Optical Coherence Tomography Angiography Metrics Monitor Severity Progression of Diabetic Retinopathy—3-Year Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Seven-Field Color Fundus Photography
2.2. Optical Coherence Tomography
2.3. OCT-Angiography
2.4. Statistical Analysis
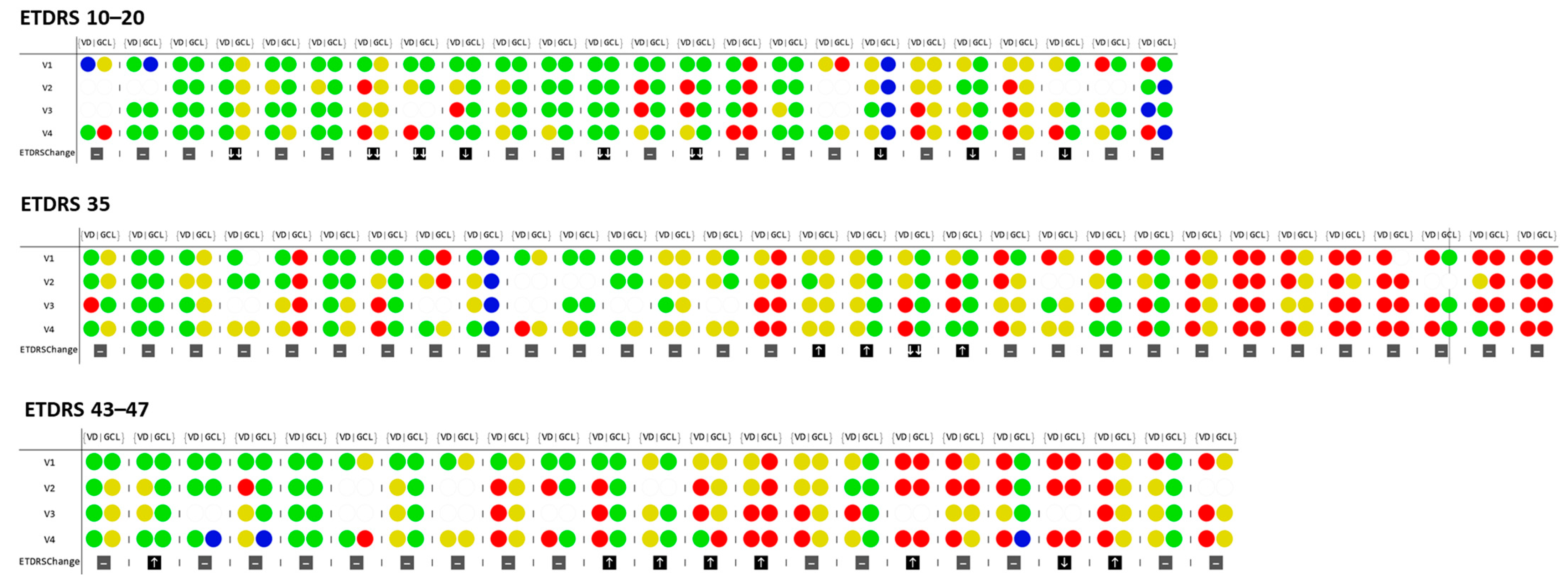
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Marques, I.P.; Alves, D.; Santos, T.; Mendes, L.; Santos, A.R.; Lobo, C.; Durbin, M.; Cunha-Vaz, J. Multimodal Imaging of the Initial Stages of Diabetic Retinopathy: Different Disease Pathways in Different Patients. Diabetes 2019, 68, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Ribeiro, L.; Lobo, C.; Cunhavaz, J.G. Three Different Phenotypes of Mild Nonproliferative Diabetic Retinopathy With Different Risks for Development of Clinically Significant Macular Edema. Investig. Opthalmology Vis. Sci. 2013, 54, 4595–4604. [Google Scholar] [CrossRef] [PubMed]
- Cunhavaz, J.G.; Bernardes, R.; Santos, T.; Oliveira, C.; Lobo, C.; Pires, I.; Ribeiro, L. Computer-Aided Detection of Diabetic Retinopathy Progression. Digit. Teleretinal Screen. 2012, 226, 59–66. [Google Scholar] [CrossRef]
- Marques, I.P.; Madeira, M.H.; Messias, A.L.; Santos, T.; Martinho, A.C.-V.; Figueira, J.; Cunha-Vaz, J. Retinopathy Phenotypes in Type 2 Diabetes with Different Risks for Macular Edema and Proliferative Retinopathy. J. Clin. Med. 2020, 9, 1433. [Google Scholar] [CrossRef]
- Marques, I.P.; Madeira, M.H.; Messias, A.L.; Martinho, A.C.-V.; Santos, T.; Sousa, D.C.; Figueira, J.; Cunha-Vaz, J. Different retinopathy phenotypes in type 2 diabetes predict retinopathy progression. Acta Diabetol. 2021, 58, 197–205. [Google Scholar] [CrossRef]
- Marques, I.P.; Alves, D.; Santos, T.; Mendes, L.; Lobo, C.; Santos, A.R.; Durbin, M.; Cunha-Vaz, J. Characterization of Disease Progression in the Initial Stages of Retinopathy in Type 2 Diabetes: A 2-Year Longitudinal Study. Investig. Opthalmology Vis. Sci. 2020, 61, 20. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- ETDRSR Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification, ETDRS Report Number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Soares, M.; Neves, C.; Marques, I.P.; Pires, I.; Schwartz, C.; Costa, M.Â.; Santos, T.; Durbin, M.; Cunha-Vaz, J. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br. J. Ophthalmol. 2016, 101, 62–68. [Google Scholar] [CrossRef]
- Figueira, J.; Fletcher, E.; Massin, P.; Silva, R.; Bandello, F.; Midena, E.; Varano, M.; Sivaprasad, S.; Eleftheriadis, H.; Menon, G.; et al. Ranibizumab Plus Panretinal Photocoagulation versus Panretinal Photocoagulation Alone for High-Risk Proliferative Diabetic Retinopathy (PROTEUS Study). Ophthalmology 2018, 125, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Moss, S.E. How Many Steps of Progression of Diabetic Retinopathy Are Meaningful? Arch. Ophthalmol. 2001, 119, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Warren, L.H.; Santos, A.R.; Marques, I.P.; Kubach, S.; Mendes, L.G.; De Sisternes, L.; Madeira, M.H.; Durbin, M.; Cunha-Vaz, J.G. Swept-source OCTA quantification of capillary closure predicts ETDRS severity staging of NPDR. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Lei, J.; Durbin, M.K.; Shi, Y.; Uji, A.; Balasubramanian, S.; Baghdasaryan, E.; Al-Sheikh, M.; Sadda, S.R. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmol. 2017, 135, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, J.; Bernardes, R.; Pires, I.; Figueira, J.; Lobo, C.; Cunha-Vaz, J. Alterations of retinal capillary blood flow in preclinical retinopathy in subjects with type 2 diabetes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 181–186. [Google Scholar] [CrossRef]
- Keith, C.G.; Cunha-Vaz, J.G.; Shakib, M. Studies on the effects of osmotically active substances on the circulation and structure of the retina. Part I. Observations in vivo. Investig. Ophthalmol. Vis. Sci. 1967, 6, 192–197. [Google Scholar]
- Hudetz, A.G.; Fehér, G.; Weigle, C.G.; E Knuese, D.; Kampine, J.P. Video microscopy of cerebrocortical capillary flow: Response to hypotension and intracranial hypertension. Am. J. Physiol. Hear. Circ. Physiol. 1995, 268, 2202–2210. [Google Scholar] [CrossRef]
- Cogan, D.G.; Kuwabara, T. Capillary Shunts in the Pathogenesis of Diabetic Retinopathy. Diabetes 1963, 12, 293–300. [Google Scholar] [CrossRef]
- Choi, W.; Waheed, N.K.; Moult, E.M.; Adhi, M.; Lee, B.; De Carlo, T.; Jayaraman, V.; Baumal, C.R.; Duker, J.S.; Fujimoto, J.G. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina 2017, 37, 11–21. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, F.; Wong, R.; Lok, J.; Szeto, S.K.H.; Chan, J.C.K.; Chan, C.K.M.; Than, C.C.; Ng, D.S.; Cheung, C.Y. OCT Angiography Metrics Predict Progression of Diabetic Retinopathy and Development of Diabetic Macular Edema: A Prospective Study. Ophthalmology 2019, 126, 1675–1684. [Google Scholar] [CrossRef]
- You, Q.S.; Wang, J.; Guo, Y.; Pi, S.; Flaxel, C.J.; Bailey, S.T.; Huang, D.; Jia, Y.; Hwang, T.S. Optical Coherence Tomography Angiography Avascular Area Association With 1-Year Treatment Requirement and Disease Progression in Diabetic Retinopathy. Am. J. Ophthalmol. 2020, 217, 268–277. [Google Scholar] [CrossRef]
- Greig, E.C.; Brigell, M.; Cao, F.; Levine, E.S.; Peters, K.; Moult, E.M.; Fujimoto, J.G.; Waheed, N.K. Macular and Peripapillary Optical Coherence Tomography Angiography Metrics Predict Progression in Diabetic Retinopathy: A Sub-analysis of TIME-2b Study Data. Am. J. Ophthalmol. 2020, 219, 66–76. [Google Scholar] [CrossRef]
| Healthy Controls | ETDRS 10–20 | ETDRS 35 | ETDRS 43–47 | p-Value (Between the Three ETDRS Groups) ** | |
|---|---|---|---|---|---|
| (n = 84) | (n = 24) | (n = 31) | (n = 23) | ||
| Sex, Male/female | 39/45 | 16/8 | 25/6 | 17/6 | 0.499 |
| 0.08 | 0.001 | 0.019 | |||
| Age, years | 69.2 ± 4.5 | 69.5 ± 5.9 | 65.4 ± 5.5 | 66.5 ± 7.2 | 0.064 |
| 0.706 | 0.002 | 0.213 | |||
| Diabetes duration, years | --- | 18.2 ± 7.1 | 16.5 ± 6.8 | 17.6 ± 5.8 | 0.577 |
| BCVA, letters | --- | 85.5 ± 4.1 | 85.3 ± 4.4 | 86.8 ± 3.4 | 0.528 |
| HbA1c, % | --- | 6.9 ± 1.1 | 7.3 ± 1.1 | 8.0 ± 1.2 | 0.005 |
| VD, SCP, inner ring, mm−1 (p-value *) | 22.3 ± 0.89 | 21.7 ± 1.1 | 20.9 ± 1.1 | 21.2 ± 1.3 | 0.033 |
| 0.058 | p < 0.001 | p < 0.001 | |||
| VD, DCP, inner ring, mm−1 (p-value *) | 17.0 ± 2.14 | 17.2 ± 1.97 | 16.2 ± 2.2 | 16.4 ± 2.2 | 0.324 |
| 0.918 | 0.078 | 0.310 | |||
| VD, FR, inner ring, mm−1 (p-value *) | 23.7 ± 0.90 | 23.5 ± 1.1 | 22.6 ± 1.1 | 22.9 ± 1.1 | 0.009 |
| 0.958 | p < 0.001 | 0.010 | |||
| PD, SCP, inner ring, mm−1 (p-value *) | 0.398 ± 0.02 | 0.403 ± 0.02 | 0.390 ± 0.02 | 0.400 ± 0.02 | 0.038 |
| 0.416 | 0.006 | 0.073 | |||
| PD, DCP, inner ring, mm−1 (p-value *) | 0.315 ± 0.04 | 0.332 ± 0.03 | 0.312 ± 0.04 | 0.318 ± 0.04 | 0.221 |
| 0.535 | 0.119 | 0.491 | |||
| PD, FR, inner ring, mm−1 (p-value *) | 0.416 ± 0.02 | 0.429 ± 0.02 | 0.414 ± 0.02 | 0.426 ± 0.02 | 0.011 |
| 0.051 | 0.059 | 0.176 | |||
| FAZ circularity index (p-value *) | 0.687 ± 0.07 | 0.638 ± 0.2 | 0.607 ± 0.1 | 0.560 ± 0.1 | 0.004 |
| 0.636 | 0.205 | p < 0.001 | |||
| GCL + IPL, Inner Ring, µm (58 healthy controls) (p-value *) | 82.7 ± 5.5 | 82.8 ± 9.1 | 77.6 ± 8.1 | 78 ± 6.8 | 0.087 |
| 0.710 | 0.003 | 0.007 |
| ≥2D Changes | ETDRS 10–20 (n = 24) | ETDRS 35 * (n = 31) | ETDRS 43–47 (n = 23) |
|---|---|---|---|
| Visit 1 | |||
| Vessel closure (VC) | 8.3% | 38.7% | 30.4% |
| Neurodegeneration (ND) | 4.2% | 22.6% | 13.0% |
| VC and ND in the same eye | 0.0% | 9.6% | 8.7% |
| Visit 4 | |||
| Vessel closure (VC) | 33.3% | 41.9% | 47.8% |
| Neurodegeneration (ND) | 8.3% | 24.1% | 21.7% |
| VC and ND in the same eye | 16.0% | 16.1% | 8.7% |
| VD (SCP) and GCL + IPL thickness correlation change (V4 − V1) | 0.07 (p = 0.739) | 0.03 (p = 0.872) | −0.25 (p = 0.246) |
| ETDRS Change | Vessel Density Inner Ring mm−1 | Layer Thickness Inner Ring, µm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SCP | DCP | FR | GCL + IPL | ||||||
| V1 | V4 − V1 | V1 | V4 − V1 | V1 | V4 − V1 | V1 | V4 − V1 | ||
| Worsening (1 and 2 steps) (n = 11) | AVG | 21.5 | −1.3 | 16.7 | −2.1 | 23.1 | −1.3 | 81.9 | −0.5 |
| SD | 0.9 | 1.0 | 1.7 | 1.5 | 0.9 | 1.1 | 8.7 | 1.2 | |
| Min | 20.0 | −2.9 | 14.6 | −4.8 | 21.3 | −3.2 | 65.0 | −2.0 | |
| Max | 22.5 | −0.3 | 19.7 | −0.1 | 24.3 | −0.1 | 98.0 | 1.0 | |
| No change + improving (n = 67) | AVG | 21.2 | −0.4 | 16.6 | −1.0 | 23.0 | −0.4 | 78.9 | −1.1 |
| SD | 1.2 | 1.0 | 2.2 | 1.7 | 1.2 | 1.0 | 8.3 | 5.6 | |
| Min | 18.5 | −1.8 | 11.4 | −4.4 | 20.3 | −1.9 | 58.0 | −26.0 | |
| Max | 24.9 | 3.1 | 22.1 | 2.9 | 26.5 | 3.1 | 110.0 | 18.0 | |
| Statistical Diff V4− V1 (p-value) | 0.014 * | 0.048 | 0.047 | 0.810 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, I.P.; Kubach, S.; Santos, T.; Mendes, L.; Madeira, M.H.; de Sisternes, L.; Tavares, D.; Santos, A.R.; Lewis, W.; Lobo, C.; et al. Optical Coherence Tomography Angiography Metrics Monitor Severity Progression of Diabetic Retinopathy—3-Year Longitudinal Study. J. Clin. Med. 2021, 10, 2296. https://doi.org/10.3390/jcm10112296
Marques IP, Kubach S, Santos T, Mendes L, Madeira MH, de Sisternes L, Tavares D, Santos AR, Lewis W, Lobo C, et al. Optical Coherence Tomography Angiography Metrics Monitor Severity Progression of Diabetic Retinopathy—3-Year Longitudinal Study. Journal of Clinical Medicine. 2021; 10(11):2296. https://doi.org/10.3390/jcm10112296
Chicago/Turabian StyleMarques, Inês P., Sophie Kubach, Torcato Santos, Luís Mendes, Maria H. Madeira, Luis de Sisternes, Diana Tavares, Ana Rita Santos, Warren Lewis, Conceição Lobo, and et al. 2021. "Optical Coherence Tomography Angiography Metrics Monitor Severity Progression of Diabetic Retinopathy—3-Year Longitudinal Study" Journal of Clinical Medicine 10, no. 11: 2296. https://doi.org/10.3390/jcm10112296
APA StyleMarques, I. P., Kubach, S., Santos, T., Mendes, L., Madeira, M. H., de Sisternes, L., Tavares, D., Santos, A. R., Lewis, W., Lobo, C., Durbin, M. K., & Cunha-Vaz, J. (2021). Optical Coherence Tomography Angiography Metrics Monitor Severity Progression of Diabetic Retinopathy—3-Year Longitudinal Study. Journal of Clinical Medicine, 10(11), 2296. https://doi.org/10.3390/jcm10112296






