Sarcopenia and Malnutrition Screening in Female Osteoporosis Patients—A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
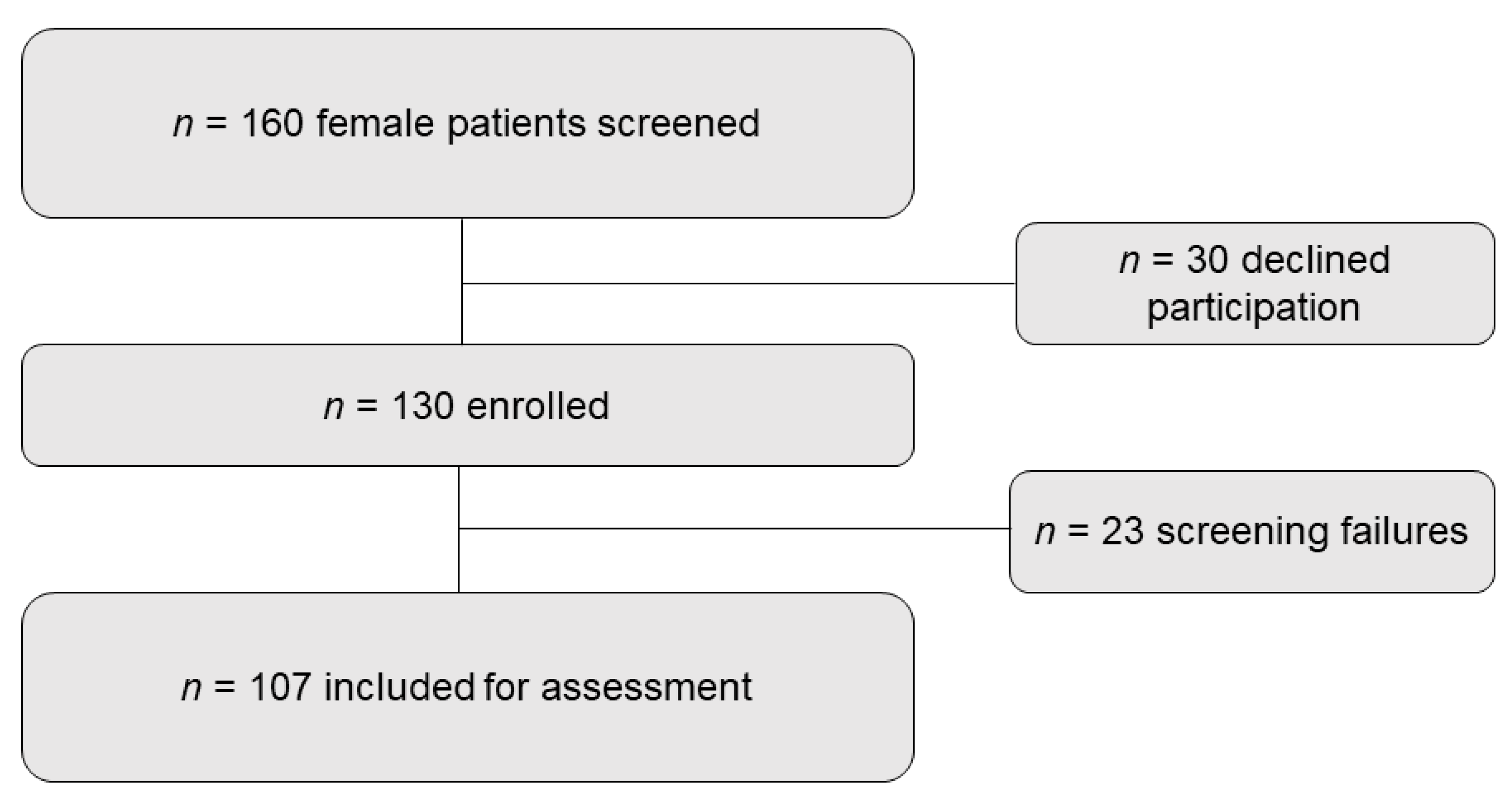
2.1. Patients and Study Design
2.2. Sarcopenia and Body Constitution
2.3. Nutritional Assessment
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Sarcopenia
3.2. Nutritional Status
3.3. Sarcopenic and Malnourished Patients
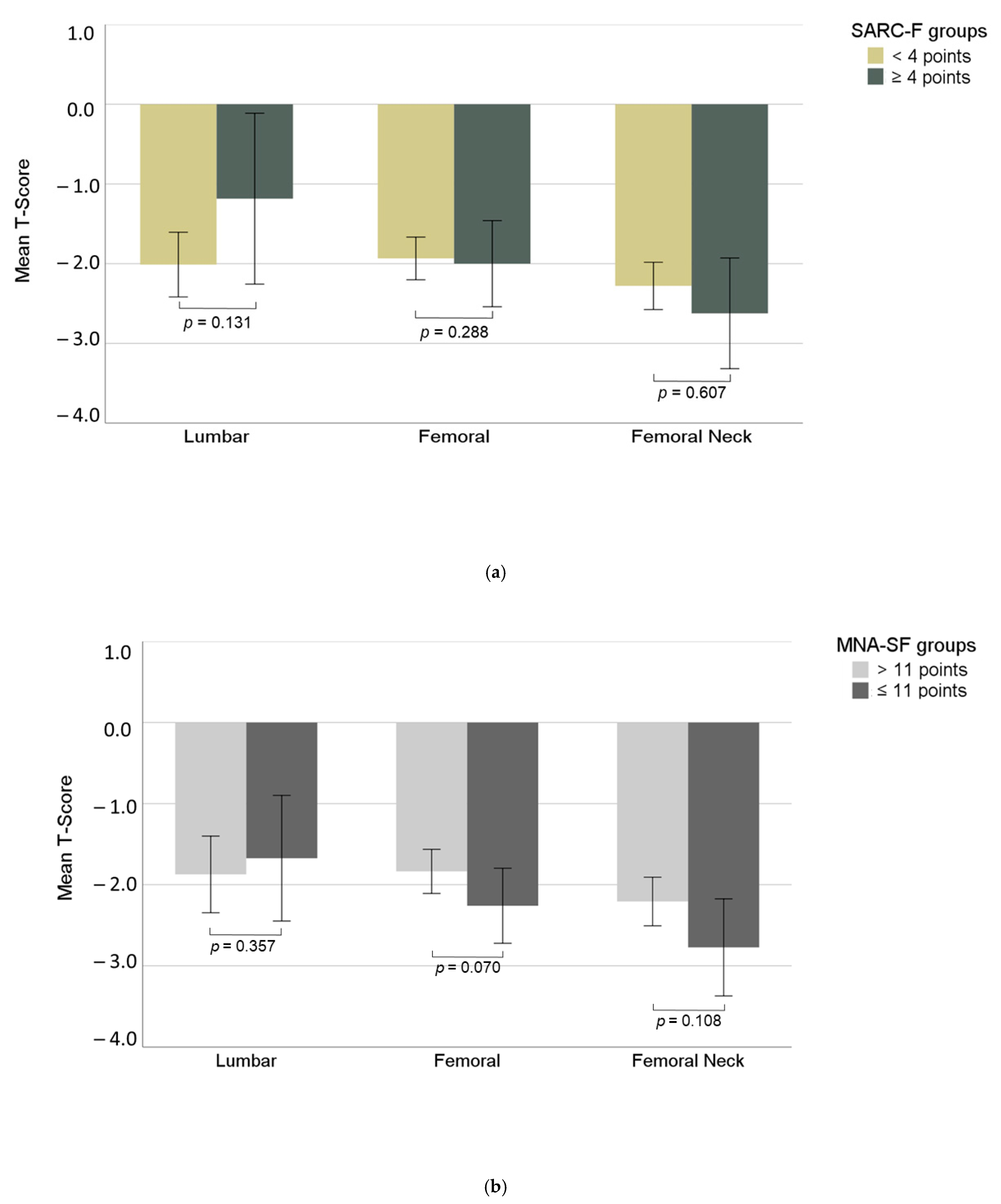
3.4. Bone Mineral Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Kanis, J.A.; Borgstrom, F.; De Laet, C.; Johansson, H.; Johnell, O.; Jonsson, B.; Oden, A.; Zethraeus, N.; Pfleger, B.; Khaltaev, N. Assessment of fracture risk. Osteoporos. Int. 2005, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Haentjens, P.; Magaziner, J.; Colón-Emeric, C.S.; Vanderschueren, D.; Milisen, K.; Velkeniers, B.; Boonen, S. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann. Intern. Med. 2010, 152, 380–390. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Achenbach, S.J.; Atkinson, E.J.; Therneau, T.M.; Amin, S. Long-term mortality following fractures at different skeletal sites: A population-based cohort study. Osteoporos Int. 2013, 24, 1689–1696. [Google Scholar] [CrossRef]
- Langdahl, B.L. Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. 2021, 178, 1891–1906. [Google Scholar] [CrossRef]
- Järvinen, T.L.N.; Sievänen, H.; Khan, K.M.; Heinonen, A.; Kannus, P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 2008, 336, 124–126. [Google Scholar] [CrossRef]
- Merlijn, T.; Swart, K.; Van der Horst, H.; Netelenbos, J.; Elders, P. Fracture prevention by screening for high fracture risk: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 251–257. [Google Scholar] [CrossRef]
- Leslie, W.D.; Morin, S.N.; Lix, L.M.; Binkley, N. Comparison of treatment strategies and thresholds for optimizing fracture prevention in Canada: A simulation analysis. Arch. Osteoporos. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Chalhoub, D.; Cawthon, P.M.; Ensrud, K.E.; Stefanick, M.L.; Kado, D.M.; Boudreau, R.; Greenspan, S.L.; Newman, A.B.; Zmuda, J.M.; Orwoll, E.S.; et al. Risk of Nonspine Fractures in Older Adults with Sarcopenia, Low Bone Mass, or Both. J. Am. Geriatr. Soc. 2015, 63, 1733–1740. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. AGE 2013, 36, 773–785. [Google Scholar] [CrossRef]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical Pathways of Sarcopenia and Their Modulation by Physical Exercise: A Narrative Review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef]
- Cesari, M.; Vellas, B.; Hsu, F.-C.; Newman, A.B.; Doss, H.; King, A.C.; Manini, T.M.; Church, T.; Gill, T.M.; Miller, M.E.; et al. A Physical Activity Intervention to Treat the Frailty Syndrome in Older Persons–Results From the LIFE-P Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 70, 216–222. [Google Scholar] [CrossRef]
- Van Wissen, J.; Van Stijn, M.F.M.; Doodeman, H.J.; Houdijk, A.P.J. Mini nutritional assessment and mortality after hip fracture surgery in the elderly. J. Nutr. Health Aging 2016, 20, 964–968. [Google Scholar] [CrossRef]
- Zanetti, M.; Cappellari, G.G.; Ratti, C.; Ceschia, G.; Murena, L.; De Colle, P.; Barazzoni, R. Poor nutritional status but not cognitive or functional impairment per se independently predict 1 year mortality in elderly patients with hip-fracture. Clin. Nutr. 2019, 38, 1607–1612. [Google Scholar] [CrossRef]
- Malafarina, V.; Reginster, J.-Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.A.; Martinez, J.A.; Zulet, M.A. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef]
- Weaver, C.; Gordon, C.; Janz, K.; Kalkwarf, H.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.; Zemel, B. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kaiser, M.J.; MNA-International Group; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Urquiza, M.; Fernandez, N.; Arrinda, I.; Sierra, I.; Irazusta, J.; Larrad, A.R. Nutritional Status Is Associated with Function, Physical Performance and Falls in Older Adults Admitted to Geriatric Rehabilitation: A Retrospective Cohort Study. Nutrients 2020, 12, 2855. [Google Scholar] [CrossRef] [PubMed]
- DVO. Prophylaxe, Diagnostik und Therapie der OSTEOPOROSE bei Postmenopausalen Frauen und bei Männern 2017. Available online: https://www.dv-osteologie.org/uploads/Leitlinie%202017/Finale%20Version%20Leitlinie%20Osteoporose%202017_end.pdf (accessed on 2 May 2019).
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- Schutz, Y.; Kyle, U.; Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466. [Google Scholar]
- Phillips, A.; Strobl, R.; Vogt, S.; Ladwig, K.-H.; Thorand, B.; Grill, E. Sarcopenia is associated with disability status—Results from the KORA-Age study. Osteoporos. Int. 2017, 28, 2069–2079. [Google Scholar] [CrossRef]
- Drey, M.; Ferrari, U.; Schraml, M.; Kemmler, W.; Schoene, D.; Franke, A.; Freiberger, E.; Kob, R.; Sieber, C. German Version of SARC-F: Translation, Adaption, and Validation. J. Am. Med. Dir. Assoc. 2020, 21, 747–751.e1. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Su, Y.; Woo, J.W.; Kwok, T.C.Y. The Added Value of SARC-F to Prescreening Using FRAX for Hip Fracture Prevention in Older Community Adults. J. Am. Med. Dir. Assoc. 2019, 20, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.-C.; Won, C.W.; Kim, M.; Chun, K.-J.; Yoo, J.-I. SARC-F as a Useful Tool for Screening Sarcopenia in Elderly Patients with Hip Fractures. J. Nutr. Health Aging 2019, 24, 78–82. [Google Scholar] [CrossRef]
- Van der Sijp, M.P.L.; van Eijk, M.; Krijnen, P.; Schipper, I.B.; Achterberg, W.P.; Niggebrugge, A.H.P. Screening for malnutrition in patients admitted to the hospital with a proximal femoral fracture. Injury 2018, 49, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Misu, S.; Tanaka, T.; Kakehi, T.; Ono, R. Acute phase nutritional screening tool associated with functional outcomes of hip fracture patients: A longitudinal study to compare MNA-SF, MUST, NRS-2002 and GNRI. Clin. Nutr. 2019, 38, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Malafarina, C.; Ugarte, A.B.; Martinez, J.A.; Goñi, I.A.; Zulet, M.A. Factors Associated with Sarcopenia and 7-Year Mortality in Very Old Patients with Hip Fracture Admitted to Rehabilitation Units: A Pragmatic Study. Nutrients 2019, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Dupuy, C.; Abellan Van Kan, G.; Cesari, M.; Vellas, B.; Faruch, M.; Dray, C.; de Suoto Barreto, P. Sarcopenia Screened by the SARC-F Questionnaire and Physical Performances of Elderly Women: A Cross-Sectional Study. J. Am. Med. Dir. Assoc. 2017, 18, 848–852. [Google Scholar] [CrossRef]
- Harvey, N.C.; Odén, A.; Orwoll, E.; Lapidus, J.; Kwok, T.; Karlsson, M.K.; Rosengren, E.B.; Ribom, E.; Cooper, C.; Cawthon, P.M.; et al. Measures of Physical Performance and Muscle Strength as Predictors of Fracture Risk Independent of FRAX, Falls, and aBMD: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. J. Bone Miner. Res. 2018, 33, 2150–2157. [Google Scholar] [CrossRef]
- Wihlborg, A.; Englund, M.; Åkesson, K.; Gerdhem, P. Fracture predictive ability of physical performance tests and history of falls in elderly women: A 10-year prospective study. Osteoporos. Int. 2015, 26, 2101–2109. [Google Scholar] [CrossRef]
- Kistler-Fischbacher, M.; Weeks, B.K.; Beck, B.R. The effect of exercise intensity on bone in postmenopausal women (part 2): A meta-analysis. Bone 2021, 143, 115697. [Google Scholar] [CrossRef]
- Peraza-Delgado, A.; Sánchez-Gómez, M.B.; Gómez-Salgado, J.; Romero-Martín, M.; Novo-Muñoz, M.; Duarte-Clíments, G. Non-Pharmacological Interventions towards Preventing the Triad Osteoporosis-Falls Risk-Hip Fracture, in Population Older than 65. Scoping Review. J. Clin. Med. 2020, 9, 2329. [Google Scholar] [CrossRef]
- Cauley, J.A.; Giangregorio, L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol. 2020, 8, 150–162. [Google Scholar] [CrossRef]

| All n = 107 (100%) | SARC-F ≥ 4 n = 33 (30.8%) | SARC-F < 4 n = 73 (68.2%) | p-Value ° | MNA-SF ≤11 n = 38 (35.5%) | MNA-SF >11 n = 69 (64.5%) | p-Value * | |
|---|---|---|---|---|---|---|---|
| Age (years) | 75.0 (5.9) | 77.1 (5.5) | 74.1 (5.9) | 0.014 | 76.6 (5.8) | 74.1 (5.9) | 0.035 |
| Height (cm) | 158.8 (6.0) | 159.8 (5.0) | 158.4 (6.4) | 0.267 | 159.6 (6.2) | 158.4 (5.9) | 0.321 |
| Weight (kg) | 64.6 (11.9) | 67.2 (12.6) | 63.0 (10.8) | 0.085 | 61.6 (14.5) | 66.1 (9.9) | 0.094 |
| BMI (kg/m2) | 25.6 (4.4) | 26.4 (5.6) | 25.1 (3.6) | 0.215 | 24.1 (5.1) | 26.4 (3.8) | 0.010 |
| SMI (kg/m2) | 7.0 (1.2) | 7.2 (1.3) | 7.0 (1.2) | 0.336 | 7.0 (1.2) | 7.1 (1.3) | 0.701 |
| SPPB (points) | 9.9 (2.6) | 7.0 (3.1) | 10.9 (1.4) | <0.001 | 8.7 (3.2) | 10.5 (2.0) | 0.005 |
| CRT (s) | 12.5 (5.2) | 17.4 (6.9) | 11.4 (4.0) | 0.004 | 12.9 (5.7) | 12.4 (4.9) | 0.673 |
| Usual Gait Speed (m/s) | 1.2 (0.3) | 0.9 (0.3) | 1.4 (0.3) | <0.001 | 1.1 (0.3) | 1.3 (0.3) | 0.003 |
| Handgrip Strength (kg) | 20.2 (4.1) | 17.4 (4.4) | 21.2 (3.6) | <0.001 | 19.5 (4.4) | 20.5 (4.0) | 0.247 |
| SARC-F (points) | 2.7 (2.8) | 6.3 (1.9) | 1.0 (1.1) | <0.001 | 3.8 (3.3) | 2.0 (2.3) | 0.006 |
| MNA-SF (points) | 11.8 (2.3) | 11.1 (2.5) | 12.2 (2.1) | 0.023 | 9.3 (1.7) | 13.2 (0.9) | <0.001 |
| Lumbar T-Score | −1.9 (1.5) | −1.5 (1.7) | −2.0 (1.3) | 0.131 | −1.6 (1.4) | −2.0 (1.5) | 0.357 |
| Femoral T-Score | −2.0 (0.9) | −1.8 (0.9) | −2.0 (0.9) | 0.288 | −2.2 (0.9) | −1.8 (0.9) | 0.070 |
| Femoral Neck T-Score | −2.4 (1.0) | −2.5 (1.2) | −2.3 (1.0) | 0.607 | −2.7 (1.2) | −2.2 (0.9) | 0.108 |
| Vitamin D (20–40 ng/mL) | 38.7 (14.3) | 36.3 (10.7) | 40.2 (15.9) | 0.225 | 37.4 (9.2) | 39.5 (16.5) | 0.444 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genest, F.; Rak, D.; Bätz, E.; Ott, K.; Seefried, L. Sarcopenia and Malnutrition Screening in Female Osteoporosis Patients—A Cross-Sectional Study. J. Clin. Med. 2021, 10, 2344. https://doi.org/10.3390/jcm10112344
Genest F, Rak D, Bätz E, Ott K, Seefried L. Sarcopenia and Malnutrition Screening in Female Osteoporosis Patients—A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(11):2344. https://doi.org/10.3390/jcm10112344
Chicago/Turabian StyleGenest, Franca, Dominik Rak, Elisa Bätz, Kerstin Ott, and Lothar Seefried. 2021. "Sarcopenia and Malnutrition Screening in Female Osteoporosis Patients—A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 11: 2344. https://doi.org/10.3390/jcm10112344
APA StyleGenest, F., Rak, D., Bätz, E., Ott, K., & Seefried, L. (2021). Sarcopenia and Malnutrition Screening in Female Osteoporosis Patients—A Cross-Sectional Study. Journal of Clinical Medicine, 10(11), 2344. https://doi.org/10.3390/jcm10112344






