An Up-Date of the Muscle Strengthening Exercise Effectiveness in Postmenopausal Women with Osteoporosis: A Qualitative Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Outcome Measure
2.6. Quality Assessment
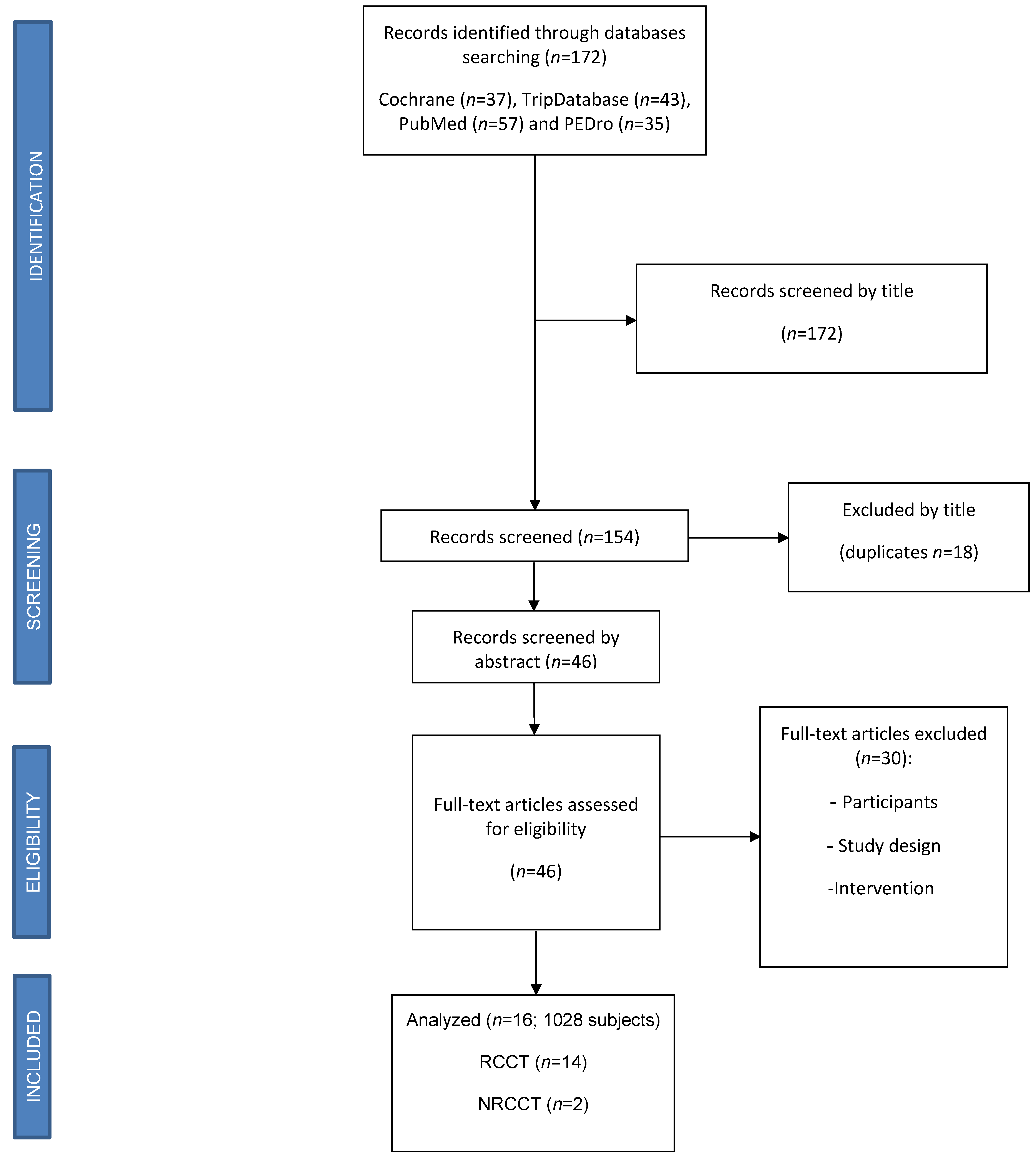
3. Results
3.1. Study Selection
3.2. Study Characteristics and Quality Assessment
4. Summary of Results
4.1. Association between Exercise Therapy and Bone Quality
4.2. Association between Exercise Therapy and Muscular Strength
4.3. Association between Exercise Therapy and Balance
4.4. Association between Exercise and Quality of Life
4.5. Association between Exercise Therapy and Functionality
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Riek, A.E.; Towler, D.A. The pharmacological management of osteoporosis. Mo. Med. 2011, 108, 118–123. [Google Scholar]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Mauro, G.L. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Villafane, J.H.; Valdes, K.; Bertozzi, L.; Negrini, S. Minimal Clinically Important Difference of Grip and Pinch Strength in Women With Thumb Carpometacarpal Osteoarthritis When Compared to Healthy Subjects. Rehabil. Nurs. 2017, 42, 139–145. [Google Scholar] [CrossRef]
- Chan, D.-C.; Chang, C.-B.; Han, D.-S.; Hong, C.-H.; Hwang, J.-S.; Tsai, K.-S.; Yang, R.-S. Effects of exercise improves muscle strength and fat mass in patients with high fracture risk: A randomized control trial. J. Formos. Med Assoc. Taiwan yi zhi. 2018, 117, 572–582. [Google Scholar] [CrossRef]
- Pillastrini, P.; Ferrari, S.; Rattin, S.; Cupello, A.; Villafañe, J.H.; Vanti, C. Exercise and tropism of the multifidus muscle in low back pain: A short review. J. Phys. Ther. Sci. 2015, 27, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Ashe, M.C.; Gorman, E.; Khan, K.M.; Brasher, P.M.; Cooper, D.M.L.; McKay, H.A.; Liu-Ambrose, T. Does frequency of resistance training affect tibial cortical bone density in older women? A randomized controlled trial. Osteoporos. Int. 2013, 24, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sanudo, B.; de Hoyo, M.; Del Pozo-Cruz, J.; Carrasco, L.; del Pozo-Cruz, B.; Tejero, S.; Firth, E. A systematic review of the exercise effect on bone health: The importance of assessing mechanical loading in perimenopausal and postmenopausal women. Menopause 2017, 24, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, T.M.; Kukkonen-Harjula, K.; Miilunpalo, S. Exercise for health for early postmenopausal women: A systematic review of randomised controlled trials. Sports Med. 2004, 34, 753–778. [Google Scholar] [CrossRef]
- Mosti, M.P.; Kaehler, N.; Stunes, A.K.; Hoff, J.; Syversen, U. Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J. Strength Cond. Res. 2013, 27, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Marchese, D.; D’Andrea, M.; Ventura, V.; Montalcini, T.; Foti, D.; Pujia, A.; Gulletta, E.; Iocco, M. Effects of a Weight-Bearing Exercise Training on Bone Mineral Density and Neuromuscular Function of Osteopenic Women. Eur. J. Inflamm. 2012, 10, 427–435. [Google Scholar] [CrossRef]
- Larpi, P.; Savanelli, A.; Cigliano, B.; De Fazio, P.; Vecchio, P.; Esposito, P. Aarskog’s facio-digital-genital syndrome. Clinical contribution. Minerva Chir. 1985, 40, 1689–1692. [Google Scholar] [PubMed]
- Borba-Pinheiro, C.J.; Dantas, E.H.M.; Vale, R.G.D.S.; Drigo, A.J.; Carvalho, M.C.G.D.A.; Tonini, T.; Meza, E.I.A.; de Figueiredo, N.M.A. Resistance training programs on bone related variables and functional independence of postmenopausal women in pharmacological treatment: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Murtezani, A.; Nevzati, A.; Ibraimi, Z.; Sllamniku, S.; Meka, V.S.; Abazi, N. The effect of land versus aquatic exercise program on bone mineral density and physical function in postmenopausal women with osteoporosis: A randomized controlled trial. Ortop. Traumatol. Rehabil. 2014, 16, 319–325. [Google Scholar] [CrossRef]
- Tolomio, S.; Ermolao, A.; Travain, G.; Zaccaria, M. Short-term adapted physical activity program improves bone quality in osteopenic/osteoporotic postmenopausal women. J. Phys. Act. Health 2008, 5, 844–853. [Google Scholar] [CrossRef]
- Bocalini, D.S.; Serra, A.J.; dos Santos, L.; Murad, N.; Levy, R.F. Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health 2009, 21, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Borba-Pinheiro, C.J.; de Alencar Carvalho, M.C.; da Silva, N.S.; Drigo, A.J.; Bezerra, J.C.; Dantas, E.H. Bone density, balance and quality of life of postmenopausal women taking alendronate participating in different physical activity programs. Ther. Adv. Musculoskelet Dis. 2010, 2, 175–185. [Google Scholar] [CrossRef]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-intensity exercise did not cause vertebral fractures and improves thoracic kyphosis in postmenopausal women with low to very low bone mass: The LIFTMOR trial. Osteoporos Int. 2019, 30, 957–964. [Google Scholar] [CrossRef]
- Brentano, M.A.; Cadore, E.L.; Da Silva, E.M.; Ambrosini, A.B.; Coertjens, M.; Petkowicz, R.; Viero, I.; Kruel, L.F.M. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J. Strength Cond. Res. 2008, 22, 1816–1825. [Google Scholar] [CrossRef]
- Marini, S.; Leoni, E.; Raggi, A.; Sanna, T.; Malavolta, N.; Angela, B.; Latessa, P.M.; Dallolio, L. Proposal of an Adapted Physical Activity Exercise Protocol for Women with Osteoporosis-Related Vertebral Fractures: A Pilot Study to Evaluate Feasibility, Safety, and Effectiveness. Int. J. Environ. Res. Public Health 2019, 16, 2562. [Google Scholar] [CrossRef]
- Koevska, V.; Nikolikj-Dimitrova, E.; Mitrevska, B.; Gjeracaroska-Savevska, C.; Gocevska, M.; Kalcovska, B. Effect of Exercises on Quality of Life in Patients with Postmenopausal Osteoporosis—Randomized Trial. Open Access Maced. J. Med. Sci. 2019, 7, 1160–1165. [Google Scholar] [CrossRef]
- Cergel, Y.; Topuz, O.; Alkan, H.; Sarsan, A.; Akkoyunlu, N.S. The effects of short-term back extensor strength training in postmenopausal osteoporotic women with vertebral fractures: Comparison of supervised and home exercise program. Arch. Osteoporos. 2019, 14, 82. [Google Scholar] [CrossRef]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.; Horan, S.A.; Beck, B.R. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.; Almasi, M.H.; Raeissadat, S.A.; Sedighipour, L.; Zamani, Y.S.; Zohoor, M.R.O. Long-term effects of back extensor strengthening exercises on quality of life in women with osteoporosis. J. Women Aging 2016, 29, 505–514. [Google Scholar] [CrossRef]
- Burke, T.N.; Franca, F.J.; Ferreira de Meneses, S.R.; Ferreira de Meneses, S.R.; Cardoso, V.I.; Marques, A.P. Postural control in elderly persons with osteoporosis: Efficacy of an intervention program to improve balance and muscle strength: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2010, 89, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.E.P.P.; Silva, K.N.G.; Imoto, A.M.; Teixeira, T.J.P.; Kayo, A.H.; Montenegro-Rodrigues, R.; Peccin, M.S.; Trevisani, V. Progressive load training for the quadriceps muscle associated with proprioception exercises for the prevention of falls in postmenopausal women with osteoporosis: A randomized controlled trial. Osteoporos. Int. 2010, 21, 589–596. [Google Scholar] [CrossRef]
- Marin-Cascales, E.; Alcaraz, P.E.; Ramos-Campo, D.J.; Rubio-Arias, J.A. Effects of multicomponent training on lean and bone mass in postmenopausal and older women: A systematic review. Menopause 2018, 25, 346–356. [Google Scholar] [CrossRef]
- Rhodes, E.C.; Martin, A.D.; E Taunton, J.; Donnelly, M.; Warren, J.; Elliot, J. Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br. J. Sports Med. 2000, 34, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; Bishop, M.D.; Pedersini, P.; Berjano, P. Physical Activity and Osteoarthritis: Update and Perspectives. Pain Med. 2019, 20, 1461–1463. [Google Scholar] [CrossRef]
- Shojaa, M.; von Stengel, S.; Kohl, M.; Schoene, D.; Kemmler, W. Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: A systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporos. Int. 2020, 31, 1427–1444. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Bacabac, R.G.; Bakker, A.D. Mechanical loading and how it affects bone cells: The role of the osteocyte cytoskeleton in maintaining our skeleton. Eur. Cells Mater. 2012, 24, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Krum, S.A. Direct transcriptional targets of sex steroid hormones in bone. J. Cell. Biochem. 2011, 112, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.M.; Takayanagi, H. The immune system, bone and RANKL. Arch. Biochem. Biophys. 2014, 561, 118–123. [Google Scholar] [CrossRef]
- Gardinier, J.D.; Mohamed, F.; Kohn, D.H. PTH Signaling During Exercise Contributes to Bone Adaptation. J. Bone Miner. Res. 2015, 30, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Merlotti, D.; Nuti, R. Selective estrogen receptor modulator (SERM) for the treatment of osteoporosis in postmenopausal women: Focus on lasofoxifene. Clin. Interv. Aging 2010, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bentz, A.T.; Schneider, C.M.; Westerlind, K.C. The relationship between physical activity and 2-hydroxyestrone, 16alpha-hydroxyestrone, and the 2/16 ratio in premenopausal women (United States). Cancer Causes Control 2005, 16, 455–461. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Tong, X.; Zhang, M.; Zhao, Y.; Guo, J.; Lei, L.; Chen, X.; Tickner, J.; Xu, J.; et al. Mechanical Stress Regulates Bone Metabolism Through MicroRNAs. J. Cell. Physiol. 2016, 232, 1239–1245. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Lopez, M.A.; Boriek, A.M. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J. Biol. Chem. 2010, 285, 29336–29347. [Google Scholar] [CrossRef]
- Shyu, K.-G.; Wang, B.-W.; Wu, G.-J.; Lin, C.-M.; Chang, H. Mechanical stretch via transforming growth factor-beta1 activates microRNA208a to regulate endoglin expression in cultured rat cardiac myoblasts. Eur. J. Heart Fail. 2013, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Y.; Liu, Y.; Liu, Y.; Zeng, Q.; Zhao, Y.; Zhang, X.; Zhang, X. MicroRNA-218, microRNA-191*, microRNA-3070a and microRNA-33 are responsive to mechanical strain exerted on osteoblastic cells. Mol. Med. Rep. 2015, 12, 3033–3038. [Google Scholar] [CrossRef]
- Yehya, N.; Yerrapureddy, A.; Tobias, J.; Margulies, S.S. MicroRNA modulate alveolar epithelial response to cyclic stretch. BMC Genom. 2012, 13, 154. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.; Zhang, L.; Wu, J.; Guo, J.; Zou, D.; Chen, B.; Sun, Z.; Shen, C.; Zou, J. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog. Biophys. Mol. Biol. 2016, 122, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Wohl, G.R.; Novack, D.V.; Lynch, J.A.; Silva, M.J. Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna. Calcif. Tissue Int. 2007, 80, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xu, Z.; Zhao, M. Effects of Oestrogen Treatment on Skeletal Response to Exercise in the Hips and Spine in Postmenopausal Women: A Meta-Analysis. Sports Med. 2015, 45, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Participants | Outcome Measures | Intervention | Results | Conclusions | PEDro Score |
|---|---|---|---|---|---|---|
| Marini et. Al (2019) [21] | n = 44 women [22 experimental group (APA), 18 control group (CG)]. Inclusion criteria:
| Health-Related Quality of Life ECOS-16 EuroQoL (EQ-5D-3L) Fear of Falling: FES-I questionnaire Lumbar Back Pain:
| Protocol duration: 6 months Frequency: 2 times per week Session: 1 h IG: Supervised -Warm-up: 15 min; multi-articular exercises, focus on joint mobilization, balance and postural control during walking. -Workout: 35 min; resistance bodyweight exercises (isometric and dynamic). -Cool down: 10 min; Stretching, Exercise in an upright and supine static position, holding a stretch position for up to 30 s. Drug exposure (% allocated subject): IG 100% (Bisphosphonates) | Adherence: 75.8% (56.4–97.8%) ECOS-16: APA group −0.5 ± 0.5, p = 0.001 *; CG +0.0 ± 0.3, ns EuroQoL VAS: APA +6.0 ± 16.6, ns; CG +1.9 ± 12.1, ns FES-I: APA −4.7 ± 7.4, p = 0.006 *; CG +0.9 ± 2.5, ns Lumbar back pain VAS: APA −1.2 ± 2.6, p = 0.029 *; CG +0.3 ± 3.3, ns Tinetti scale: APA +2.8 ± 5.2, p = 0.003 *; CG −0.7 ± 2.4. ns 6MWT: APA +52.2 ± 42.1, p < 0.001 *; CG −8.5 ± 45.2, ns Chair Sit-and-Reach right/left: APA +6.5 and +7.3, p = 0.002 */0.001 *; CG −0.6 and −0.2, ns * whitin group difference p < 0.05 | The feasibility, the safety and the positive effect of the proposed exercise protocol on quality of life, fear of falling, balance and functional exercise capacity show that APA programs should be extended also to patients whit OP and a history of vertebral fracture. | 6 /10 |
| Koevska et al. (2019) [22] | n = 92 women Inclusion criteria:
| Quality of life QUALEFFO-41 | Protocol duration: 12 months Frequency: 3 times per week IG: Exercise and physical modalities (interferent currents and magnetic therapy for 3 week, each day with a weekend break). CG 1: Exercise CG 2: No exercise Exercise: 5 to 8 times -Respiratory -Strengthening of the paraverterbral muscles, upper and lower extremities muscles, abdominal muscle -Active exercise for maintaining the range of motion of the joints of upper and lower extremities and spine -Balance Drug exposure (% allocated subject): IG 100%; CG 100% (Bisphosphonates, calcium and vitamin D) | Pain: III vs. I, 59.3 ± 21.3 vs. 40.87 ± 20.6 (p = 0.004 *) III vs. II, 59.3 ± 21.3 vs. 31.0 ± 23.2 (p < 0.0001 *) Physical function: III vs. I, 41.8 ± 19.3 vs. 19.95 ± 13.3 (p < 0.0001 *) III vs. II, 41.8 ± 19.3 vs. 19.99 ± 15.4 (p < 0.0001 *) Social Life: III vs. I, 67.06 ± 27.9 vs. 34.8 ± 19.9 (p < 0.0001 *) III vs. II, 67.06 ± 27.9 vs. 27.65 ± 21.64 (p < 0.0001 *) Health perception: III vs. I, 78.2 ± 21.2 vs. 45.88 ± 22.1 ( p < 0.0001 *) III vs. II, 78.2 ± 21.2 vs. 41.5 ± 21.9 (p < 0.000 *) * whitin group difference p < 0.05 | The exercise program for OP has significantly improved the quality of life in patients after one year of practicing in all four domains: pain, physical activities and mobility, social activities and perception about general health condition. | 8/10 |
| Çergel et al. (2019) [23] | n = 60 women Inclusion criteria
| Spinal pain -VAS Back extensor muscle Strength - Hand dynamometer Trunk muscle endurance - Timed Loaded Standing test Thoracic kyphosis - Digital inclinometer Functional mobilty - Time Up and Go test (TUG) Health-related quality of life - QUALEFFO-41 | Protocol duration: 6 weeks Frequency: 3 times per week IG: Supervised exercise group (SE) with full supervision of physiatrist. CG1: Home-based exercise group (HE) with instructional booklet and asked to apply the program at home. CG2: Daily life activities -Workkout: Back extensor Strengthening exercises In prone position: trunk extension, alternate arm raises, opposing arm and legs On the hands and knees position: opposing arm and leg raises. I-II weeks: 3 set of 8 rep III-IV weeks: 3 set of 10 rep V-VI weeks: 3 set of 12 rep Drug exposure (% allocated subject): IG 100%; CG 100% (Bisphosphonates) at least 6 months | VAS Rest: SE 2.80 ± 1.57 *, HE 5.15 ± 1.56 *, Control 5.75 ± 1.61 Activity: SE 2.75 ± 1.65 *, HE 5.85 ± 1.42, Control 6.30 ± 1.49 TUG (s): SE 8.5 ± 1.31 *, HE 12.10 ± 2.84 *, Control 12.40 ± 2.06 Back extensor strength (N): SE 45.2 ± 7.15 *, HE 38.5 ± 6.63 *, Control 34.75 ± 5.80 Trunk endurance (s): SE 108.05 ± 17.4 *, HE 56.80 ± 22.27 *, Control 47.10 ± 21.11 ** QUALEFFO-41: SE 32.48 ± 7.31 *, HE 44.32 ± 8.17 **, Control 45.44 ± 7.76 * p < 0.01 ** p < 0.05 | This study demonstrated that short-term supervised back extensor strengthening training is superior to home-based program in terms of spinal pain, back extensor muscle stgrength, trunk endurance, functional mobility, and QoL for postmenopausal osteoporotic women with vertebral fractures. | 6/10 |
| Watson et al. (2019) [19] | n = 51 women Inclusion criteria - Women older than 58 years - Low bone mass (T-score < −1.0 at the hip and/or spine). | Magnitude of kyphosis:
- DXA and Cobb angle | Protocol duration: 8 months Frequency: 2/week Session: 30 min Intervention group (HiRIT): Supervised Warm-up: 2 sets of deadlift at 50% to 7% 1RM First month: Body weight and low-load exercise variants, with focus on progressively learning the movement patterns. 4 fundamental exercise within 2 months Remainder intervention period: Resistance exercise (deadlift, overhead press, back squat) Training: 5 sets of 5 repetition Intensity: >80% to 85% 1 RM Drug exposure (% allocated subject): IG 100%; CG 100% (Bisphosphonates, calcium and vitamin D) | Height (cm): CON −0.1 ± 0.6, HiRIT +0.2 ± 0.6, p = 0.140 Inclinometer (°)
Cobb endplate angle (°): CON −0.6 ± 4.3, HiRIT +0.4 ± 4.4, p = 0.631 Cobb body angle (°): CON +0.5 ± 4.5, HiRIT −1.0 ± 4.5, p = 0.276 * whitin group difference p < 0.05 | Observations from the LIFTMOR trial indicate that brief, twice-weekly, supervides HiRIT exercise for 8 months did not cause fragility fractures and improved thoracic kyphosis in postmenopausal women with low to very low bone mass. | 7/10 |
| Watson et al. (2018) [24] | n = 101 women Mean age: 65 ± 5 Inclusion criteria - Women older than 58 years - Low bone mass (T-score < −1.0 at the hip and/or spine). | Bone measures -Femoral neck BMD -Lumbar spine BMD -QUS -Calcaneal BUA -SOS -SI Physical performance -LES -BES -TUG -FTSTS -FRT -Maximal vertical jump test | Protocol duration: 8 months Frequency: 2/week Session: 30 min IG: Supervised First month: Body weight and low-load exercise variants, with focus on progressively learning the movement patterns. 4 fundamental exercise within 2 months Remainder intervention period: Resistance exercise: deadlift, overhead press, back squat Warm-up: 2 sets of deadlift at 50% to 7% 1RM Reps: 5 sets of 5 repetition Intensity: >80% to 85% 1 RM CG: Home-based 8-month, twice-weekly, 30-min Warm-up: walking (10 min) Cool down (5 min) Resistance exercise: low-load resistance training (lunges, calf raises, standing forward raise, and shrugs) Stretching: side-to-side neck stretch, static calf stretch, shoulder stretch, and side-to-side lumbar spine stretch. Reps: 10 to 15 Intensity: <60% 1 RM Drug exposure (% allocated subject): IG 100%; CG 100% (Bisphosphonates, calcium and vitamin D) | LS BMD (g/cm2): CON −1.2 ± 3.1%, HiRIT +2.9 ± 3.1 %, p < 0.001 * FN BMD (g/cm2): CON −2.0 ± 3.0 %, HiRIT +0.3 ± 3.0 %, p = 0.025 * BUA (dB/MHz): CON +0.8 ± 7.6 %, HiRIT +1.0 ± 7.6 %, p = 0.534 SI: CON +2.0 ± 6.8 %, HiRIT +2.7 ± 6.8 %, p = 0.200 SOS (m/s): +0.2 ± 1.1 %, HiRIT +0.3 ± 1.1, p = 0.006 * FN total BMC (g): CON −0.2 ± 23.6%, HiRIT +1.7 ± 23.7%, p = 0.077 FN total vBMD (g/cm3): CON −0.3 ± 24.3%, HiRIT +3.7 ± 24.3, p = 0.830 * whitin group difference p < 0.05 | LIFTMOR trial showed a brief, supervised, twice-weekly HiRIT exercise intervention was efficacious and superior to previous programs for enhancing bone at clinically relevant sites, as well as stature and functional performance of relevance to falls in postmenopausal women with low to very low bone mass | 7/10 |
| Borba-Pinheiro et al. (2016) [14] | n = 52 women Inclusion criteria - Female volunteers -Aged over 50 years -Low BMD: T-score <1SD (low bone density) -Different ethnic population (descendants of Europeans, Blacks and Indians) -Patient being treated with sodium alendronate [70 mg] and/or vitamin D3 -No previous history of fractures -No history for at least 1 year of regular practice of physical activity -Indication/medical clearance for physical exercises practice. | BMD DXA Functional autonomy Latin American Development Group for Maturity (GDLAM): −10-m walk (10MW) -Rising from sitting position (RSP) -Rising frorm ventral decubitus position (RVDP) -Rising from a chair and walking around the house (RCWH) -Putting on and take off a shirt (PRTS) Muscular Strength 10 maximum repetitions test (10MR) Quality of life Osetoporosis Assessment Questionnaire (OPAQ) | Protocol duration: 13 months Session: 60 min IG: RT3 (3 times a week) CG: RT2 (two times a week) Exercises: leg press 45°; knee extension; plantar flexion; squats; hip adduction; gluts (machine for gluts); elbow flexion; elbow extension; shoulder adduction Posology: 3 sets per exercise. Repetitions numers/sets, rest intervals between exercises and sessions respected the scientific principle of inter-dependence volume x intensity (American College Sports Medicine). Intensity: between 60% and 90%; 7 months cycles (60%, 65%, 70%, 75%, 80%, 85% and 90%); in addition 3 bimonthly cycles (70%, 80%, 90%) Drug exposure (% allocated subject): IG 100%; CG 100% (Sodium alendronate [70 mg] and/or vitamin D3) | Total BMD: RT3 +0.10% * vs. CG +0.09%, p < 0.05 T2 +0.06% vs. CG, p = 0.046 Leg press 45°: RT3 * vs. RT2 * = +24.97% favorable to RT3 RT3/RT2 vs. CG = +84.1% / +59.1% favorable to RT3/RT2 Knee extension: RT3 */RT2 * vs. CG = +15.28% / +20.37% favorable to RT3/RT2 OPAQ total score: RT3 369.05 ± 19.9 *,§, RT2 348.8 ± 22.6 *,§,°, CG 311.4 ± 35.7 §,° * intra-group p < 0.05 § p < 0.05 inter-groups favorable RT3 ° p < 0.05 inter-groups faborable RT2 | Both experimental groups presented favorable results for BMD, strength, FA and QoL. However, the RT3 showed the best results compared to other groups after 13 months of intervention. | 8/10 |
| Khalili et al. (2016) [25] | n = 183 women Inclusion criteria - Women with primary OP (with DEXA bone densitometry) - 30 to 50 degrees kyphosis (with lateral standard wiew thoracic spine x-ray graphs). | Quality of life - Persian version of the SF-36 QOL questionnaire | Protocol duration: 6 months Session: 30 min Frequency: 5 times a week IG Warm-up: Walk and back extensors Resistance exercise: Home-base Reps: 10 contractions of back extensor without increasing the low back lordosis Drug exposure (% allocated subject): IG 100%; CG 100% (Calcium [1000 mg], vitamin D [800 IU] daily, sodium alendronate [70 mg] weekly) | Phisical Component Score: Intervention group 270.55 ± 58.72 *, Control group 233.30 ± 67.47 *, p = 0.00 Mental Component Score: Intervention group 255.78 ± 61.19 *, Control group 239.58 ± 73.60, p = 0.39 * intra-group p < 0.05 | Home-based exercise with no direct supervision improved QOL in elderly women whith OP at a 6-month follow-up. | 8/10 |
| Murtezani et al. (2014) [15] | n = 62 women Inclusion criteria Women recently diagnosed (within the past 6 months) with OP on account of a DEXA scan T score below −2.5 Aged 50–70 years No history of vertebral fractures or lower extremities fractures No endoprostheses or fixation materials Capable of signing written informed consent | Muscle Strength -GS -QS Flexibility - BRPT Balance - Berg Balance Scale (BBS) Gait time - 6MWT Pain - VAS | Protocol duration: 10 months Session: 55 min Frequency: 3 times a week IG (Land exercise) Warm-up: 10 min. Stretching and balance exercise at 70–80% Exercises: leg press 45°; knee extension; plantar flexion; squats; hip adduction; gluts (machine for gluts); elbow flexion; elbow extension; shoulder adduction Posology: 3 sets per exercise. Repetitions numers/sets, rest intervals between exercises and sessions respected the scientific principle of inter-dependence volume x intensity (American College Sports Medicine). Intensity: between 60% and 90%; 7 months cycles (60%, 65%, 70%, 75%, 80%, 85% and 90%); in addition 3 bimonthly cycles (70%, 80%, 90%) Drug exposure (% allocated subject): IG 100%; CG 100% (Dietary restriction and supplementation (Calcium [1000 mg] daily and Vitamin D [800–1000 IU] daily) | VAS: LE −81.26% *, Control −32.28%, p < 0.001 GS: LE −4.54% *, Control −2.35%, p = 0.002 QS: LE +4.4% *, Control +1.1% *, p = 0.002 BBS: LE +3.24% *, Control +3.04%, p = 0.38 6MWT: LE +18.72% *, Control +12.29% *, p < 0.001 BMD: LE +5.35% *, Control +3.92%, p < 0.001 T-score: LE −12.04% *, Control −6.44%, p < 0.001 * whitin group difference p < 0.05 | Significant improvements in physical function and BMD suggest that land exercise is a possible alterative for postmenopausal women with OP. | 6/10 |
| Mosti et al. (2013) [11] | n = 16 women Inclusion criteria At least 2 years postmenopausal Age < 75 years old BMD t-score between −1.5 and −4.0 at the lumbar spine, femoral neck or total hip | MS, RFD and PF - Squat exercise machine BMC and BMD - Lumbar spine - Femoral neck - Total hip Vitamin D and Markers of Bone Metabolism Treadmill Testing - Peak oxygen consumption (VO2 peak) | Protocol duration: 12 weeks Frequency: 3 times a week IG: (Maximal Strength Training MST) Workout: supervised maximal strength training, focused on high acceleration during the concentric phase, resulting in a high RFD during muscle contraction. Posology: Each set was separated by 2–3 min rest. Intensity: If the participants could perform >5 repetitions, the training load was increased by 2.5 kg. Drug exposure (% allocated subject): IG 100%; CG 100% (Calcium and Vitamin D) | 1RM (kg): TG 93.13 ± 8.10 *,°, CG 62.19 ± 14.36 Dynamic RFD (N/s): TG 1103.35 ± 282.75 *, CG 1386.02 ± 595.00 Peak force (N): TG 1397.23 ± 123.84 *, CG 1389 ± 260.00 BMC (g): TG lumbar +2.9 ± 2.8% (p = 0.012); femoral neck +4.9% ± 5.6% (p = 0.043), No change in CG Serum bone markers: - Vitamin D (nmol/L) TG 80.7 ± 29.2; CG 99.5 ± 16.5). P1NP and CTX no significant changes P1NP/CTX ratio TG +21.5 ± 40.5%, p = 0.093 * Difference within group, p < 0.05 ° Difference between group, p < 0.01 | This study demonstrates that squat exercise MST, applying only one exercise, improves 1RM, RFD, and BMC in patients with OP and osteopenia. | 6/10 |
| Marchese et al. (2012) [12] | n = 22 women Inclusion criteria Diagnosis of osteopenia by DXA performed within 6 months Age between 40 and 80 years old | BMD - Lumbar spine - Proximal femoral epiphysis Osteocalcin and CTX in serum Electromyographic signal - Quadriceps femoris - Hip adductors - Adbominal - Paravertebral Static Balance - LC - MAO 6MWT Disability and Quality of Life - EuroQoL | Protocol duration: 24 weeks Frequency: 3 times a week Session: 60 min IG: Training group A combination of exercised designed to improve strength and muscle tropism, aerobic capacity, coordination and balance, designed to stimulate bone tissue in an atypical and intermittent compression, bending and tensile multi-directional stress. Drug exposure (% allocated subject): IG 18.1%; CG 27.2% (Antiresorptives) | Balance LC: TG −49.79%, CG +7.33%, p < 0.0001 * MAO: TG −45.92%, CG +0.33%, p = 0.002 * Muscle Strength (s-EMG, μV) Quadriceps femoris: TG +45.49%, CG −1.60%, p < 0.00001 * Adductors: TG +33.66%, CG −1.13%, p < 0.00001 Extensors of Trunk: TG +53.35%, CG −1.58%, p < 0.00001 * 6MWT: TG +33.33%, CG +16.18%, p < 0.0001 * EuroQoL Score: TG +34.52%, CG −12.30%, p = 0.0002 * BMD Lumbar spine TG +14.90%, CG −6.60%, p = 0.0005 * Hip TG +5.06%, CG −8.60%, p = 0.03 Markers CTX: TG −24.52%, CG +11.32%, p = 0.002 * Osteocalcin: TG −15.06%, CG +25.28%, p = 0.0003 * * whitin group difference p < 0.05 | A improve strength and muscle tropism, coordination and balance, can provide advantages of unquestioned importance in bone mass, neuromuscular function, reduced risk of falling and general health.rehabilitation program of group exercise based on gravitational load, designed to | 5/10 |
| Burke et al. (2012) [26] | n = 33 women Inclusion criteria Women from 65 to 79 year of age Diagnosis of OP (according to the WHO criteria) BMD reduced at leat 2.5 SD compared with young adults (region of lumbar spine) | Postural control LOS CTSIBm Inferior Members Strength (Isometric Strength) Ankle dorsiflexion Knee extension Kn ee flexion | Protocol duration: 8 weeks Frequency: 2 times a week Session: 60 min IG (Strength group) Warm-up: 10 min walking at low intensity Exercises: Balance exercise (20 min): walking in the tandem position, on the tips the toes and heel, sideways, while raising the leg and controlateral arm; standing on one leg, in the tandem position; Strengthening exercises for lower limb (30 min): exercise for knee extensor muscle, hip flexors muscles and akle extensor muscles. Posology: 10 repetitions, 1 min between sets. CG1 (Stretching group) CG2 (Education) Drug exposure (% allocated subject): IG 94%; CG1 52%; CG2 56% (Medication and calcium supplementation) | Adherence: 82.3% Isometric strength: Ankle flexion IG +4.4 kg, CG +0.3 kg, p = 0.012 * Knee extension IG +4.43 kg, CG +0.1 kg, p = 0.003 * Knee flexion IG +1.71 kg, CG +0.22 kg, p = 0.003 * Postural control: COP velocity IG +2.34°/s, CG 0.01°/s, p = 0.009 * Directional control IG +5.34 %, CG 0.44 %, p = 0.002 * CTSIBm (closed eyes) IG −0.21°/s, CG +0.05°/s, p = 0.021 * * whitin group difference p < 0.05 | Our study suggests that, in old woman with OP, 8 weeks of exercises improving balance and inferior member strength yielded improvement of postural control and of muscular strength. | 6/10 |
| Borba-Pinheiro et al. (2010) [18] | n = 28 women Inclusion criteria Women with OP and/or osteopenia in at least one of the measurements of BMD T-score Patients treated with sodium alendronate (70 mg) No history of fractures No history for at least 1 year of regular practice of physical activity Good physical and mental health | BMD - Lumbar spine - Proximal femur Body balance - Static Balance Test with Visual Control Quality of Life - OPAQ | Protocol duration: 12 months Frequency: 3 times a week Session: 60 min IG: RTG Exercises: leg press 45°; knee extension; plantar flexion; squats; hip adduction; gluts (machine for gluts); elbow flexion; elbow extension; shoulder adduction Posology: 10 maximum repetitions (10RM) test Intensity: 70–90% CG1: JUG Exercises: Traditional methodology for judo classes CG2: WAG Exercises: in a 25-m pool, 1.45 m deep; dislocations (previous, posterior and lateral), shoulder adduction/abduction, short jumps with knee extension, alternate elbow flexion, alternate knee flexion, alternate elbow extension, hip adduction/abduction, shoulder abduction/adduction, squats. Drug exposure (% allocated subject): IG 100%; CG 100% (Sodium alendronate [70 mg] weekly) | BMD Lumbar: RTG 0.091, JUG 0.079, WUG 0.034, CG −0.024, p = 0.002, p = 0.003, ns Neck of femur: RTG 0.083, JUG 0.019, WUG −0.007, CG −0.06, p = 0.002, ns, ns Great trochanter: RTG 0.049, JUG 0.015, WUG, 0.018, CG −0.029, p = 0.002, ns, ns Body balance RTG 5.74, JUG 5.30, WUG 0.018, CG −1.06, p = 0.018, p = 0.022, ns OPAQ RTG 30.56, JUG 53.09, WUG 7.63, CG −7.29, p = 0.006, p = 0.000, ns * whitin group difference p < 0.05 | The type of physical activity examined in this study could be raccomended alone or as adjunvtive therapy to a biphosponate in postmenopausal women with low BMD, especially resistance training. | 5/10 |
| Teixeira et al. (2010) [27] | n = 100 women Inclusion criteria - Aged from 55 to 75 years old - Individuals with postmenopausal OP. - BMD T-score of −2.5 SD in the lumbar spine, femoral neck or total femur region | Quality of life SF-36 Functional mobility - TUG Balance Berg Balance Muscular strength - Dynamic strength of the quadriceps muscle (1-RM) | Protocol duration: 18 weeks Frequency: 2 times a week IG Warm-up: 5–10 min treadmill, static stretching exercises (global and segmentary) for upper and lower limbs, lumbar, cervical, and thoracic region; 2 series of 3 rep for each muscle; 30 s maintening. Workout: Functional exercises (proprioception and balance) Strengthening exercises included leg extension, load up to 80% 1RM (following a two week protocol, from 50% to 80%) Drug exposure (% allocated subject): IG 100%; CG 100% (Antiresorptives) | SF-36: Δ in all subscales > 13.5 points, p ≤ 0.0018 Berg Scale: Δ 3.58 [2.75;4.42], p < 0.0001 Maximum load (kg): Δ 3.65 [2.74;4.57], p < 0.0001 Time Up and Go test (s): Δ −3.96 [−4.63; −3.29], p < 0.0001 | The progressive muscle strength training for the quadriceps associated to the proprioceptive training is effective in increasing muscle strength in quadriceps, improvement in static and dynamic balance, speed of the motor responses, therefore improving the performance of daily activities and reducing the frequency of falls in women with postmenopausal OP. | 6/10 |
| Bocalini et al. (2009) [17] | n = 35 women Inclusion criteria Women older than 55 years Able to train 3 times per week in the course of 24 weeks of the protocol | Body composition BMI Body fat percentage BMD - Lumbar spine - Femur neck Muscle Strength (1RM) - Chest press - Leg extension | Protocol duration: 24 weeks Frequency: 3 times a week Session: 1 h supervised IG Warm-up: 10 min of running with low impact at 50% of maximum hearth rate; 1 set at 50% 1RM Workout (TR): Focus on eccentric muscle action. Leg press, chest press, leg curl, latissumus pull down, elbow flexion, elbow extension, leg extension, upper back row, military press, hip abductor, hip adductor, abdominal curls. Drug exposure (% allocated subject): IG 100%; CG 100% (Antiresorptives) | MS: TR 62 ± 5 kg, +39%, p < 0.001 lower limb; 37 ± 6 kg, +46%, p < 0.001 upper body UN 38 ± 7 kg, −2.5%, p > 0.05 lower limb; 23.5 ± 5 kg, +4.5%, p > 0.05 upper body BMD: TR 0.880 ± 0.001 g/cm2, p > 0.05 lumbar spine, 0.704 ± 0.001 g/cm2 femoral neck UN 0.873 ± 0.002 g/cm2, p < 0.05 lumbar spine, 0.695 ± 0.001 g/cm2 femoral neck | We demonstrated the positive effects of strength training on the body composition parameters, muscular strength, and bone health of postmenopausal women without hormone replacement therapy. | 6/10 |
| Tolomio et al. (2008) [16] | n = 64 women Inclusion criteria Postmenopausal women (age between 50 and 70 years) Diagnosis of osteopenia or OP (t-score determined by ultrasounds < 1.0SD Lack of any disease that affect bone metabolism No previous skeletal fractures Lack of any controindication to perform physical activity | Bone quality Phalangeal quantitative osteosonography As-so UBPS Muscle Strength (1RM) - Knee extensor muscles | Protocol duration: 20 weeks Frequency: 3 times a week Session: two 60-min sessions and one 45-min session IG 60-min session: Warm-up: 20–25 min of walking, stretching, small jumps. Workout: 30-min training; callistheni/isometric exercises and exercises with dumbells, Thera-Bands, balls aimed to improve range of motion, overall Strength, balance and aerobic capacity. Cool down: 5–10 min; stretching, breathing, postural exercises Volume: graded increase of intensity and number of rep/series starting after the fifth week of training. CG 45-min session: Combination of aerobic endurance and Strength exercises. Workout: Circuit training of 6 bouts of exercise lasting 5 min each; treadmill, leg extension, arm ergometer, horizontal leg press, bike, lat machine. Indication to progressively increase repetitions or load lifted in during each 5-min Strength exercise. Drug exposure (% allocated subject): IG 58,6%; CG: 55% (Bisphosphonates, calcium and Raloxifene) | Ad-Sos: EG 1988.8 ± 74.4 m/s, p < 0.05; CG ns UBPS: EG 36.8 ± 21.3, p < 0.05; CG 36.5 ± 17.2, ns T-score: EG −2.1 ± 1.1, p < 0.05; CG ns Knee extension: 52.7 ± 9.5 kg, p < 0.05; CG ns | In a group of postmenopausal women, a supervised, multidimensional exercise program improved bone quality, evaluated at the finger, in a relatively short period of time. | 6/10 |
| Brentano et al. (2008) [20] | n = 28 women Inclusion criteria No neuromuscular injury or engaged in any tipe of competitive exercise Practiced sports occasionally at a recreational level. | Body composition BM FFM FM SF VO2 max TE Dynamic Strength (1RM) Arm curl exercises Knee extension exercises Isometric Strength MVC Electromyographic Signal Vastus lateralis Vastus medialis BMD Lumbar spine Femur | Protocol duration: 24 weeks Frequency: 3 times a week Session: 1 h supervised Warm-up: 5 min; cycloergometer or treadmill Workout: leg press, hip abduction, hip adduction, knee extension, chest fly, reverse fly, arm curl, triceps push-down, sit-ups, back extension. IG: STG The exercises were performed separately, with a 2-min rest between sets. Posology: 20–6 repetitions and 45–80% 1RM, 2–4 sets for each exercises. CG: CTG The exercises were performed with no rest between exercises Posology: 23–10 repetitions and 45–60% 1RM; 2–3 sets for each exercise. Drug exposure (% allocated subject): IG 50%; CG: 50% (Hormone therapy (HT)) | VO2max and TE: increased significantly in both training group after 24 weeks Dynamic strength: LDS and UDS increased significantly in STG and CTG, greater than the CON group. Isometric strength: Increased significantly in both training group after 24 weeks BMD: no alteration in BMD lumbar, BMD neck, BMD inter, BMD troc, BMD ward in all groups after the 24-week period. Correlations: LLS and VO2max: r = 0.73, p = 0.000 LLS and TE: r = 0.72, p = 0.000 IS and VO2max: r = 0.59, p < 0.01 IS and TE: r = 0.54, p < 0.01 | Circuit weight training is an effective training in strategy to improve neuromuscular and cardiorespiratory conditioning of postmenopausal women with no history of resistance training. | 6/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso Pérez, J.L.; Martín Pérez, S.; Battaglino, A.; Villafañe, J.H.; Alonso-Sal, A.; Sánchez Romero, E.A. An Up-Date of the Muscle Strengthening Exercise Effectiveness in Postmenopausal Women with Osteoporosis: A Qualitative Systematic Review. J. Clin. Med. 2021, 10, 2229. https://doi.org/10.3390/jcm10112229
Alonso Pérez JL, Martín Pérez S, Battaglino A, Villafañe JH, Alonso-Sal A, Sánchez Romero EA. An Up-Date of the Muscle Strengthening Exercise Effectiveness in Postmenopausal Women with Osteoporosis: A Qualitative Systematic Review. Journal of Clinical Medicine. 2021; 10(11):2229. https://doi.org/10.3390/jcm10112229
Chicago/Turabian StyleAlonso Pérez, Jose Luis, Sebastián Martín Pérez, Andrea Battaglino, Jorge H. Villafañe, Alexandra Alonso-Sal, and Eleuterio A. Sánchez Romero. 2021. "An Up-Date of the Muscle Strengthening Exercise Effectiveness in Postmenopausal Women with Osteoporosis: A Qualitative Systematic Review" Journal of Clinical Medicine 10, no. 11: 2229. https://doi.org/10.3390/jcm10112229
APA StyleAlonso Pérez, J. L., Martín Pérez, S., Battaglino, A., Villafañe, J. H., Alonso-Sal, A., & Sánchez Romero, E. A. (2021). An Up-Date of the Muscle Strengthening Exercise Effectiveness in Postmenopausal Women with Osteoporosis: A Qualitative Systematic Review. Journal of Clinical Medicine, 10(11), 2229. https://doi.org/10.3390/jcm10112229







