The Treatment Gap in Osteoporosis
Abstract
1. Introduction
2. Definition & Diagnosis
3. Who to Treat
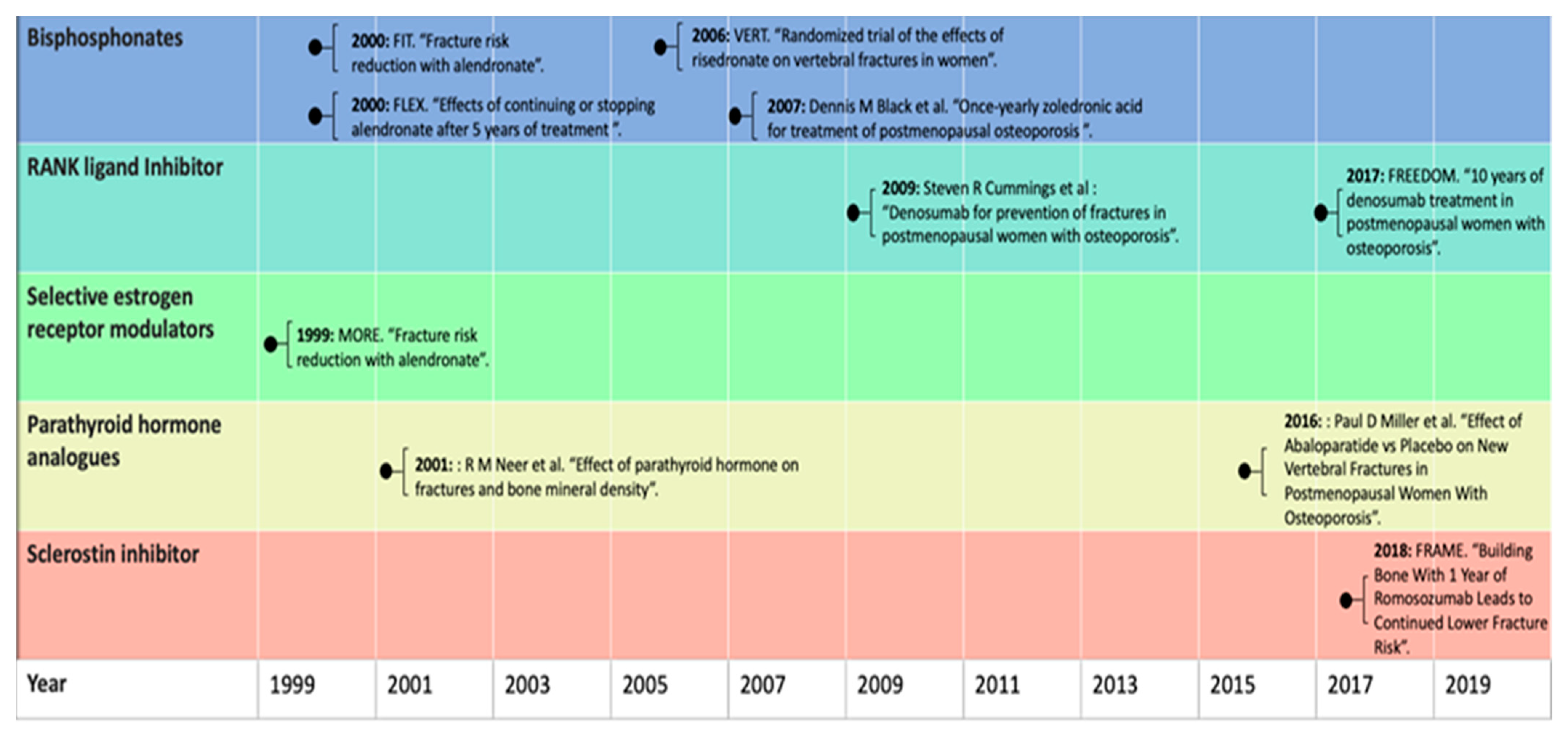
4. Pharmacological Treatment
5. The Growing Gap in Treatment Options
5.1. Rare Side Effects and Growing Treatment Gap
5.2. Underestimation of the Fracture Risk and Growing Treatment Gap
6. Bisphosphonate Use, Mortality, and Vascular Calcification
7. Glucocorticoid-Induced Osteoporosis
8. Future Directions
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weycker, D.; Li, X.; Barron, R.; Bornheimer, R.; Chandler, D. Hospitalizations for osteoporosis-related fractures: Economic costs and clinical outcomes. Bone Rep. 2016, 5, 186–191. [Google Scholar] [CrossRef]
- Borgström, F.; International Osteoporosis Foundation; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, G.; Bartstra, J.W.; Weijmans, M.; de Jong, P.A.; Mali, W.P.; Verhaar, H.J.; Visseren, F.; Spiering, W. Bisphosphonates for cardiovascular risk reduction: A systematic review and meta-analysis. Atherosclerosis 2016, 252, 106–115. [Google Scholar] [CrossRef]
- Stone, K.L.; Seeley, D.G.; Luy, L.-Y.; Cauley, L.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R.; Osteoporotic Fractures Research Group. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Frac-tures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Koromani, F.; Breda, S.J.; Schousboe, J.T.; Clark, E.M.; Van Meurs, J.B.; Ikram, M.A.; Waarsing, J.H.; Van Rooij, F.J.; Zillikens, M.C.; et al. Osteoporotic Vertebral Fracture Prevalence Varies Widely Between Qualitative and Quantitative Radiological Assessment Methods: The Rotterdam Study. J. Bone Miner. Res. 2018, 33, 560–568. [Google Scholar] [CrossRef]
- O’Neill, T.W.; Felsenberg, D.; Varlow, J.; Cooper, C.; Kanis, J.A.; Silman, A.J. The prevalence of vertebral deformity in European men and women: The european vertebral osteoporosis study. J. Bone Miner. Res. 2009, 11, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Trajanoska, K.; Schoufour, J.D.; De Jonge, E.A.; Kieboom, B.C.; Mulder, M.; Stricker, B.H.; Voortman, T.; Uitterlinden, A.G.; Oei, E.H.; Ikram, M.A.; et al. Fracture incidence and secular trends between 1989 and 2013 in a population based cohort: The Rotterdam Study. Bone 2018, 114, 116–124. [Google Scholar] [CrossRef]
- Cooper, C.; Melton, L. Epidemiology of osteoporosis. Trends Endocrinol. Metab. 1992, 3, 224–229. [Google Scholar] [CrossRef]
- A Kanis, J.; Svedbom, A.; Harvey, N.; McCloskey, E.V. The Osteoporosis Treatment Gap. J. Bone Miner. Res. 2014, 29, 1926–1928. [Google Scholar] [CrossRef]
- Ji, M.-X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Painter, S.E.; Kleerekoper, M.; Camacho, P.M. Secondary Osteoporosis: A Review of The Recent Evidence. Endocr. Pract. 2006, 12, 436–445. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Thompson, D.E.; Bauer, D.C.; Ensrud, K.; Musliner, T.; Hochberg, M.C.; Nevitt, M.C.; Suryawanshi, S.; Cummings, S.R. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. J. Clin. Endocrinol. Metab. 2000, 85, 4118–4124. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Schwartz, A.V.; Ensrud, K.; A Cauley, J.; Levis, S.; Quandt, S.A.; Satterfield, S.; Wallace, R.B.; Bauer, D.C.; Palermo, L.; et al. Effects of Continuing or Stopping Alendronate After 5 Years of Treatment. The Fracture Intervention Trial Long-term Extension (FLEX): A Randomized Trial. Obstet. Gynecol. Surv. 2007, 62, 251–252. [Google Scholar] [CrossRef]
- Reginster, J.; Minne, H.W.; Sorensen, O.H.; Hooper, M.; Roux, C.; Brandi, M.L.; Lund, B.; Ethgen, D.; Pack, S.; Roumagnac, I.; et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteo-porosis. Osteoporos. Int. 2000, 11, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.; Boonen, S.; A Cauley, J.; Cosman, F.; Lakatos, P.L.; Leung, P.C.; Man, Z.; et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of Vertebral Fracture Risk in Postmenopausal Women with Osteoporosis Treated with RaloxifeneResults From a 3-Year Randomized Clinical Trial. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporo-sis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Ferrari, S.; Khan, A.; E Lane, N.; Lippuner, K.; Matsumoto, T.; E Milmont, C.; Libanati, C.; Grauer, A. FRAME Study: The Foundation Effect of Building Bone with 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J. Bone Miner. Res. 2018, 33, 1219–1226. [Google Scholar] [CrossRef]
- Khosla, S.; Bilezikian, J.P.; Dempster, D.W.; Lewiecki, E.M.; Miller, P.D.; Neer, R.M.; Recker, R.R.; Shane, E.; Shoback, D.; Potts, J.T. Benefits and Risks of Bisphosphonate Therapy for Osteoporosis. J. Clin. Endocrinol. Metab. 2012, 97, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. J. Formul. Manag. 2018, 43, 92–104. [Google Scholar]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Kapoor, E.; Asi, N.; Alahdab, F.; Mohammed, K.; Benkhadra, K.; Almasri, J.; Farah, W.; Sarigianni, M.; Muthusamy, K.; et al. Efficacy of Pharmacological Therapies for the Prevention of Fractures in Postmenopausal Women: A Network Meta-Analysis. J. Clin. Endocrinol. Metab. 2019, 104, 1623–1630. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis. Endocr. Pract. 2016, 22, 1–42. [Google Scholar] [CrossRef]
- Shakeri, A.; Adanty, C. Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review. J. Popul. Ther. Clin. Pharmacol. 2020, 27, e25–e31. [Google Scholar] [CrossRef]
- Khosla, S.; Shane, E. A Crisis in the Treatment of Osteoporosis. J. Bone Miner. Res. 2016, 31, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic Burden: A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Hiligsmann, M.; Cornelissen, D.; Vrijens, B.; Abrahamsen, B.; Al-Daghri, N.; Biver, E.; Brandi, M.; Bruyère, O.; Burlet, N.; Cooper, C.; et al. Determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment: Results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Osteoporosis Foundation (IOF). Osteoporos. Int. 2019, 30, 2155–2165. [Google Scholar] [CrossRef]
- Solomon, D.H.; Johnston, S.S.; Boytsov, N.N.; McMorrow, D.; Lane, J.M.; Krohn, K.D. Osteoporosis Medication Use After Hip Fracture in U.S. Patients Between 2002 and 2011. J. Bone Miner. Res. 2014, 29, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata, J.B.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; et al. The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef]
- Shane, E.; Burr, D.; Abrahamsen, B.; A Adler, R.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical Subtrochanteric and Diaphyseal Femoral Fractures: Second Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef]
- A Khan, A.; Morrison, A.; A Hanley, D.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of Osteonecrosis of the Jaw in Patients with Oral Bisphosphonate Exposure. J. Oral Maxillofac. Surg. 2010, 68, 243–253. [Google Scholar] [CrossRef]
- Meier, R.P.H.; Perneger, T.V.; Stern, R.; Rizzoli, R.; Peter, R.E. Increasing Occurrence of Atypical Femoral Fractures Associated with Bisphosphonate Use. Arch. Intern. Med. 2012, 172, 930–936. [Google Scholar] [CrossRef]
- Dell, R.M.; Adams, A.L.; Greene, D.F.; Funahashi, T.T.; Silverman, S.L.; O Eisemon, E.; Zhou, H.; Burchette, R.J.; Ott, S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012, 27, 2544–2550. [Google Scholar] [CrossRef]
- Schilcher, J.; Koeppen, V.; Aspenberg, P.; Michaëlsson, K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop. 2015, 86, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical Femur Fracture Risk vs. Fragility Fracture Prevention with Bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef]
- Glusman, J.E.; Huster, W.J.; Paul, S. Raloxifene effects on vasomotor and other climacteric symptoms in postmenopausal women. Prim. Care Updat. OB/GYNS 1998, 5, 166. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Bonanni, B.; Costantino, J.P.; Cummings, S.; DeCensi, A.; Dowsett, M.; Forbes, J.F.; Ford, L.; LaCroix, A.Z.; et al. Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual partici-pant data. Lancet 2013, 381, 1827–1834. [Google Scholar]
- Vahle, J.L.; Long, G.G.; Sandusky, G.; Westmore, M.; Ma, Y.L.; Sato, M. Bone Neoplasms in F344 Rats Given Teriparatide [rhPTH(1-34)] Are Dependent on Duration of Treatment and Dose. Toxicol. Pathol. 2004, 32, 426–438. [Google Scholar] [CrossRef]
- Andrews, E.B.; Gilsenan, A.; Midkiff, K.; Sherrill, B.; Wu, Y.; Mann, B.H.; Masica, D. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: Study design and findings from the first 7 years. J. Bone Miner. Res. 2012, 27, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Hattersley, G.; Riis, B.J. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef]
- Asadipooya, K.; Weinstock, A. Cardiovascular Outcomes of Romosozumab and Protective Role of Alendronate. Arter. Thromb. Vasc. Biol. 2019, 39, 1343–1350. [Google Scholar] [CrossRef]
- van Geel, T.A.; Eisman, J.A.; Geusens, P.P.; van den Bergh, J.P.; Center, J.R.; Dinant, G.J. The utility of absolute risk prediction using FRAX(R) and Garvan Fracture Risk Calculator in daily practice. Maturitas 2014, 77, 174–179. [Google Scholar] [CrossRef]
- Domiciano, D.S.; Machado, L.G.; Lopes, J.B.; Figueiredo, C.P.; Caparbo, V.F.; Oliveira, R.M.; Scazufca, M.; McClung, M.R.; Pereira, R.M. Bone Mineral Density and Parathyroid Hormone as Independent Risk Factors for Mortality in Communi-ty-Dwelling Older Adults: A Population-Based Prospective Cohort Study in Brazil. J. Bone Miner. Res. 2016, 31, 1146–1157. [Google Scholar] [CrossRef]
- Johansson, H.; Odén, A.; Kanis, J.; McCloskey, E.; Lorentzon, M.; Ljunggren, Ö.; Karlsson, M.K.; Orwoll, E.; Tivesten, Å.; Ohlsson, C.; et al. Low bone mineral density is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos. Int. 2010, 22, 1411–1418. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos. Int. 2009, 21, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.; Center, J.; A Eisman, J.; Nguyen, T.V. Bone Loss, Weight Loss, and Weight Fluctuation Predict Mortality Risk in Elderly Men and Women. J. Bone Miner. Res. 2007, 22, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.M.; Browner, W.S.; Blackwell, T.; Gore, R.; Cummings, S.R. Rate of Bone Loss Is Associated with Mortality in Older Women: A Prospective Study. J. Bone Miner. Res. 2000, 15, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Persy, V.; D’Haese, P. Vascular calcification and bone disease: The calcification paradox. Trends Mol. Med. 2009, 15, 405–416. [Google Scholar] [CrossRef]
- Ahmadi, N.; Mao, S.S.; Hajsadeghi, F.; Arnold, B.; Kiramijyan, S.; Gao, Y.; Flores, F.; Azen, S.; Budoff, M. The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos. Int. 2018, 29, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Campos-Obando, N.; Kavousi, M.; van Lennep, J.E.R.; Rivadeneira, F.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Zillikens, M.C. Bone health and coronary artery calcification: The Rotterdam Study. Atherosclerosis 2015, 241, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Ballanti, P.; Mantella, D.; Manni, M.; Lippi, B.; Pierantozzi, A.; Di Giulio, S.; Pellegrino, L.; Romagnoli, A.; Simonetti, G.; et al. Bone Turnover, Osteopenia and Vascular Calcifications in Hemodialysis Patients. Am. J. Nephrol. 2009, 29, 145–152. [Google Scholar] [CrossRef]
- Kim, D.H.; Rogers, J.R.; Fulchino, L.A.; Kim, C.A.; Solomon, D.H.; Kim, S. Bisphosphonates and Risk of Cardiovascular Events: A Meta-Analysis. PLoS ONE 2015, 10, e0122646. [Google Scholar] [CrossRef]
- Casula, M.; Olmastroni, E.; Galimberti, F.; Tragni, E.; Corrao, G.; Scotti, L.; Catapano, A.L. Association between the cumulative exposure to bisphosphonates and hospitalization for atherosclerotic cardiovascular events: A population-based study. Atherosclerosis 2020, 301, 1–7. [Google Scholar] [CrossRef]
- van Staa, T.P.; Leufkens, H.G.; Abenhaim, L.; Begaud, B.; Zhang, B.; Cooper, C. Use of oral corticosteroids in the United Kingdom. QJM 2000, 93, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Overman, R.; Yeh, J.-Y.; Deal, C.L. Prevalence of oral glucocorticoid usage in the United States: A general population perspective. Arthritis Rheum. 2013, 65, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Benedek, T.G. History of the development of corticosteroid therapy. Clin. Exp. Rheumatol. 2011, 29, 5–12. [Google Scholar]
- DeMartini, F.; Grokoest, A.W.; Ragan, C. Pathological fractures in patients with rheumatoid arthritis treated with cortisone. JAMA 1952, 149, 750–752. [Google Scholar] [CrossRef]
- Teicher, R.; Nelson, C.T. Osteoporosis and Pathological Fractures Following Treatment with Acth and Cortisone. J. Investig. Dermatol. 1952, 19, 205–210. [Google Scholar] [CrossRef]
- Curtiss, P.H.; Clark, W.S.; Herndon, C.H. Vertebral fractures resulting from prolonged cortisone and corticotropin therapy. J. Am. Med. Assoc. 1954, 156, 467–469. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chotiyarnwong, P.; McCloskey, E.V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 2020, 16, 437–447. [Google Scholar] [CrossRef]
- Allen, C.S.; Yeung, J.H.; VanderMeer, B.; Homik, J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst. Rev. 2016, 2016, CD001347. [Google Scholar] [CrossRef]
- Adami, G.; Saag, K.G. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos. Int. 2019, 30, 1145–1156. [Google Scholar] [CrossRef]
- Peat, I.D.; Healy, S.; Reid, D.M.; Ralston, S.H. Steroid induced osteoporosis: An opportunity for prevention? Ann. Rheum. Dis. 1995, 54, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.R.; Green, B. Osteoporosis prophylaxis during corticosteroid treatment: Failure to prescribe. Postgrad. Med. J. 2002, 78, 242–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neogi, T.; Allison, J.J. Suboptimal osteoporosis management: Moving beyond detection. J. Rheumatol. 2004, 31, 413–415. [Google Scholar] [PubMed]
- Compston, J.; The National Osteoporosis Guideline Group (NOGG); Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Oste-oporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Lekamwasam, S.; Joint IOF-ECTS GIO Guidelines Working Group; Adachi, J.D.; Agnusdei, D.; Bilezikian, J.; Boonen, S.; Borgström, F.; Cooper, C.; Perez, A.D.; Eastell, R.; et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos. Int. 2012, 23, 2257–2276. [Google Scholar] [CrossRef]
- Naunton, M.; Peterson, G.M.; Jones, G.; Griffin, G.M.; Bleasel, M.D. Multifaceted educational program increases prescribing of preventive medication for corticosteroid induced osteopo-rosis. J. Rheumatol. 2004, 31, 550–556. [Google Scholar] [PubMed]
- Carter, M. Prevention of Glucocorticoid-Induced Osteoporosis: Clinical audit to evaluate the implementation of National Osteoporosis Guideline Group 2017 guidelines in a primary care setting. J. Clin. Densitom. 2019, 22, 25–30. [Google Scholar] [CrossRef]
- van de Laarschot, D.M.; Smits, A.A.; Buitendijk, S.K.; Stegenga, M.T.; Zillikens, M.C. Screening for Atypical Femur Fractures Using Extended Femur Scans by DXA. J. Bone Miner. Res. 2017, 32, 1632–1639. [Google Scholar] [CrossRef]
| Category | T-Score |
|---|---|
| Normal | −1 or higher |
| Osteopenia (low bone mass) | Between −1 and −2.5 |
| Osteoporosis | −2.5 or lower |
| Class/Medication | Dose | Route of Administration | Major Action | Type of Fracture Reduction | FDA Indication |
|---|---|---|---|---|---|
| Bisphosphonate | |||||
| Alendronate | 10 mg daily/70 mg weekly | Oral | Anti-resorptive | Vertebral, nonvertebral, hip | Treatment and prevention |
| Risedronate | 5 mg daily/35 mg weekly/150 mg monthly | Oral | Anti-resorptive | Vertebral, nonvertebral, hip | Treatment and prevention |
| Ibandronate | 2.5 mg daily/150 mg monthly/3 mg every three months | Oral/intravenous | Anti-resorptive | Vertebral | Treatment and prevention |
| Zoledronic acid | 5 mg yearly | Intravenous | Anti-resorptive | Vertebral, nonvertebral, hip | Treatment and prevention |
| RANK Ligand inhibitor | |||||
| Denosumab | 60 mg every six months | Subcutaneous | Anti-resorptive | Vertebral, nonvertebral, hip | Treatment |
| Selective estrogen receptor modulators | |||||
| Raloxifene | 60 mg daily | Oral | Anti-resorptive | Vertebral | Treatment and prevention |
| Parathyroid hormone analogs | |||||
| Teriparatide | 20 ug daily | Subcutaneous | Osteoanabolic | Vertebral, non-vertebral | Treatment |
| Abaloparatide | 80 ug daily | Subcutaneous | Osteoanabolic | Vertebral, non-vertebral | Treatment |
| Sclerostin inhibitor | |||||
| Romosozumab | 210 mg monthly | Subcutaneous | Osteoanabolic/ anti-resorptive | Vertebral | Treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayub, N.; Faraj, M.; Ghatan, S.; Reijers, J.A.A.; Napoli, N.; Oei, L. The Treatment Gap in Osteoporosis. J. Clin. Med. 2021, 10, 3002. https://doi.org/10.3390/jcm10133002
Ayub N, Faraj M, Ghatan S, Reijers JAA, Napoli N, Oei L. The Treatment Gap in Osteoporosis. Journal of Clinical Medicine. 2021; 10(13):3002. https://doi.org/10.3390/jcm10133002
Chicago/Turabian StyleAyub, Nazia, Malak Faraj, Sam Ghatan, Joannes A. A. Reijers, Nicola Napoli, and Ling Oei. 2021. "The Treatment Gap in Osteoporosis" Journal of Clinical Medicine 10, no. 13: 3002. https://doi.org/10.3390/jcm10133002
APA StyleAyub, N., Faraj, M., Ghatan, S., Reijers, J. A. A., Napoli, N., & Oei, L. (2021). The Treatment Gap in Osteoporosis. Journal of Clinical Medicine, 10(13), 3002. https://doi.org/10.3390/jcm10133002






