Role of Iron Chelation and Protease Inhibition of Natural Products on COVID-19 Infection
Abstract
1. Introduction
2. Iron and SARS-CoV-2
3. Protease Inhibition and SARS-CoV-2
4. Materials and Methods
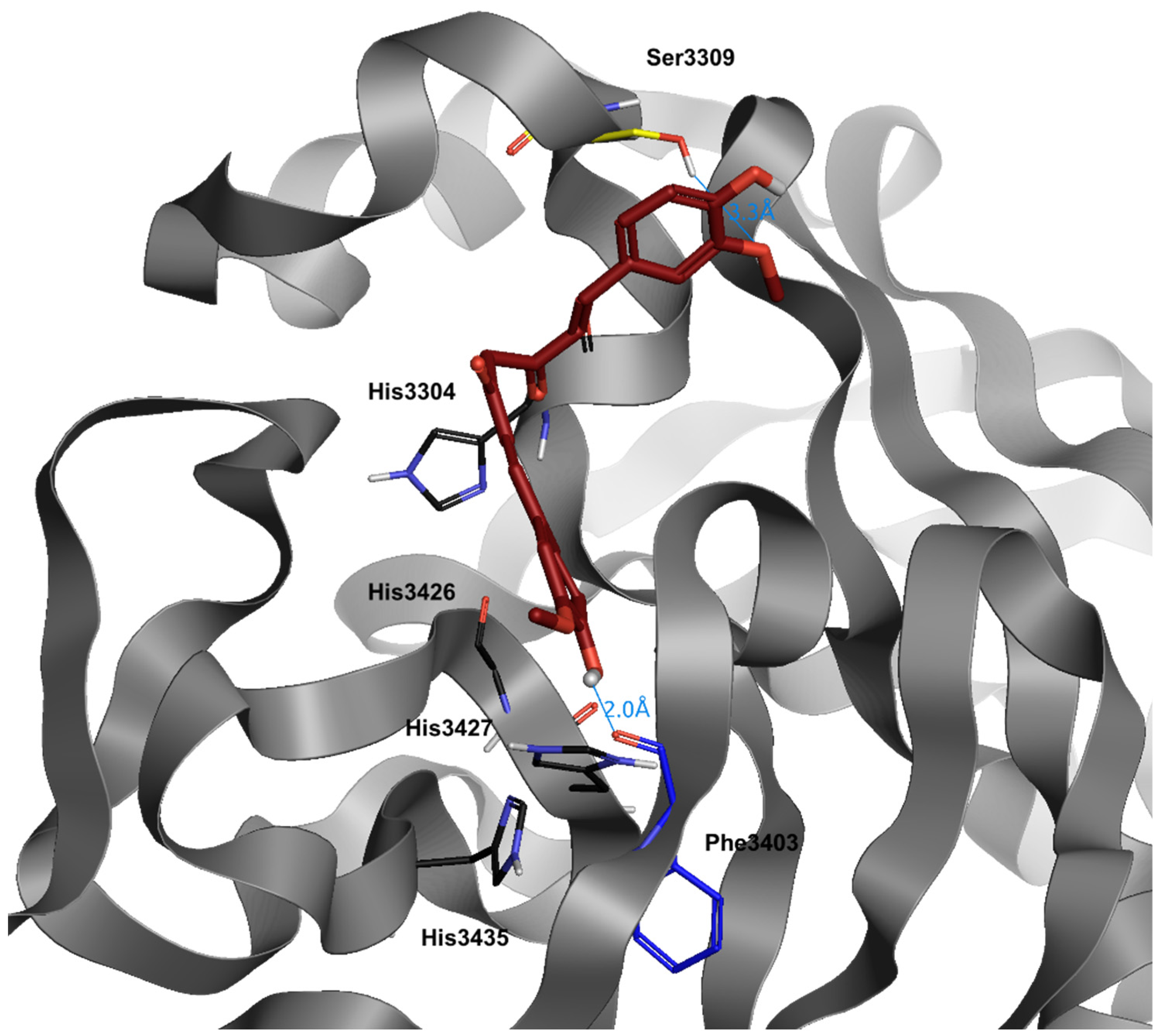
5. α-Lipoic Acid
6. Quercetin
7. Caffeic Acid
8. Phytic Acid
9. Curcumin
| Natural Product | Effect | Reference |
|---|---|---|
| Lactoferrin | Reduction of oxidative stress and inflammation through iron chelation properties | [17] |
| Glycyrrhizin | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Tryptanthrine | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Rhein | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Berberin | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Quercetin | Inhibition of SARS-CoV-2 protease 3CLpro | [66] |
| Caffeic acid | Inhibition of SARS-CoV-2 protease Mpro | [76] |
| Curcumin | Inhibition of SARS-CoV-2 protease 3CLpro | [97] |
| Type of Study | Iron Metabolism | Reference |
|---|---|---|
| Clinical | Anemic patients had increased levels of inflammation markers; Hyperferritinemia; Correlation between increased ferritin levels and cytokine mRNA over-expression | [33] |
| Meta-analysis | Elevated levels of serum ferritin have been found in non-survivors compared with survivors | [34] |
| Meta-analysis | The ferritin level was significantly increased in severe patients compared with non-severe patients | [35] |
| Clinical | High ferritin levels | [36] |
| Clinical | High ferritin levels | [37] |
| Meta-analysis | SARS-CoV-2 attacks one of the beta chains of the hemoglobin, causing dissociation of iron from the porphyrins and its release into the circulation | [38] |
| Clinical | Iron overload and Hepcidin overexpression | [40] |
| Computational | Similarity between hepcidin and the coronavirus spike glycoprotein | [42] |
| Name | Structure | dG |
|---|---|---|
| α-Lipoic Acid | 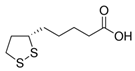 | −8.0 |
| Quercetin | 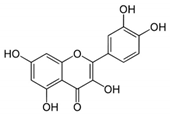 | −8.0 |
| Caffeic Acid | 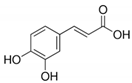 | −6.3 |
| Phytic Acid | 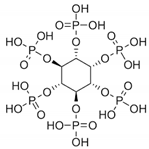 | −1.5 |
| Curcumin | 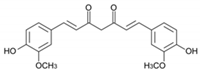 | −8.1 |
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, S.R.; Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Working Group of Novel Coronavirus, P.U.M.C.H. Diagnosis and clinical management of 2019 novel coronavirus infection: An operational recommendation of Peking Union Medical College Hospital (V2.0). Zhonghua Nei Ke Za Zhi 2020, 59, 186–188. [Google Scholar] [CrossRef]
- Ali, M.J.; Hanif, M.; Haider, M.A.; Ahmed, M.U.; Sundas, F.; Hirani, A.; Khan, I.A.; Anis, K.; Karim, A.H. Treatment Options for COVID-19: A Review. Front. Med. 2020, 7, 480. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef]
- Ali, M.K.; Kim, R.Y.; Brown, A.C.; Donovan, C.; Vanka, K.S.; Mayall, J.R.; Liu, G.; Pillar, A.L.; Jones-Freeman, B.; Xenaki, D.; et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020, 251, 49–62. [Google Scholar] [CrossRef]
- Khiroya, H.; Turner, A.M. The role of iron in pulmonary pathology. Multidiscip. Respir. Med. 2015, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carter, J.D.; Richards, J.H.; Richer, L.D.; Grissom, C.K.; Elstad, M.R. Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit. Care Med. 2003, 31, 395–400. [Google Scholar] [CrossRef]
- Cappellini, M.D. Exjade(R) (deferasirox, ICL670) in the treatment of chronic iron overload associated with blood transfusion. Clin. Risk. Manag. 2007, 3, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Abobaker, A. Can iron chelation as an adjunct treatment of COVID-19 improve the clinical outcome? Eur. J. Clin. Pharm. 2020, 76, 1619–1620. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Ng, T.B.; Sun, W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Mann, J.K.; Ndung’u, T. The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19. Future Virol. 2020, 15, 609–624. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef]
- Parisi, G.F.; Carota, G.; Castruccio Castracani, C.; Spampinato, M.; Manti, S.; Papale, M.; Di Rosa, M.; Barbagallo, I.; Leonardi, S. Nutraceuticals in the Prevention of Viral Infections, including COVID-19, among the Pediatric Population: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 2465. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharm. 2021, 136, 111228. [Google Scholar] [CrossRef] [PubMed]
- Jedinak, A.; Maliar, T.; Grancai, D.; Nagy, M. Inhibition activities of natural products on serine proteases. Phytother. Res. 2006, 20, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Niwa, Y.; Beppu, Y.; Towatari, T. Cellular proteases involved in the pathogenicity of enveloped animal viruses, human immunodeficiency virus, influenza virus A and Sendai virus. Adv. Enzym. Regul. 1996, 36, 325–347. [Google Scholar] [CrossRef]
- Wruck, W.; Adjaye, J. SARS-CoV-2 receptor ACE2 is co-expressed with genes related to transmembrane serine proteases, viral entry, immunity and cellular stress. Sci. Rep. 2020, 10, 21415. [Google Scholar] [CrossRef]
- Chandwani, A.; Shuter, J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Clin. Risk Manag. 2008, 4, 1023–1033. [Google Scholar] [CrossRef]
- Patick, A.K.; Potts, K.E. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 1998, 11, 614–627. [Google Scholar] [CrossRef]
- Anderson, J.; Schiffer, C.; Lee, S.K.; Swanstrom, R. Viral protease inhibitors. Handb. Exp. Pharm. 2009, 189, 85–110. [Google Scholar] [CrossRef]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef]
- Sagawa, T.; Inoue, K.I.; Takano, H. Use of protease inhibitors for the prevention of COVID-19. Prev. Med. 2020, 141, 106280. [Google Scholar] [CrossRef]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat. Prod. Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Khodour, Y.; Kaguni, L.S.; Stiban, J. Iron-sulfur clusters in nucleic acid metabolism: Varying roles of ancient cofactors. Enzymes 2019, 45, 225–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic against Viral Survival. Curr. Clin. Microbiol. Rep. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Pretorius, E.; Kell, D.B. Diagnostic morphology: Biophysical indicators for iron-driven inflammatory diseases. Integr. Biol. 2014, 6, 486–510. [Google Scholar] [CrossRef]
- Perricone, C.; Bartoloni, E.; Bursi, R.; Cafaro, G.; Guidelli, G.M.; Shoenfeld, Y.; Gerli, R. COVID-19 as part of the hyperferritinemic syndromes: The role of iron depletion therapy. Immunol. Res. 2020, 68, 213–224. [Google Scholar] [CrossRef]
- Garrick, M.D.; Ghio, A.J. Iron chelation may harm patients with COVID-19. Eur. J. Clin. Pharm. 2021, 77, 265–266. [Google Scholar] [CrossRef]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstatter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020, 21, 276. [Google Scholar] [CrossRef]
- Taneri, P.E.; Gomez-Ochoa, S.A.; Llanaj, E.; Raguindin, P.F.; Rojas, L.Z.; Roa-Diaz, Z.M.; Salvador, D., Jr.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Liu, W.L.H. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Heme Metabolism. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Abobaker, A. Reply: Iron chelation may harm patients with COVID-19. Eur. J. Clin. Pharm. 2021, 77, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Banchini, F.; Vallisa, D.; Maniscalco, P.; Capelli, P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020, 91, e2020013. [Google Scholar] [CrossRef]
- Daher, R.; Manceau, H.; Karim, Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Med. 2017, 46, e272–e278. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, S. COVID-19 and iron dysregulation: Distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol. Direct 2020, 15, 19. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Ragia, G.; Manolopoulos, V.G. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: A promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharm. 2020, 76, 1623–1630. [Google Scholar] [CrossRef]
- Bhatnagar, T.; Murhekar, M.V.; Soneja, M.; Gupta, N.; Giri, S.; Wig, N.; Gangakhedkar, R. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: Protocol for restricted public health emergency use. Indian J. Med. Res. 2020, 151, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Peng, W.; Tang, D.; Dai, Y. Protease Inhibitor Use in COVID-19. Sn Compr. Clin. Med. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Doi, K.; Ikeda, M.; Hayase, N.; Moriya, K.; Morimura, N.; Group, C.-U.S. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: A case series. Crit. Care 2020, 24, 392. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kiso, M.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Takeda, M.; Kinoshita, N.; Ohmagari, N.; Gohda, J.; Semba, K.; et al. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses 2020, 12, 629. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Muller, M.A.; Drosten, C.; Pohlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 2020, 95, 1989–1999. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Cheeseright, T.; Mackey, M.; Rose, S.; Vinter, A. Molecular field extrema as descriptors of biological activity: Definition and validation. J. Chem. Inf. Model. 2006, 46, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharm. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Camiolo, G.; Tibullo, D.; Giallongo, C.; Romano, A.; Parrinello, N.L.; Musumeci, G.; Di Rosa, M.; Vicario, N.; Brundo, M.V.; Amenta, F.; et al. alpha-Lipoic Acid Reduces Iron-induced Toxicity and Oxidative Stress in a Model of Iron Overload. Int. J. Mol. Sci. 2019, 20, 609. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of alpha-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, C.; Song, D.; Li, Y.; Song, Y.; Su, G.; Dunaief, J.L. Systemic administration of the antioxidant/iron chelator alpha-lipoic acid protects against light-induced photoreceptor degeneration in the mouse retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5979–5988. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Zheng, Q.; Zhai, S.; Cai, T.; Xu, L.; Yang, L.; Jiao, L.; Zhang, C. Alpha-Lipoic Acid Mediates Clearance of Iron Accumulation by Regulating Iron Metabolism in a Parkinson’s Disease Model Induced by 6-OHDA. Front. Neurosci. 2020, 14, 612. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharm. 2003, 65, 1199–1206. [Google Scholar] [CrossRef]
- Lesjak, M.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur. J. Nutr. 2019, 58, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.; Ameen, S.H.; AbdEl-Fatah, S.S. Ameliorating Iron Overload in Intestinal Tissue of Adult Male Rats: Quercetin vs Deferoxamine. J. Toxicol. 2018, 2018, 8023840. [Google Scholar] [CrossRef] [PubMed]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral. Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Yao, C.; Xi, C.; Hu, K.; Gao, W.; Cai, X.; Qin, J.; Lv, S.; Du, C.; Wei, Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, H.; Kim, S.; Shin, D.H.; Kim, M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019, 94, 2023–2030. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Mehrbod, P.; Hudy, D.; Shyntum, D.; Markowski, J.; Los, M.J.; Ghavami, S. Quercetin as a Natural Therapeutic Candidate for the Treatment of Influenza Virus. Biomolecules 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Hynes, M.J.; O’Coinceanainn, M. The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J. Inorg. Biochem. 2004, 98, 1457–1464. [Google Scholar] [CrossRef]
- Yamanaka, N.; Oda, O.; Nagao, S. Prooxidant activity of caffeic acid, dietary non-flavonoid phenolic acid, on Cu2+-induced low density lipoprotein oxidation. FEBS Lett. 1997, 405, 186–190. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Mauricio, A.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Tian, W.Y.; Sakharkar, M.K.; Liu, Q.; Yang, X.; Zhou, Y.Z.; Mou, C.L.; Cai, G.L.; Yang, J. Characterization of Nine Compounds Isolated from the Acid Hydrolysate of Lonicera fulvotomentosa Hsu et S.C. Cheng and Evaluation of Their In Vitro Activity towards HIV Protease. Molecules 2019, 24, 4526. [Google Scholar] [CrossRef]
- Shen, H.; Yamashita, A.; Nakakoshi, M.; Yokoe, H.; Sudo, M.; Kasai, H.; Tanaka, T.; Fujimoto, Y.; Ikeda, M.; Kato, N.; et al. Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus. PLoS ONE 2013, 8, e82299. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020, 39, 1–13. [Google Scholar] [CrossRef]
- Adem, S.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2020, 153310. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.V.; Tetens, I.; Meyer, A.S. Potential of phytase-mediated iron release from cereal-based foods: A quantitative view. Nutrients 2013, 5, 3074–3098. [Google Scholar] [CrossRef]
- Graf, E.; Empson, K.L.; Eaton, J.W. Phytic acid. A natural antioxidant. J. Biol. Chem. 1987, 262, 11647–11650. [Google Scholar] [CrossRef]
- Xu, Q.; Kanthasamy, A.G.; Reddy, M.B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology 2008, 245, 101–108. [Google Scholar] [CrossRef]
- Kamp, D.W.; Israbian, V.A.; Yeldandi, A.V.; Panos, R.J.; Graceffa, P.; Weitzman, S.A. Phytic acid, an iron chelator, attenuates pulmonary inflammation and fibrosis in rats after intratracheal instillation of asbestos. Toxicol. Pathol. 1995, 23, 689–695. [Google Scholar] [CrossRef]
- Zajdel, A.; Wilczok, A.; Weglarz, L.; Dzierzewicz, Z. Phytic acid inhibits lipid peroxidation in vitro. BioMed Res. Int. 2013, 2013, 147307. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kuwata, G.; Imai, M.; Nagao, A.; Terao, J. Protective effect of phytic acid hydrolysis products on iron-induced lipid peroxidation of liposomal membranes. Lipids 2000, 35, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Messner, D.J.; Surrago, C.; Fiordalisi, C.; Chung, W.Y.; Kowdley, K.V. Isolation and characterization of iron chelators from turmeric (Curcuma longa): Selective metal binding by curcuminoids. Biometals 2017, 30, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin Attenuates Iron Accumulation and Oxidative Stress in the Liver and Spleen of Chronic Iron-Overloaded Rats. PLoS ONE 2015, 10, e0134156. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wilkinson, J.t.; Christine Pietsch, E.; Buss, J.L.; Wang, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef]
- Wallander, M.L.; Leibold, E.A.; Eisenstein, R.S. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim. Biophys. Acta 2006, 1763, 668–689. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J.T.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef]
- Dijiong, W.; Xiaowen, W.; Linlong, X.; Wenbin, L.; Huijin, H.; Baodong, Y.; Yuhong, Z. Iron chelation effect of curcumin and baicalein on aplastic anemia mouse model with iron overload. Iran. J. Basic Med. Sci. 2019, 22, 660–668. [Google Scholar] [CrossRef]
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; Ortiz de Montellano, P.R. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415–422. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015, 6, 3412–3419. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Morris, G.M.; Olson, A.J. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg. Med. Chem. Lett. 2005, 15, 3364–3368. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Dang, M.; Roy, A.; Kang, J.; Song, J. Curcumin Allosterically Inhibits the Dengue NS2B-NS3 Protease by Disrupting Its Active Conformation. ACS Omega 2020, 5, 25677–25686. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ying, Y. The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia. Front. Cell Dev. Biol. 2020, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) from Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020. [Google Scholar] [CrossRef]
- Das, S.; Sarmah, S.; Lyndem, S.; Singha Roy, A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Futur J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, Y.; Haridas, V.; Vasanthakumar, K.C.; Muthu, S.; Thavoorullah, F.F.; Shetty, P. Curcumin: A Wonder Drug as a Preventive Measure for COVID19 Management. Indian J. Clin. Biochem. 2020, 35, 373–375. [Google Scholar] [CrossRef] [PubMed]
- McKee, D.L.; Sternberg, A.; Stange, U.; Laufer, S.; Naujokat, C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharm. Res. 2020, 157, 104859. [Google Scholar] [CrossRef] [PubMed]
- Keretsu, S.; Bhujbal, S.P.; Cho, S.J. Rational approach toward COVID-19 main protease inhibitors via molecular docking, molecular dynamics simulation and free energy calculation. Sci. Rep. 2020, 10, 17716. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carota, G.; Ronsisvalle, S.; Panarello, F.; Tibullo, D.; Nicolosi, A.; Li Volti, G. Role of Iron Chelation and Protease Inhibition of Natural Products on COVID-19 Infection. J. Clin. Med. 2021, 10, 2306. https://doi.org/10.3390/jcm10112306
Carota G, Ronsisvalle S, Panarello F, Tibullo D, Nicolosi A, Li Volti G. Role of Iron Chelation and Protease Inhibition of Natural Products on COVID-19 Infection. Journal of Clinical Medicine. 2021; 10(11):2306. https://doi.org/10.3390/jcm10112306
Chicago/Turabian StyleCarota, Giuseppe, Simone Ronsisvalle, Federica Panarello, Daniele Tibullo, Anna Nicolosi, and Giovanni Li Volti. 2021. "Role of Iron Chelation and Protease Inhibition of Natural Products on COVID-19 Infection" Journal of Clinical Medicine 10, no. 11: 2306. https://doi.org/10.3390/jcm10112306
APA StyleCarota, G., Ronsisvalle, S., Panarello, F., Tibullo, D., Nicolosi, A., & Li Volti, G. (2021). Role of Iron Chelation and Protease Inhibition of Natural Products on COVID-19 Infection. Journal of Clinical Medicine, 10(11), 2306. https://doi.org/10.3390/jcm10112306








