Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Assessment
2.3. Laboratory Determinations
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features of Vitamin D Supplemented and Non-Supplemented COVID-19 Outpatients at Baseline
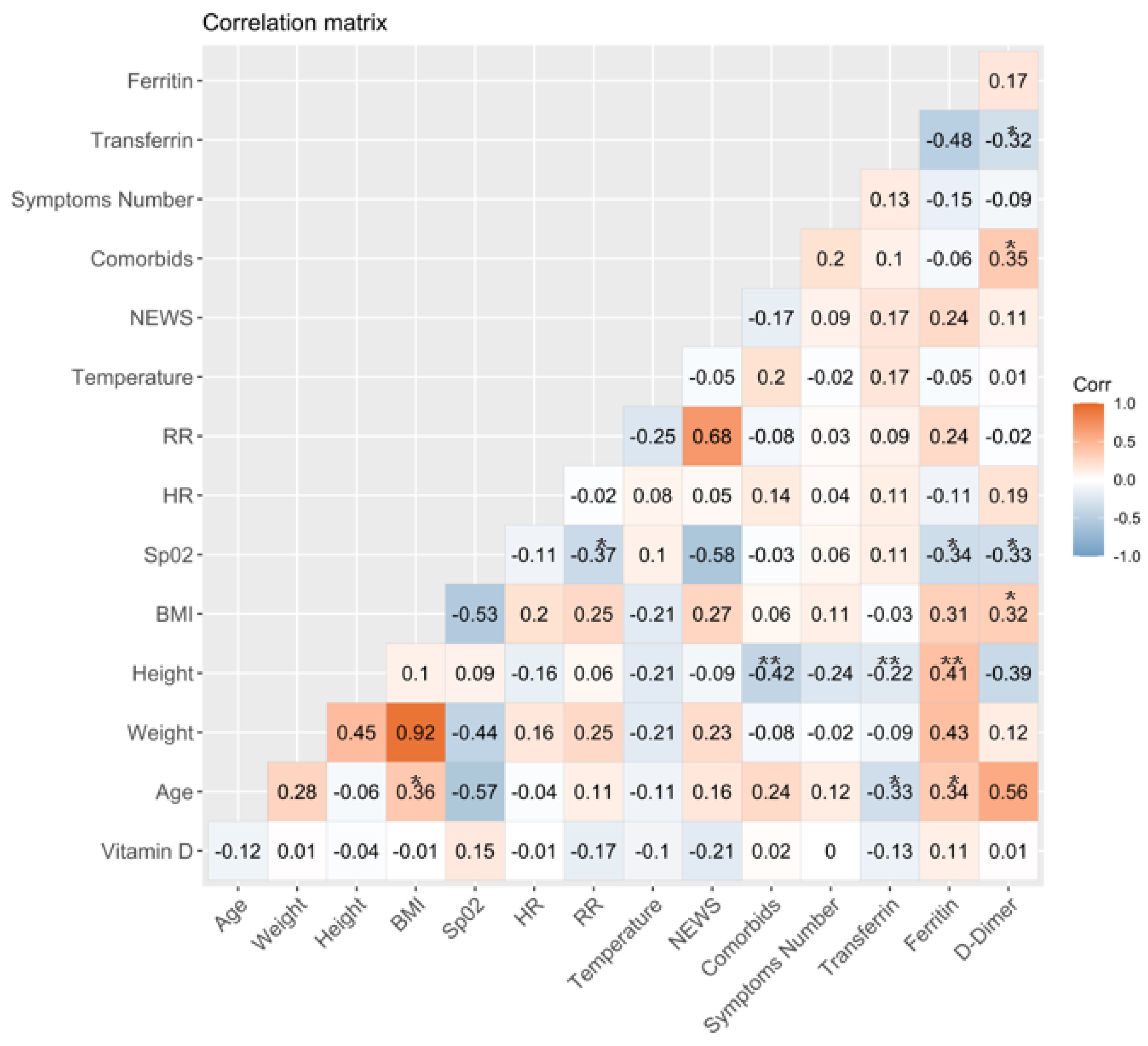
3.2. Association between Vitamin D Serum Levels and Clinical and Laboratory Variables at Baseline
3.3. Comparison between Laboratory Parameters and Clinical Features of Outpatients with or without Sufficient Levels of Total Vitamin D
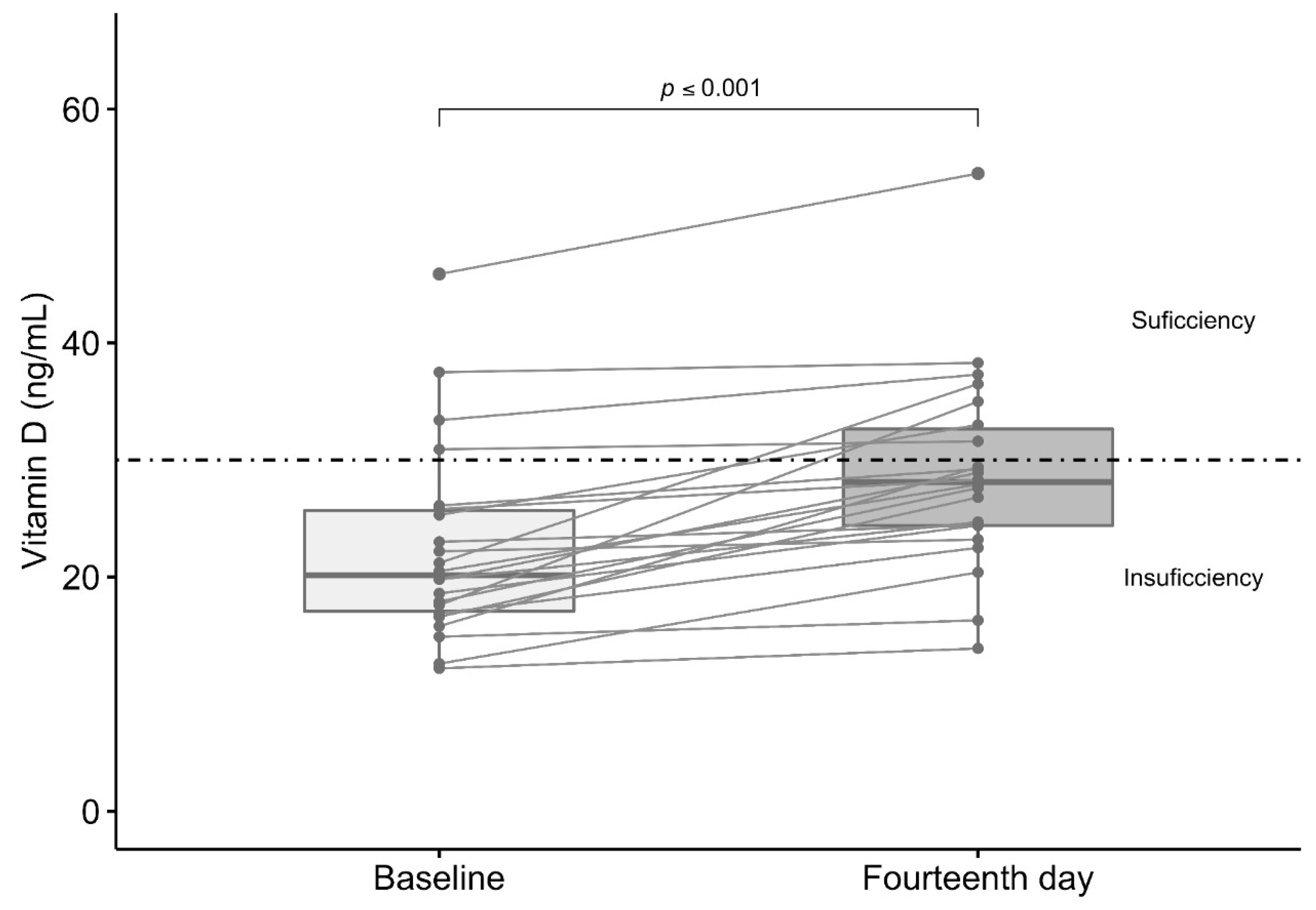
3.4. Effect of Vitamin D3 Supplementation in Total Serum Levels of Vitamin D
3.5. Comparison between Symptoms, Treatment, and Viral Load in Supplemented and Non-Supplemented Outpatients Study Groups
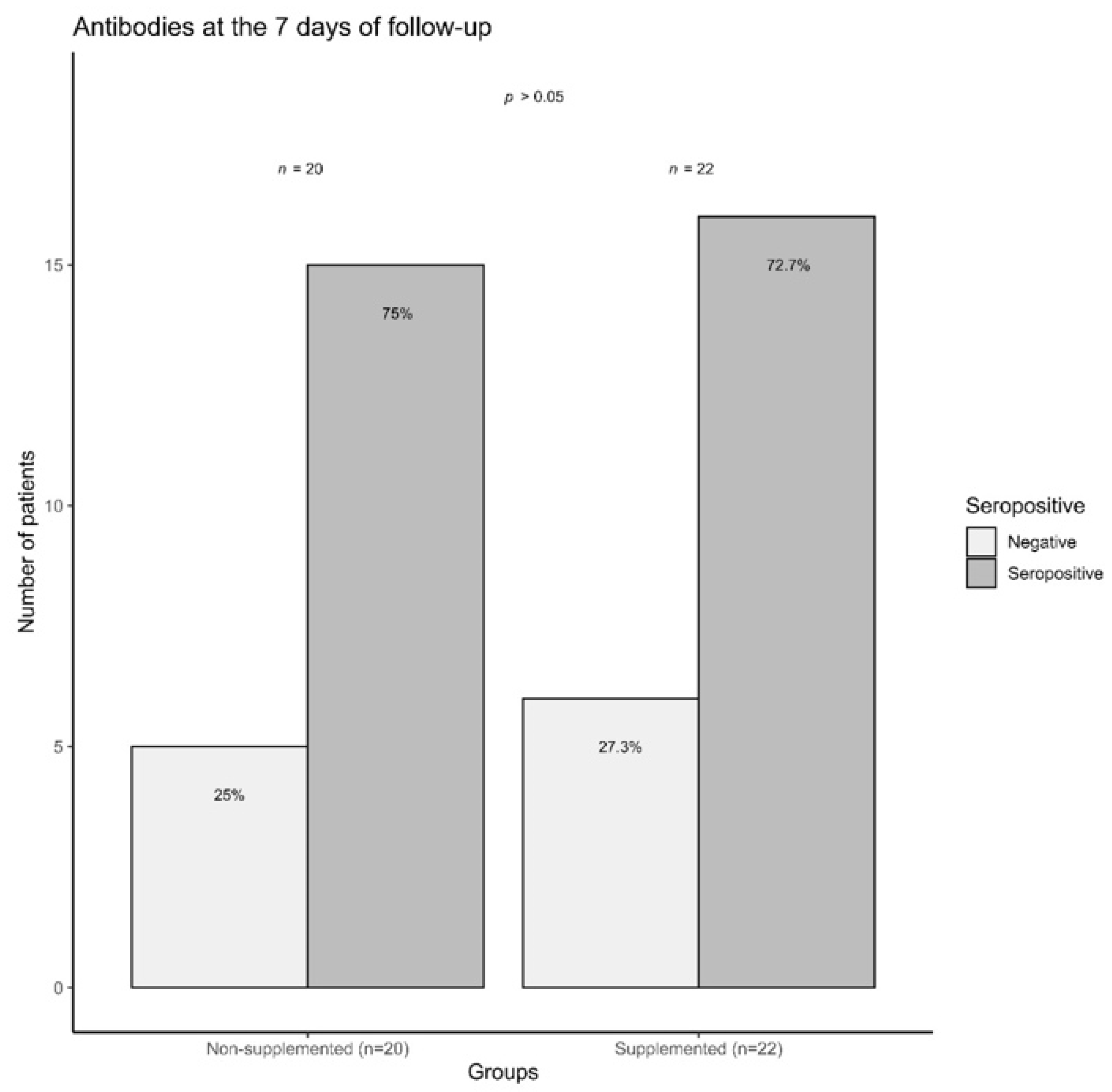
3.6. COVID-19 Outpatients Seropositivity Rate on the Seventh Day of Follow-Up
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://covid19.who.int (accessed on 15 March 2021).
- Mortality Analyses—Johns Hopkins Coronavirus Resource. Available online: https://coronavirus.jhu.edu/data/mortality (accessed on 15 March 2021).
- Conti, P. Induction of Pro-Inflammatory Cytokines (IL-1 and IL-6) and Lung Inflammation by COVID-19: Anti-Inflammatory Strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Montano-Loza, A.J. Perspective: Improving Vitamin D Status in the Management of COVID. Eur. J. Clin. Nutr. 2020, 74, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. Mild versus Severe COVID-19: Laboratory Markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Bolondi, G.; Russo, E.; Gamberini, E.; Circelli, A.; Meca, M.C.C.; Brogi, E.; Viola, L.; Bissoni, L.; Poletti, V.; Agnoletti, V. Iron Metabolism and Lymphocyte Characterisation during Covid-19 Infection in ICU Patients: An Observational Cohort Study. World J. Emerg. Surg. 2020, 15, 41. [Google Scholar] [CrossRef]
- Dai, X. ABO Blood Group Predisposes to COVID-19 Severity and Cardiovascular Diseases. Eur. J. Prev. Cardiol. 2020, 27, 1436–1437. [Google Scholar] [CrossRef]
- Latz, C.A.; DeCarlo, C.; Boitano, L.; Png, C.Y.M.; Patell, R.; Conrad, M.F.; Eagleton, M.; Dua, A. Blood Type and Outcomes in Patients with COVID-19. Ann. Hematol. 2020, 99, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- de Lucena, T.M.C.; da Silva Santos, A.F.; de Lima, B.R.; de Albuquerque Borborema, M.E.; de Azevêdo Silva, J. Mechanism of Inflammatory Response in Associated Comorbidities in COVID. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 597–600. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and Its Impact on Patients with COVID. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID -19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef]
- Malaguarnera, L. Vitamin D3 as Potential Treatment Adjuncts for COVID. Nutrients 2020, 12, 3512. [Google Scholar] [CrossRef]
- Turrubiates-Hernández, F.; Sánchez-Zuno, G.; González-Estevez, G.; Hernández-Bello, J.; Macedo-Ojeda, G.; Muñoz-Valle, J. Potential Immunomodulatory Effects of Vitamin D in the Prevention of Severe Coronavirus Disease 2019: An Ally for Latin America (Review). Int. J. Mol. Med. 2021, 47, 32. [Google Scholar] [CrossRef]
- Shi, Y.-Y.; Liu, T.-J.; Fu, J.-H.; Xu, W.; Wu, L.-L.; Hou, A.-N.; Xue, X.-D. Vitamin D/VDR Signaling Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Maintaining the Integrity of the Pulmonary Epithelial Barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Regulation of the Renin-Angiotensin System. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Greiller, C.; Martineau, A. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, K.; Şen, V. Is Vitamin D Deficiency a Risk Factor for COVID-19 in Children? Pediatr. Pulmonol. 2020, 55, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring Links between Vitamin D Deficiency and COVID. PLoS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Whittemore, P.B. COVID-19 Fatalities, Latitude, Sunlight, and Vitamin D. Am. J. Infect. Control 2020, 48, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.M.; O’Shea, P.M.; Faul, J.L.; Healy, M.J.; Byrne, G.; Griffin, T.P.; Walsh, J.B.; Byrne, D.G.; Kenny, R.A. Vitamin D and SARS-CoV-2 Infection—Evolution of Evidence Supporting Clinical Practice and Policy Development: A Position Statement from the Covit-D Consortium. Ir. J. Med. Sci. 1971. 2020. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Smith, G.B.; Prytherch, D.R.; Meredith, P.; Schmidt, P.E.; Featherstone, P.I. The Ability of the National Early Warning Score (NEWS) to Discriminate Patients at Risk of Early Cardiac Arrest, Unanticipated Intensive Care Unit Admission, and Death. Resuscitation 2013, 84, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kuriacose, R.; Olive, K.E. Vitamin D Insufficiency/Deficiency Management. South. Med. J. 2014, 107, 66–70. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-analysis. J. Clin. Lab. Anal. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological Findings and Complications of COVID. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Huang, W.; Ye, B.; Chen, C.; Huang, R.; Wu, F.; Wei, Q.; Zhang, W.; Hu, J. Changes of Hematological and Immunological Parameters in COVID-19 Patients. Int. J. Hematol. 2020, 112, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Fredenburgh, J.C.; Eikelboom, J.W. A Test in Context: D-Dimer. J. Am. Coll. Cardiol. 2017, 70, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Linkins, L.-A.; Takach Lapner, S. Review of D-Dimer Testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017, 39, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Taneri, P.E.; Gómez-Ochoa, S.A.; Llanaj, E.; Raguindin, P.F.; Rojas, L.Z.; Roa-Díaz, Z.M.; Salvador, D.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and Iron Metabolism in COVID-19: A Systematic Review and Meta-Analysis. Eur. J. Epidemiol. 2020, 35, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. Serum Ferritin Is an Important Inflammatory Disease Marker, as It Is Mainly a Leakage Product from Damaged Cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Bruce, K.E.; Wu, H.; Giedroc, D.P. The S2 Cu( i ) Site in CupA from Streptococcus Pneumoniae Is Required for Cellular Copper Resistance. Metallomics 2016, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.K.; Kwon, M.-J.; Ham, S.-Y.; Kim, J.M.; Lim, S.-Y.; Song, J.-U. Decreased Lung Function Is Associated with Elevated Ferritin but Not Iron or Transferrin Saturation in 42,927 Healthy Korean Men: A Cross-Sectional Study. PLoS ONE 2020, 15, e0231057. [Google Scholar] [CrossRef]
- Franco, C.K.; Silva, D.R.; Barreto, S.S.M. Relationship of Body Mass Index and Waist-to-Hip Ratio with Fibrinolytic Activity Measured as d-Dimer. Obes. Res. Clin. Pract. 2011, 5, e37–e41. [Google Scholar] [CrossRef]
- Shitrit, D.; Peled, N.; Shitrit, A.B.-G.; Meidan, S.; Bendayan, D.; Sahar, G.; Kramer, M.R. An Association between Oxygen Desaturation and D-Dimer in Patients with Obstructive Sleep Apnea Syndrome. Thromb. Haemost. 2005, 94, 544–547. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short Term, High-Dose Vitamin D Supplementation for COVID-19 Disease: A Randomised, Placebo-Controlled, Study (SHADE Study). Postgrad. Med. J. 2020. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, i6583. [Google Scholar] [CrossRef]
- Dancer, R.C.A.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D Deficiency Contributes Directly to the Acute Respiratory Distress Syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D Supplementation to Prevent Asthma Exacerbations: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef]
- Bedolla-Barajas, M.; López-Hernández, J.C.; García-Padilla, L.F.; Morales-Romero, J.; Velarde-Rivera, F.A.; Robles-Figueroa, M.; Ortiz-Peregrina, J.R. Prevalencia de insuficiencia y deficiencia de vitamina D en adultos mexicanos con asma alérgica. Rev. Alerg. México 2017, 64, 178. [Google Scholar] [CrossRef] [PubMed]
- Azrielant, S.; Shoenfeld, Y. Vitamin D and the Immune System. Israel Med. Assoc. J. 2017, 19, 510–511. [Google Scholar]
- Hansen, K.E.; Johnson, M.G. An Update on Vitamin D for Clinicians. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Vivanco-Muñoz, N.; Piña, J.T.; Rivas-Ruiz, R.; Huitrón, G.; Chico-Barba, G.; Reza-Albarrán, A.A. High Prevalence of Hypovitaminosis D in Mexicans Aged 14 Years and Older and Its Correlation with Parathyroid Hormone. Arch. Osteoporos. 2015, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Pinzon, R.T.; Angela; Pradana, A.W. Vitamin D Deficiency among Patients with COVID-19: Case Series and Recent Literature Review. Trop. Med. Health 2020, 48, 102. [Google Scholar] [CrossRef]
- Nowaczewska, M.; Wiciński, M.; Osiński, S.; Kaźmierczak, H. The Role of Vitamin D in Primary Headache–from Potential Mechanism to Treatment. Nutrients 2020, 12, 243. [Google Scholar] [CrossRef]
- Ye, K.; Tang, F.; Liao, X.; Shaw, B.A.; Deng, M.; Huang, G.; Qin, Z.; Peng, X.; Xiao, H.; Chen, C.; et al. Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity?-A Case-Control Study. J. Am. Coll. Nutr. 2020, 1–8. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of Vitamin D Level among Asymptomatic and Critically Ill COVID-19 Patients and Its Correlation with Inflammatory Markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Ajabshir, S. The Effects of Vitamin D on the Renin-Angiotensin System. Eff. Vitam. Renin. Angiotensin Syst. 2014. [Google Scholar] [CrossRef]
- Martínez-Zavala, N.; López-Sánchez, G.N.; Vergara-Lopez, A.; Chávez-Tapia, N.C.; Uribe, M.; Nuño-Lámbarri, N. Vitamin D Deficiency in Mexicans Have a High Prevalence: A Cross-Sectional Analysis of the Patients from the Centro Médico Nacional 20 de Noviembre. Arch. Osteoporos. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
| Variable | n = 42 | Supplemented Outpatients n = 22 | Non-Supplemented Outpatients n = 20 | p-Value |
|---|---|---|---|---|
| Age (years) a | 43.0 (20–74) | 44.0 (20.0–71.0) | 43.0 (21.0–78.0) | 0.66 |
| Females b | 22 (52.3) | 7 (31.8) | 6 (30.0) | 1.00 |
| BMI (kg/m2) a | 25.5 (18.1–41.2) | 25.4 (19.7–41.2) | 26.3 (18.1–35.0) | 0.95 |
| Comorbidities | ||||
| 7 (16.7) | 4 (18.2) | 3 (15.0) | 0.55 |
| 4 (9.5) | 2 (9.1) | 2 (10.0) | 1.00 |
| 2 (4.8) | 0 (0.0) | 2 (10.0) | 0.22 |
| 1 (2.4) | 1 (2.4) | 0 (0.0) | 1.00 |
| Treatment | 30 (71.4) | 15 (68.2) | 15 (75.0) | 0.88 |
| 22 (52.4) | 12 (54.5) | 10 (50.0) | 1.00 |
| 17 (40.5) | 7 (31.8) | 10 (50.0) | 0.37 |
| 8 (19.0) | 2 (9.1) | 6 (30.0) | 0.12 |
| 6 (14.3) | 3 (13.6) | 3 (15.0) | 1.00 |
| 5 (11.9) | 1 (4.5) | 4 (20.0) | 0.17 |
| 10 (23.8) | 5 (22.7) | 5 (25.0) | 1.00 |
| Laboratory parameters | ||||
| 129.5 (6.62–842.0) | 72.8 (8.6–419.0) | 153.0 (6.62–842) | 0.05 |
| 286.1 (100–2825.6) | 306.7 (100–2825.6) | 263.6 (186.0–1038.5) | 0.89 |
| 236.0 (171.0–376.0) | 254.0 (193.0–376.0) | 226.0 (171.0–297) | 0.03 |
| Total vitamin D (ng/mL) a | 22.4 (12.1–45.9) | 20.2 (12.2–45.9) | 23.4 (12.1–45.6) | 0.06 |
| Sufficient vitamin D b | 8 (19.0) | 4 (18.2) | 4 (20.0) | 1.00 |
| Variable | Outpatients with Insufficient Levels of Vitamin D n = 34 | Outpatients with Sufficient Levels of Vitamin D n = 8 | p-Values |
|---|---|---|---|
| Age (years) a | 45 (20–74) | 38.5 (36–64) | 0.42 |
| Comorbid b | 11 (32.4) | 2 (25.0) | 0.70 |
| BMI (kg/m2) a | 25.5 (18.1–39.2) | 25.9 (19.6–41.2) | 0.75 |
| Symptoms b | 34 (100.0) | 6 (75.0) | 0.03 |
| >1 symptom b | 31 (91.2) | 5 (62.5) | 0.03 |
| >2 symptoms b | 27 (79.4) | 5 (62.5) | 0.37 |
| >3 symptoms b | 23 (67.6) | 4 (50.0) | 0.42 |
| >4 more symptoms b | 18 (52.9) | 4 (50.0) | 0.59 |
| Number of symptoms a | 6 (0–11) | 5 (0–10) | 0.36 |
| Treatment b | 24 (72.7) | 6 (75.0) | 0.89 |
| Laboratory parameters | |||
| 237.0 (178.0–376.0) | 234 (171.0–289.0) | 0.72 |
| 119–5 (6.6–842.0) | 186.5 (70.5-453) | 0.18 |
| 278.4 (100.0–1239.9) | 3310.4 (128.9–2825.6) | 0.56 |
| Baseline | 7 Days | 14 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Supplemented Outpatients n = 22 | Non-supplemented Outpatients n = 20 | p-Value | Supplemented Outpatients n = 22 | Non-supplemented Outpatients n = 20 | p-Value | Supplemented Outpatients n = 22 | Non-Supplemented Outpatients n = 20 | p-Value |
| Presence of symptoms | 21 (95.5) | 19 (95.0) | 1.00 | 13 (59.1) | 9 (45.0) | 0.53 | 14 (63.6) | 8 (40.0) | 0.22 |
| >1 symptom a | 18 (81.8) | 18 (90.0) | 0.66 | 5 (22.7) | 6 (30.0) | 0.43 | 6 (27.3) | 6 (30.0) | 1.00 |
| >2 symptoms a | 17 (77.3) | 15 (75.0) | 1.00 | 2 (9.1) | 4 (20.0) | 0.28 | 4 (18.2) | 4 (20.0) | 0.59 |
| >3 symptoms a | 14 (63.6) | 13 (65.0) | 1.00 | 0 (0.0) | 4 (20.0) | 0.04 | 0 (0.0) | 4 (20.0) | 0.04 |
| NEWS score b | 4 (0–9) | 3 (0–7) | 0.14 | --- | ---- | --- | --- | ---- | --- |
| Treatment a | 15 (68.2) | 15 (75.0) | 0.88 | 7 (31.8) | 8 (40.0) | 0.81 | 4 (18.2) | 4 (20.0) | 0.88 |
| Analgesic a | 12 (54.5) | 10 (50.0) | 1.00 | 2 (9.1) | 3 (15.0) | 0.57 | 2 (9.1) | 3 (15.0) | 0.65 |
| Antipyretic a | 7 (31.8) | 10 (50.0) | 0.35 | 4 (18.2) | 6 (30.0) | 0.53 | 1 (4.5) | 3 (15.0) | 0.27 |
| Antibiotic a | 2 (9.1) | 6 (30.0) | 0.12 | 0 (0.0) | 2 (10.0) | 0.12 | 1 (4.5) | 0 (0.0) | 1.00 |
| Antihistamine a | 3 (13.6) | 3 (15.0) | 1.00 | 1 (4.5) | 0 (0.0) | 1.00 | 0 (0.0) | 0 (0.0) | --- |
| Anticoagulant a | 1 (4.5) | 4 (20.0) | 0.17 | 0 (0.0) | 1 (5.0) | 1.00 | 0 (0.0) | 0 (0.0) | --- |
| Other drugs a | 5 (22.7) | 5 (25.0) | 1.00 | 4 (18.8) | 3 (15.0) | 1.00 | 0 (0.0) | 0 (0.0) | --- |
| Positive RT-PCR test a | 22 (100.0) | 20 (100.0) | --- | 12 (60.0) | 12 (54.5) | 0.97 | 1 (5.0) | 0 (0.0) | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Zuno, G.A.; González-Estevez, G.; Matuz-Flores, M.G.; Macedo-Ojeda, G.; Hernández-Bello, J.; Mora-Mora, J.C.; Pérez-Guerrero, E.E.; García-Chagollán, M.; Vega-Magaña, N.; Turrubiates-Hernández, F.J.; et al. Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation. J. Clin. Med. 2021, 10, 2378. https://doi.org/10.3390/jcm10112378
Sánchez-Zuno GA, González-Estevez G, Matuz-Flores MG, Macedo-Ojeda G, Hernández-Bello J, Mora-Mora JC, Pérez-Guerrero EE, García-Chagollán M, Vega-Magaña N, Turrubiates-Hernández FJ, et al. Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation. Journal of Clinical Medicine. 2021; 10(11):2378. https://doi.org/10.3390/jcm10112378
Chicago/Turabian StyleSánchez-Zuno, Gabriela Athziri, Guillermo González-Estevez, Mónica Guadalupe Matuz-Flores, Gabriela Macedo-Ojeda, Jorge Hernández-Bello, Jesús Carlos Mora-Mora, Edsaúl Emilio Pérez-Guerrero, Mariel García-Chagollán, Natali Vega-Magaña, Francisco Javier Turrubiates-Hernández, and et al. 2021. "Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation" Journal of Clinical Medicine 10, no. 11: 2378. https://doi.org/10.3390/jcm10112378
APA StyleSánchez-Zuno, G. A., González-Estevez, G., Matuz-Flores, M. G., Macedo-Ojeda, G., Hernández-Bello, J., Mora-Mora, J. C., Pérez-Guerrero, E. E., García-Chagollán, M., Vega-Magaña, N., Turrubiates-Hernández, F. J., Machado-Sulbaran, A. C., & Muñoz-Valle, J. F. (2021). Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation. Journal of Clinical Medicine, 10(11), 2378. https://doi.org/10.3390/jcm10112378










