |
| Smoking, n/N (%) | ||
| Smoker at inclusion | 11/143 (17.9) | 25/140 (7.7) |
| Quit smoking prior to pregnancy | 12/143 (8.4) | 24/140 (17.1) |
| Social class, n/N (%) | ||
| I and II | 49/124 (39.5) | 47/126 (37.3) |
| III | 42/124 (33.9) | 33/126 (26.2) |
| IV and V | 33/124 (26.6) | 46/126 (36.5) |
| Educational level, n/N (%) | ||
| Primary school | 8/147 (5.4) | 14/143 (9.8) |
| Secondary school | 61/147 (41.5) | 54/143 (37.8) |
| University | 78/147 (51.7) | 74/143 (51.7) |
| IPAQ (MET-min/week), mean ± SD | 1008.0 ± 14,033.3 | 1047 ± 10,33.3 |
| IPAQ category score, n/N (%) | ||
| Low | 63/145 (43.4) | 75/141 (53.2) |
| Moderate | 76/145 (52.4) | 60/141 (42.6) |
| High | 6/145 (4.1) | 6/141 (4.3) |
| MOS sleep, mean ± SD | ||
| Sleep disturbance | 29.9 ± 22.6 | 25.9 ± 20.6 |
| Snoring | 12.6 ± 22.6 | 16.4 ± 28.2 |
| Awakening with short breath or headache | 12.0 ± 19.2 | 13.6 ± 22.2 |
| Sleep adequacy | 60.6 ± 28.1 | 60.9 ± 29.2 |
| Day-time somnolence | 37.4 ± 16.9 | 37.2 ± 19.2 |
| Sleep Problems Index | 20.8 ± 13.7 | 21.6 ± 14.4 |
| MOS sleep: optimal sleep | 93/147 (63.3) | 91/145 (62.8) |
| EQ5D dimension | ||
| Mobility problems | 7/147 (4.8) | 7/144 (4.9) |
| Self-care problems | 1/147 (0.7) | 2/144 (1.4) |
| Usual activity problems | 7/146 (4.8) | 11/142 (7.7) |
| Pain/discomfort problems | 46/147 (31.3) | 55/144 (38.2) |
| Anxiety/depression | 20/147 (13.6) | 15/144 (10.4) |
| EQ VAS, mean ± SD | 86.4 ± 77.9 | 89.6 ± 76.8 |
| Intervention | Control | OR (95% CI) | p | ITT Analysis: Imputed OR (95% CI) | p | |
|---|---|---|---|---|---|---|
| EQ5D dimension, n/N (%) | ||||||
| Mobility problems | 5/139 (3.6) | 7/132 (5.3) | 0.66 (0.20, 2.14) | 0.491 | 0.66 (0.20, 2.14) | 0.497 |
| Self-care problems | 2/139 (1.4) | 0/132 (0) | NA | - | NA | - |
| Usual activity problems | 4/137 (2.9) | 9/130 (6.9) | 0.41 (0.12, 1.38) | 0.151 | 0.41 (0.12, 1.38) | 0.158 |
| Pain/discomfort problems | 21/139 (15.1) | 31/132 (23.5) | 0.59 (0.32, 1.10) | 0.102 | 0.59 (0.32, 1.10) | 0.11 |
| Anxiety/depression | 16/139 (11.5) | 30/132 (22.7) | 0.42 (0.21, 0.82) | 0.011 | 0.42 (0.21, 0.82) | 0.02 |
| Beta (95% CI) | Imputed Beta | |||||
| (95% CI) | ||||||
| EQ VAS, mean ± SD | 86.8 (14.4) | 82.5 (13.7) | −19.45 (−61.72, 22.83) | 0.366 | −19.44 (−61.28, 22.41) | 0.361 |
| Depression Edinburgh questionnaire total (EDPS) | 6.1 ± 1.9 | 6.8 ± 2.4 | −0.68 (−1.20, −0.15) | 0.012 | −0.70 (−1.24, −0.17) | 0.01 |
| OR (95% CI) | p | ITT analysis: Imputed OR (95% CI) | p | |||
| Risk Depression Edinburgh questionnaire | 2/136 (1.5) | 8/132 (6.1) | 0.231 (0.048, 1.11) | 0.067 | 0.211 (0.044, 1.01) | 0.052 |
| Beta (95% CI) | Imputed Beta | p | ||||
| (95% CI) | ||||||
| MOS-Sleep | ||||||
| Sleep disturbance | 38.4 ± 22.9 | 40.9 ± 24.8 | −3.99 (−9.47, 1.58) | 0.161 | −3.95 (−9.46,1.55) | 0.159 |
| Snoring | 26.5 ± 33.8 | 36.1 ± 37.5 | −5.78 (−13.03, 1.48) | 0.118 | −5.95 (−13.2, 1.31) | 0.108 |
| Awakening with short breath | 7.2 ± 16.1 | 7.1 ± 14.3 | 0.25 (−3.35, 3.86) | 0.89 | 0.26 (−3.3, 3.87) | 0.886 |
| or headache | ||||||
| Sleep adequacy | 56.0 ± 28.5 | 56.1 ± 30.1 | −0.87 (−7.43, 5.68) | 0.793 | −0.88 (−7.46, 5.68) | 0.791 |
| Day-time somnolence | 32.0 ± 16.2 | 34.4 ± 18.3 | −1.80 (−5.77, 2.18) | 0.375 | −1.69 (−5.67, 2.27) | 0.4 |
| Sleep Problems Index | 32.0 ± 18.6 | 31.2 ± 18.0 | −0.74 (−4.71, 3.22) | 0.713 | −0.65 (−4.58, 3.28) | 0.746 |
| OR (95% CI) | p | ITT analysis: Imputed OR (95% CI) | p | |||
| MOS-sleep optimal sleep | 64/130 (49.2) | 74/129 (57.4) | 0.69 (0.42, 1.14) | 0.148 | 0.69 (0.42, 1.14) | 0.152 |
| Mother (Adverse Event), n/N (%) | Intervention | Control | p |
|---|---|---|---|
| Urinary tract infection | 7/139 (5.0) | 11/132 (8.3) | 0.276 |
| Back pain | 3/139 (2.2) | 1/132 (0) | 0.623 |
| Bleeding | 3/139 (2.2) | 2/132 (1.5) | 1 |
| Contractions | 3/139 (2.2) | 0/132 (0) | 0.248 |
| Abortion/fetal death | 1/139 (0.7) | 1/132 (0.8) | 1 |
| Fetal admission to intensive care | 10/139 (7.2) | 5/132 (3.8) | 0.288 |
| Mother admission to intensive care | 1/139 (0.7) | 0/132 (0) | 1 |
| Newborn, n/N (%) | |||
| Weight, g ± SD | 3367 ± 799.7 | 3281 ± 497.1 | 0.283 |
| Intrapartum fetal distress, n (%) | 25/120 (20.8) | 28/119 (23.5) | 0.365 |
| Weeks of gestation | 39.9 ± 2.0 | 39.8 ± 2.0 | 0.739 |
| At least 41 weeks gestation | 20/139 (14.4) | 17/132 (12.9) | 0.725 |
| Apgar score | |||
| 1 min | 8.7 ± 1.3 | 8.7 ± 0.9 | 0.813 |
| 5 min | 9.8 ± 0.99 | 9.8 ± 0.4 | 0.398 |
| pH of umbilical cord blood | 7.27 ± 0.09 | 7.26 ± 0.07 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navas, A.; Carrascosa, M.d.C.; Artigues, C.; Ortas, S.; Portells, E.; Soler, A.; Yañez, A.M.; Bennasar-Veny, M.; Leiva, A. Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial. J. Clin. Med. 2021, 10, 2432. https://doi.org/10.3390/jcm10112432
Navas A, Carrascosa MdC, Artigues C, Ortas S, Portells E, Soler A, Yañez AM, Bennasar-Veny M, Leiva A. Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(11):2432. https://doi.org/10.3390/jcm10112432
Chicago/Turabian StyleNavas, Araceli, María del Carmen Carrascosa, Catalina Artigues, Silvia Ortas, Elena Portells, Aina Soler, Aina M. Yañez, Miquel Bennasar-Veny, and Alfonso Leiva. 2021. "Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 11: 2432. https://doi.org/10.3390/jcm10112432
APA StyleNavas, A., Carrascosa, M. d. C., Artigues, C., Ortas, S., Portells, E., Soler, A., Yañez, A. M., Bennasar-Veny, M., & Leiva, A. (2021). Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial. Journal of Clinical Medicine, 10(11), 2432. https://doi.org/10.3390/jcm10112432








