Abstract
Atrial fibrillation (AF) is the most prevalent form of cardiac arrhythmia. It is often related to diverse pathological conditions affecting the atria and leading to remodeling processes including collagen accumulation, fatty infiltration, and amyloid deposition. All these events generate atrial fibrosis, which contribute to beget AF. In this scenario, cardiac imaging appears as a promising noninvasive tool for monitoring the presence and degree of LA fibrosis and remodeling. The aim of this review is to comprehensively examine the bench mechanisms of atrial fibrosis moving, then to describe the principal imaging techniques that characterize it, such as cardiac magnetic resonance (CMR) and multidetector cardiac computed tomography (MDCT), in order to tailor atrial fibrillation ablation to each individual.
1. Introduction
Atrial fibrillation (AF) is the most prevalent form of cardiac arrhythmia [1]. Its prevalence has increased over time and continues to rise in a pandemic manner. In fact, estimated AF cases in Europe are expected to move from 8.8 million in 2010 to 17.9 million in 2060 [2]. During the last 50 years, age-adjusted prevalence of AF quadrupled from 20.4 to 96.2 cases per 1000 person-years in men and from 13.7 to 49.4 cases per 1000 person-years in women [3]. The longer survival has increased the risk of age-related diseases such as atrial fibrillation and its related mortality. Indeed, although this arrhythmia is frequently asymptomatic, it is far from a benign condition because is associated with increased risk of stroke [4], heart failure [5], cognitive dysfunction [6], and cardiovascular or all-cause death [7]. An understanding of the underlying pathophysiological mechanisms is needed to implement preventive strategies for AF development, and improve medical and interventional treatment of this epidemic disease. The aim of this review is to comprehensively examine the bench mechanisms underpinning atrial fibrosis, then to describe the principal imaging techniques that characterize it, to tailor atrial fibrillation ablation for everyone.
2. Atrial Fibrosis as Major Determinant of Atrial Fibrillation Genesis and Sustenance
Historically, AF has been classified into three patterns: paroxysmal, persistent, and permanent [8]. The first one is often not associated with cardiomyopathy and is triggered by pulmonary veins fires, or, in 10–20% of cases, by non-pulmonary ectopic foci, such as the left atrial appendage, coronary sinus, superior vena cava, and crista terminalis [9]. The other two forms are generally related to diverse pathological conditions affecting the atria, such as ischemic, valvular, genetic, and idiopathic cardiomyopathies [10,11]. All these conditions lead to remodeling processes, including collagen accumulation, fatty infiltration, and amyloid deposition. All these events generate atrial fibrosis, which contribute to AF [12].
Technically, fibrosis means the excessive deposition of the extracellular matrix (ECM), which is the fibrous tissue supporting muscular cells. When ECM exceeds, it alters myocytes milieu, losing the cardiac architectural integrity [13]. Different types of cardiac fibrosis have been characterized.
Histopathological classification divides fibrosis into two types:
- (1)
- Replacement fibrosis: the process occurring after myocardial injury (as myocardial acute infarct) when necrotic cells are substituted by collagen and ECM. This is also called reparative fibrosis [14];
- (2)
- Reactive fibrosis: an inflammatory process (mostly triggered by volume or pressure overload or genetic-mediated) of fibrous tissue deposition between cells (interstitial) and/or vessels (perivascular) causing disarray [15].
Depending on fibrotic tissue pattern, size, and distribution, we can describe fibrosis as [16]:
- (1)
- Interstitial: widening and thickening of the ECM;
- (2)
- Compact: consists of areas of dense collagen, deprived of any myocardial tissue;
- (3)
- Diffuse: involves mixed areas of myocardial and collagen fibers;
- (4)
- Patchy: consists of myocardial and collagen bundles and long strands.
Each type of pattern is not mutually exclusive. Different patterns and types of fibrosis may coexist in the same atrium. There are several clinical implications of these different types of fibrotic architecture on the related arrhythmogenesis. For example, compact fibrosis, although acutely severe, is less proarrhythmic than diffuse, patchy, and interstitial, and mainly promotes organized reentry (flutter) as circus movements around the fibrosis area due to unidirectional block [17]. On the contrary, patchy fibrosis is most prone to arrhythmogenesis because of development of zig-zag electrical conduction between the various bundles and long strands [18]. Diffuse fibrosis can, importantly, decrease the conduction velocity, leading to spiral wave formations, enhancing AF initiation and perpetuation [19]. Purely interstitial fibrosis, separating myocardial bundles, impairs transverse conduction without impacting longitudinal conduction and provoking anisotropic conduction [20]. In fact, the propagation perpendicular to the fiber direction becomes asynchronous, because activation must follow a tortuous route between the electrical barriers imposed by the collagen fibers. It has been shown that the presence of thick interstitial collagen strands is highly related to persistent AF [21].
3. Molecular Mechanisms
3.1. Cellular and Molecular Signaling of Fibrogenesis
Several cellular and molecular pathways are involved in the fibrogenesis (Figure 1). Atrial fibroblasts, different from their ventricular counterpart, show a higher response to mitogenic factors, such as the platelet-derived growth factor (PDGF), angiotensin II (Ang II) and tissue growth factor (TGF-β-1) [22]. Angiotensin (AT) is one of the most important molecular mechanisms, promoting inflammatory chemokine expression in atrial fibroblasts, and inducing fibroblast proliferation by increasing the expression of profibrotic TGF-β-1 [23]. Moreover, AT-1 induces reactive oxygen species (ROS) activation and proinflammatory responses. ROS are involved in the profibrotic differentiation of fibroblasts into myofibroblasts regulating collagen synthesis and matrix metalloproteinase (MMPs) activity: the main enzymes of ECM degradation [24]. Recently, some translational studies identified the pivotal role of non-coding microRNAs (miRNAs) in cardiac fibrosis. For example, microRNA-30c suppresses the pro-fibrogenic effects of cardiac fibroblasts induced by TGF-β1 and prevents atrial fibrosis by targeting TGFβRII. Thus, a decreased expression of miRNA-30c favors ECM deposition [25]. Furthermore, cardiac fibroblast proliferation is modulated by micro-RNA-10a through the TGF-β 1/SMADs pathway. Small Mothers Against Decapentaplegic (SMADs) comprise a family of structurally similar proteins that are the main signal transducers for receptors of the transforming growth factor beta (TGF-β) superfamily and of the mitogen-activated protein kinase (MAPK) cascade, which are critically important for regulating cell development and growth [26].
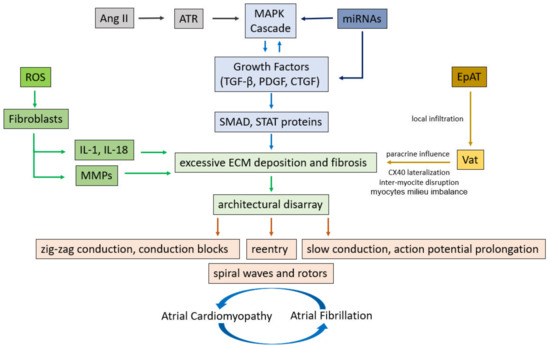
Figure 1.
Cellular and molecular pathways involved in the structural and electrical remodeling leading to atrial fibrillation. Ang II = Angiotensin II, ATR = Angiotensin receptor, MAPK = mitogen-activated protein kinase, TGF-β = Transforming growth factor-β, PDGF = platelet derived growth factor, CTGF = connective tissue growth factor, SMAD = Small mothers against decapentaplegic, STAT = signal transducer and activator of transcription, ROS = reactive oxygen species, IL-1 = interleukin-1, IL-18 = Interleukin-18, MMPs = matrix metalloproteinases, ECM = extra cellular matrix, miRNAs = microRiboNucleicAcids, EpAT = Epicardial Adipose Tissue, VaT = Visceral adipose Tissue.
The continuous evolution in the knowledge of all these interconnected cellular and molecular factors, promoting atrial fibrosis, is a promising field in the understanding of mechanisms, characterization of their pathophysiology, and development of therapeutic perspectives in atrial fibrillation.
3.2. Adipose Tissue as a Novel Risk Factor of Fibrosis and Atrial Fibrillation
It is well known that an increased body mass index (BMI) often coexists with several cardiovascular (CV) risk factors, such as arterial hypertension, insulin resistance and low-grade inflammation. Morbidity and mortality in these patients is sensibly amplified [27]. The prevalence of obesity is escalating alarmingly, and the number of affected people follows an exponential growth curve [28]. Data from the Framingham Heart Study clearly demonstrated the association between obesity and AF risk over a 14-year observation period, with a 4–5% increase in AF risk for every unit increase in BMI [29]. Several factors contribute to explaining the increased risk of AF in patients with obesity and metabolic syndrome. These include changes in volume status, cardiac loading, energy substrate utilization, tissue metabolism and systemic inflammation. Obese patients display a significant increase in the left atrial size and pressure, which is a potent predictor of AF occurrence over 10-year follow-up [27]. The accumulation of both visceral (VaT) and epicardial adipose tissue (EpAT) leads to a condition of chronic low-grade inflammation, characterized by increased levels of profibrotic and pro-hypertrophic cytokines, such as TNF-α, iL-6 and iL-1β [30]. Besides atrial fibrosis and hypertrophy, fat-related inflammatory stimuli can disturb calcium homeostasis and channel function, mainly via the activation of Nad(P)H oxidase activity, nitric oxide synthase uncoupling, eventually leading to the accumulation of reactive oxygen species (ROS) [31]. Obesity and insulin resistance also lead to the activation of the renin-angiotensin-aldosterone system (RAAS), transforming growth factor beta (TGF-β), connective tissue growth factor (cTGF) and endothelin-1, all leading to interstitial collagen deposition and subsequent defects of atrial conduction due to a substrate favoring re-entry and AF perpetuation [32]. Moreover, recent studies suggest that epicardial fat—which strongly correlates with visceral fat—is actively involved in the secretion of several proinflammatory cytokines which contribute to adverse left atrial remodeling, AF development and poor AF-related outcome [33]. In fact, local epicardial adipose tissue (EpAT) depots may contiguously infiltrate the atrial myocardium, leading to architectural disarray. This phenomenon may determine action potential prolongation and conduction slowing. Furthermore, EpAT is endocrinologically active in protein synthesis and release (paracrine effect), causing fibrosis and the lateralization of connexin-40, with inter-myocite disruption losing the regular architecture (Figure 1). This finally results in aberrant excitability and conduction heterogeneity and, thus, atrial fibrillation [34]. Important therapeutic perspectives can be derived from this knowledge. For example, targeting the EpAT adipokine population with antibody therapies may reduce the large inflammatory component decrementing AF genesis and maintenance. Indeed, reducing growth factors and profibrotic cytokines levels as well as local oxidative stress may influence the milieu that increases AF risk [35].
3.3. From Atrial Fibrosis to Atrial Cardiomyopathy
The atrium has always been the neglected heart structure in favor of its counterpart, the ventricle. However, in recent years, awareness of atrial pathologies, which may have a substantial impact on cardiac performance, arrhythmia occurrence, and stroke risk, has been raised. In 2016, an intercontinental working group defined, for the first time, the term “atrial cardiomyopathy”, indicating “any complex of structural, architectural, contractile or electrophysiological changes affecting the atria with the potential to produce clinically relevant manifestations” [36]. They even defined a working histological/pathophysiological classification scheme using the acronym EHRAS (EHRA: European Heart Rhythm Society/HRS: Hear Rhythm society/APHRS: Asian Pacific Heart Rhythm Society/SOLAECE: Sociedad Latino Americana de Estimulacion Cardiaca y Electrofisiologia) recognizing four classes:
- (1)
- Primarily cardiomyocytes changes (typical of Lone AF, genetic disease, and diabetes);
- (2)
- Primarily fibroblast changes (due to ageing or smoking);
- (3)
- Combined cardiomyocyte-fibroblast dependent fibrosis (present in valvular disease and chronic heart failure);
- (4)
- Primarily non-collagen deposits (as in isolated atrial amyloidosis, granulomatosis, inflammatory infiltrates, glycosphingolipids).
Although this classification is purely descriptive, it has the aim of describing pathological changes in biopsies and correlating pathologies with results obtained from imaging technologies.
The term atrial remodeling is used to indicate the effective change in atrial wall thickness/characterization and/or atrial chamber size and shape [37]. There are several clinical scenarios in which left atrial remodeling predisposes the development of atrial cardiomyopathy (i.e.: hypertension, diabetes, myocarditis, ageing). Atrial remodeling is considered a marker for adverse cardiovascular outcomes, such as, for example, in atrial fibrillation and in patients with left ventricular diastolic or systolic dysfunction. Left atrial enlargement and dysfunction usually represents maladaptive structural remodeling, which has prognostic relevance in these patients [37]. Maladaptive remodeling often promotes electrical remodeling that, in turn, facilitates the occurrence of atrial fibrillation. Two main mechanisms accelerate left atrial remodeling develop, fast atrial arrhythmias and both, pressure, and volume overload. Left atrial remodeling can be classified into three singular entities, which are often interrelated:
- (a)
- Structural remodeling, consequence of increases in interstitial fibrosis that result in atrial dilatation;
- (b)
- Functional remodeling, characterized by a left atrial failure;
- (c)
- Electrical remodeling that predisposes to atrial arrhythmias due to modifications to the substrate that promote reentry due to the heterogeneity of current conduction, shortening of action potentials, depolarization of resting cardiomyocytes, and induction of spontaneous phase 4 depolarization [38].
Presence of an isolated kind of remodeling is possible. However, often, the three forms coexist at the same time and enhance each other. There is rising interest in monitor left atrial remodeling in the clinical practice, with two main arguments:
- (a)
- First, it is a potential reversible process. Modifying cardiovascular conditions, including hypertension or heart failure, can reverse the remodeling process, especially in the early stages of LA structural and functional remodeling. Moreover, some medical interventions leading to a reduction in the LA arrhythmic burden, for example, isolation of the pulmonary veins, have been shown to improve LA function [39]. Patients free of AF recurrence after catheter ablation can show a significant reduction in LA fibrosis burden in follow-up cardiac magnetic resonance (CMR) studies [40];
- (b)
- LA remodeling is a prognostic factor. LA volume and function are related to the probability of developing atrial fibrillation [41] and are an independent prognostic marker in patients with heart failure and reduced ejection fraction [42]. Atrial fibrosis has been demonstrated to be associated with stroke and the presence of spontaneous echo contrast [43,44].
In this scenario, cardiac imaging appears to be a promising noninvasive tool for monitoring the presence and degree of LA remodeling. Moreover, imaging information can be incorporated into the clinical decision-making. Hypothetically, in the case of a severe atrial dilatation with extensive atrial fibrosis, it might not be justified to schedule the patients for an invasive approach as atrial fibrillation unless no other treatment options are available. On the other hand, even a patient with a longstanding AF can be a good candidate for ablation if imaging evidences a low degree of remodeling.
4. Evaluation of Fibrosis for Prognosis and Imaging to Guide Ablations
4.1. Late Gadolinium Enhancement Cardiac Magnetic Resonance (LGE-CMR)
In the last decade, CMR has emerged as a novel, fascinating imaging tool for evaluating LA remodeling. CMR image delivers a precise endocardial border definition, and its strength allows a complete measure of the LA atrial volume, rather than a simple area length. CMR is becoming more and more available and does not exposes patient to ionizing radiation. In fact, in recent years, CMR has become the gold standard for volumetric analysis. However, the more fascinating aspect of CMR is tissue characterization. LGE-CMR can identify, quantify, and characterize atrial fibrosis.
4.2. LGE-CMR Image Acquisition
LGE-CMR is considered a standard imaging technique to characterize atrial scarring. Gadolinium is used as a contrast agent to highlight areas of fibrosis which differentiate healthy tissue (with high gadolinium washout velocity) from scarred tissue (slower washout). A good quality of the acquired images is dependent on heart movements and breathing. Therefore, multiple images during consecutive breath holds are recorded, obtaining multiple short axis planes along the axis of the left atrium. Due to the high chances of recording artifacts which may impair the reconstruction of the scar, 2 and 3-dimensional acquisition protocols have been developed, based on 2- and 3D navigator-gated inversion recovery sequences, avoiding slice-shifting artifacts [45]. Moreover, the acquisition is usually electrocardiogram (ECG)-triggered to acquire images in the same moment of the cardiac cycle. Data acquisition is performed in atrial diastole to minimize motion artefact and extend data acquisition duration. Due to the irregularity of the cardiac cycle, evaluating atrial fibrosis during atrial fibrillation remains a challenge. For this reason, an external cardioversion is usually performed if irregular rhythms are present. Irregular respiratory pattern prolongs acquisition time and was also recognize as a frequent cause of poor image quality [46]. It was estimated that an appropriate image for fibrosis characterization can be obtained in 90% of CMR [47].
4.3. CMR Left Atrial Fibrosis Characterization
Following the acquisition of LGE-CMR scan, images should be post-processed based on the identified pixel signal intensities (PSI). Endocardial and epicardial borders are usually semiautomatically demarcated for segmentation (Figure 2) by using the left atrial blood pool as a reference to identify enhanced LA pixels [48].
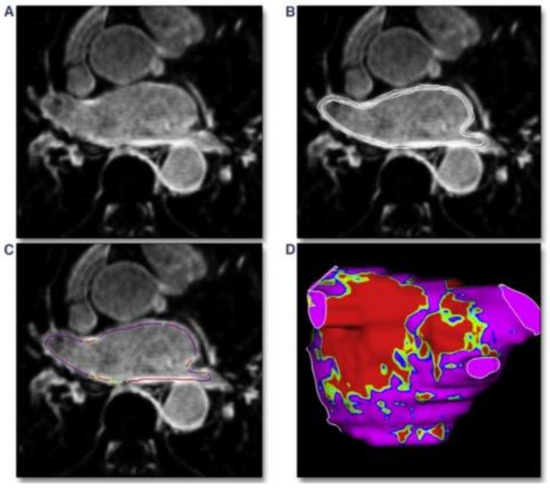
Figure 2.
Post-processing phase of left atrium detected with cardiac MRI basing on pixel signal intensity. (A): MRI-LGE scan of the left atrium. (B): manual segmentation of the atrial wall with analysis of multiple layers from endocardium to epicardium. (C): pixel intensity map creation along the atrial wall. (D): 3-dimensional shell of the left atrium with pixel intensity map based on different colors; red: dense scar, green/yellow: border zone, purple: healthy tissue. Requested permission from [49].
LA fibrosis distribution and shape are usually visualized using a 3D shell of the left atrium, applying a color code based on the PSI to distinguish healthy myocardium from scaring areas. As a result of the LA tissue characterization process, quantitative and qualitative information on the LA fibrosis is available. For a quantitative analysis of the scar, data are usually presented in grams of fibrosis or, alternatively, in volume/area percentage. The group of Marrouche use the Utah stage classification for a quantitative analysis of the fibrosis, based on LA wall enhancement, as a percentage of the total LA wall: stage I, defined as <10%, stage II >10 to <20%, stage III >20 to <30%, and stage IV >30% (Table 1). A quantitative analysis of the LA fibrosis has been shown to be related to the response to AF catheter ablation, independently of the presence of other comorbidities or AF behavior [50]. Moreover, the potential regression of structural alterations is possible when ablation is performed in early Utah stages [51]. The initial data also suggest that a decision-making approach for the selection of patients for AF ablation can be made based on the quantification of fibrosis [52]. A quantitative analysis of the fibrosis was also related to hard clinical outcomes, such as stroke [43]. The qualitative analysis of fibrosis (distribution and shape) can be used to plan AF intervention, since 3D pixel intensity maps can be imported into the navigator system to help in catheter ablation procedures. Bisbal and colleagues showed that LGE-CMR-delivered maps can successfully guide repeated PVI procedures by accurately identifying and localizing gaps and may reduce the procedural duration and radiofrequency application time [49] (Figure 3). A further hypothesis proposed for some groups is that areas of fibrosis identified in the LGE-CMR could become a target for ablation. In the recently published ALICIA trial, patients were randomized to receive pulmonary vein isolation (PVI) plus CMR-guided fibrosis ablation vs. PVI alone. After 12 months follow-up, there were no differences between groups in the recurrence rate. The results of the ongoing delayed-enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF II, ClinicalTrials.gov Identifier: NCT02529319) trial might shed light on the role of atrial fibrosis as a potential target for catheter ablation of AF.

Table 1.
Utah stage classification of left atrial fibrosis with cardiac MRI and correlation with outcomes after atrial fibrillation ablation in the DECAAF study.
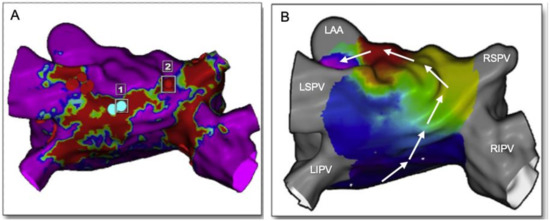
Figure 3.
Usefulness of cardiac MRI in redo procedures of atrial fibrillation and atrial flutter ablation. (A): MRI-LGE of the left atrium in a patient with previous ablation of the roof presenting with atypical flutter. Red areas represent scar due to previous ablation (blue dots = 1). In point 2 (red dot), there is a portion of healthy tissue (purple) between the roof line and the RSPV. (B): Activation mapping during flutter with CARTO system depicts a circuit directed upwards along the posterior wall and crossing the gap identified at the MRI (white arrows). One-shot ablation in point 2 interrupted the roof-dependent atrial flutter. LAA: left atrial appendage, LSPV: left superior pulmonary vein, LIPV: left inferior pulmonary vein, RSPV: right superior pulmonary vein, RIPV: right inferior pulmonary vein. Modified from [49].
4.4. Multidetector Cardiac Computed Tomography (MDCT)
MDCT has a greater spatial resolution (0.5 mm) compared with LGE-CMR, better defining the cardiac anatomy. On the other hand, it has a low contrast-to-noise ratio, a feature that reduces its ability to distinguish between normal myocardium and scar. Cardiac CT estimates LA volume without geometric assumptions, and thereby provides a more accurate evaluation of LA volume compared to TTE [53]. It can accurately define the course of the pulmonary veins, the atrial appendage location and morphology [54]. Moreover, MDCT permits the identification of sensitive extracardiac structures, i.e., the left phrenic nerve. As a consequence, MDTC is of great value for evaluation prior to pulmonary vein isolation for AF ablation. Moreover, due to its great spatial resolution, MDCT is able to accurately measure the left atrial wall thickness. The left atrial wall is a thin structure with heterogeneous thickness ranging from <1 mm to >5 mm. AF ablation durability can be limited by its inability to create transmural lesions in certain anatomical sites where left atrial wall thickness (LAWT) is higher [55]. In fact, it is an independent predictor of reconnection and AF recurrence after 12 months follow-up [56,57]. Berruezo and colleagues proposed a new approach for guiding pulmonary vein isolation by the LAWT. They recently report the feasibility of incorporating 3D LAWT maps into the navigation system, allowing a direct estimation of the WT at any point of the left atrium during the procedure [58]. They tailor radiofrequency energy, also delivering an ablation line design depending on the LAWT (Figure 4). This new approach to AF ablation can potentially increase the efficiency and safety of the ablation procedure. MDCT can also accurately identify and quantify epicardial adipose tissue (EpAT). It is, as previously cited, a potentially important player for atrial remodeling [35]. MDCT not only allows the identification of the EpAT location, it also allows a volumetric quantification defining the attenuation value range (usually −195 to −45 HU) for EAT segmentation. A recent study shows that regional EpAT affects local myocardial electrophysiology by direct infiltration in intermyocyte disruption, tissue fibrosis and gap junction remodeling [34]. It seems that the EpAT volume is correlated with a local slower activation time and an increased number of complex local potentials. A concordance could exist between the location of the fat and the electrical properties of the mapping during the ablation (slower conduction speed, fragmentation). Indeed, the properties of the fat could influence the risk of recurrence after ablation. However, further studies are needed to integrate this instrument in clinical practice.
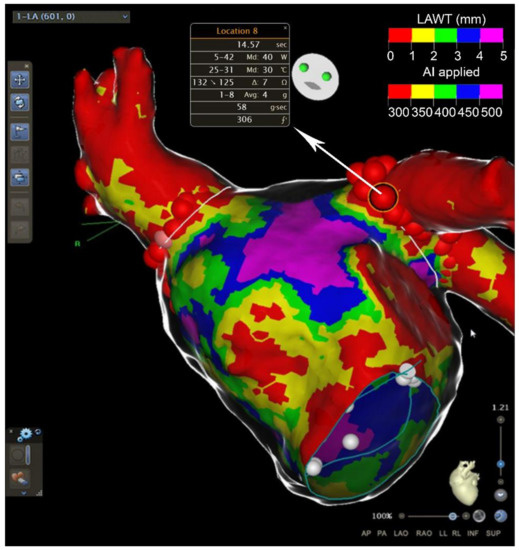
Figure 4.
Usefulness of cardiac CT in atrial fibrillation ablation. Anterior view of the left atrium after atrial fibrillation ablation around the pulmonary veins. Atrial wall thickness (in mm) is depicted with different colors on the map. Major values in radiofrequency applications (AI) are used in thicker areas. AI = ablation index. LAWT = left atrial wall thickness.
4.5. Echocardiographic Strain
Left atrial strain measures local deformation of the myocardium and have been used to evaluate atrial function in various disease states. It has been shown that alteration in atrial function, measured by strain, precede atrial dilatation [59,60]. Therefore, left atrial strain emerged as a potential tool for detecting subclinical atrial dysfunction. Indeed, LA strain can be used as a subrogate of left atrial fibrosis. A previous study including 148 patients undergoing AF ablation showed that more than 60% of patients exhibited LA reverse remodeling after catheter ablation, and baseline LA strain, as an indirect marker of fibrosis, was an independent predictor of LA reverse remodeling [61]. It has been shown that the LA strain measured in patients with paroxysmal AF can predict progression to persistent AF, as well as atrial fibrillation recurrence after catheter ablation [62]. Yasuda and colleagues previously reported a series of 100 patients undergoing AF ablation [63]. The baseline LA strain was the most useful parameter in the prediction of AF recurrence, with a 0.84 area under the curve. Finally, LA strain has recently been shown to identify patients at high risk of AF among those that suffered from cryptogenic stroke, suggesting that echocardiographic quantification of LA remodeling has the potential for secondary prevention in this population [64].
5. Atrial Substrate Characterization and Its Implications for Clinical Management of the Patient with AF
The complexity of the mechanisms leading to atrial remodelling makes each AF patient very different. Thus, AF must be interpreted as the same electrocardiografic manifestation of a protean multifaced clinical syndrome. Its treatment must be considered beyond rhythm and/or rate control and the dichotomy of anticoagulate or not anticoagulate in an AF patient. In fact, a holistic management of AF must also comprise the identification of concomitant cardiovascular risk factors and comorbidity. In this sense, the renowned classification based on AF episode duration and temporal patterns (first episode, paroxysmal, persistent, permanent) presents some limitations. A new multidimensional AF classification must be sought, addressing specific domains with treatment and prognostic implications. For this reason, the new 4S-AF scheme proposed by the 2020 international guidelines is a characterization (more than a classification) based on pathophysiology. It includes four AF- and patient-related domains: Stroke risk, Symptoms, Severity of AF burden, and Substrate severity [65]. Concerning this last point, cardiac imaging undoubtedly plays a pivotal role in estimating the grade of atrial disease and could be included in the AF management decision-making process. It represents a paradigm shift in the integrated care approach which comprises five main principal actions in the CC to ABC pathway (Atrial fibrillation Better Care):
- (1)
- C = confirm AF (with ECG recordings, cardiac devices or mobile health technology);
- (2)
- C = characterize AF (with the 4S-AF scheme);to
- (3)
- A = avoiding stroke (with anticoagulation or left atrial appendage closure);
- (4)
- B = better symptoms (with rate or rhythm control);
- (5)
- C = Cardiovascular risk and Comorbidity optimization (with aggressive risk factors interventions).
Compliance with such a management scheme has shown to improve population-based clinical outcomes in several nationwide AF cohorts [66,67]. Furthermore, using mobile health technology in the mobile Atrial Fibrillation App-II trial Guo and colleagues demonstrated the integrated holistic ABC approach, applied on an adult population of 1261 subjects (mean age 67.0 years), could reduce the composite outcome of ischaemic stroke/systemic thromboembolism, death, and rehospitalization [68].
6. Future Perspectives and New Research Areas
While progress has been made in atrial fibrosis etiopathology and characterization, major gaps persist, which need further research, especially in the field of cardiac imaging and its potential role in the management of patient with AF, particularly those undergoing ablation. In fact, contemporary techniques do not allow:
- (1)
- Explanation of the single (or multiple) arrhythmia mechanisms in each patient. Often, also during the ablative procedure, it is not possible to discern between ectopic (triggered) activity and/or re-entry. Furthermore, before the procedure, it is not possible to define AF as pulmonary vein (PV)-dependent vs. non-PV-dependent. New technologies, including integration between imaging and ECG and using artificial intelligence, are promising in this sense [69];
- (2)
- Complete understanding of the prevalent molecular pathway leading to atrial fibrosis. In fact, if, in the future, it becomes possible to identify each mechanism in each patient, new therapeutical techniques could be applied to develop a specific therapy targeting that pathway (i.e., genic therapy) [70];
- (3)
- Possible identification of a prevalent etiology of the AF (atrial fibrosis, circadian rhythm, ischaemia, autonomic imbalance, inflammation, and adiposity). Therefore, we have to manage all these factors together, without specificity. Functional methodologies studying atria innervation and perfusion are welcome in this field;
- (4)
- Individuation of a pre-state of AF to carry out a primary prevention of AF development (and its related complication as heart failure and stroke), which, at present, is left to clinical discretion. Studying a score system including clinical and imaging factors to identify people with higher chances of developing AF could be useful. Whether this is reflected by improved atrial fibrosis needs further study.
7. Conclusions
In recent years, awareness of atrial cardiomyopathies has raised. Atrial remodeling is a complex phenomenon that impacts the atrial wall and chamber, including collagen accumulation, fibrosis formation and fatty infiltration. All these conditions have a substantial impact on cardiac performance, arrhythmia occurrence, and stroke risk. Atrial fibrosis characterization, with imaging methods such as CMR, MDTC and echography, can be useful to choose the appropriate patient undergoing AF ablation and to tailor the approach to each individual.
Author Contributions
F.D.S.: Conceptualization, original draft preparation, figures preparation, review and editing, project administration, final approvement; D.P.: writing/original draft preparation, figures preparation, final approvement; D.S.-I.: figures preparation, review, and editing, final approvement; A.B.: conceptualization, review and editing, supervision, final approvement; U.L.: conceptualization, supervision, project administration, final approvement. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.H.; Franco, O.H.; Hofman, A.; Witteman, J.C.M.; Stricker, B.H.; Heeringaet, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart, J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 11, 154–162. [Google Scholar]
- De Sensi, F.; De Potter, T.; Cresti, A.; Severi, S.; Breithardt, G. Atrial fibrillation in patients with diabetes: Molecular mechanisms and therapeutic perspectives. Cardiovasc. Diagn. Ther. 2015, 5, 364–373. [Google Scholar] [PubMed]
- Ruddox, V.; Sandven, I.; Munkhaugen, J.; Skattebu, J.; Edvardsen, T.; Otterstad, J.E. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, V.; Graves, K.G.; Bunch, T.J. Anticoagulant use in atrial fibrillation and risk of dementia: Review of contemporary knowledge. Expert Rev Cardiovasc. Ther. 2017, 15, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Choi, E.K.; Han, K.D.; Lee, H.; Choe, W.S.; Lee, S.R.; Cha, M.J.; Lim, W.H.; Kim, Y.J.; Oh, S. Mortality and causes of death in patients with atrial fibrillation: A nationwide population-based study. PLoS ONE. 2018, 13, e0209687. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 74, 104–132. [Google Scholar]
- Santangeli, P.; Marchlinski, F.E. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017, 14, 1087–1096. [Google Scholar] [CrossRef]
- Parkash, R.; Verma, A.; Tang, A.S. Persistent atrial fibrillation: Current approach and controversies. Curr. Opin. Cardiol. 2010, 25, 1–7. [Google Scholar] [CrossRef]
- Lau, D.H.; Linz, D.; Schotten, U.; Mahajan, R.; Sanders, P.; Kalman, J.M. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Heart Lung Circ. 2017, 26, 887–893. [Google Scholar] [CrossRef]
- De Jong, S.; van Veen, T.A.; van Rijen, H.V.; de Bakker, J.M. Fibrosis and cardiac arrhythmias. J. Cardiovasc. Pharmacol. 2011, 57, 630–638. [Google Scholar] [CrossRef]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Hansen, B.J.; Zhao, J.; Fedorov, V.V. Fibrosis and Atrial Fibrillation: Computerized and Optical Mapping; A View into the Human Atria at Submillimeter Resolution. JACC Clin. Electrophysiol. 2017, 3, 531–546. [Google Scholar] [CrossRef]
- Fast, V.G.; Kléber, A.G. Cardiac tissue geometry as a determinant of unidirectional conduction block: Assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc. Res. 1995, 29, 697–707. [Google Scholar] [CrossRef]
- De Bakker, J.M.; van Capelle, F.J.; Janse, M.J.; Tasseron, S.; Vermeulen, J.T.; de Jonge, N.; Lahpor, J.R. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation 1993, 88, 915–926. [Google Scholar] [CrossRef]
- Ten Tusscher, K.H.; Panfilov, A.V. Influence of diffuse fibrosis on wave propagation in human ventricular tissue. Europace 2007, 9, vi38–vi45. [Google Scholar] [CrossRef]
- Nezlobinsky, T.; Solovyova, O.; Panfilov, A.V. Anisotropic conduction in the myocardium due to fibrosis: The effect of texture on wave propagation. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Spach, M.S.; Miller, W.T.; Dolber, P.C.; Kootsey, J.M.; Sommer, J.R.; Mosher, C.E., Jr. The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ. Res. 1982, 50, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Rahmutula, D.; Marcus, G.M.; Wilson, E.E.; Ding, C.H.; Xiao, Y.; Paquet, A.C.; Barbeau, R.; Barczak, A.J.; Erle, D.J.; Olgin, J.E. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-b1. Cardiovasc. Res. 2013, 99, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, X.; Wang, Q.X.; Tan, H.W.; Guo, M.; Jiang, W.-F.; Zhou, L. Angiotensin II increases CTGF expression via MAPKs/TGF-B1/TRAF6 pathway in atrial fibroblasts. Exp. Cell Res. 2012, 318, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.; Pagano, P.; Colucci, W. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. 2001, 280, C53–C60. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, H.; Chen, S.; Qi, B.; Zhou, G.; Cai, L.; Zhao, L.; Wei, Y.; Liu, S. MicroRNA-30c suppresses the pro-fibrogenic effects of cardiac fibroblasts induced by TGF-b1 and prevents atrial fibrosis by targeting TGFbRII. J. Cell Mol. Med. 2018, 22, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-F.; He, R.-H.; Shi, S.-B.; Li, R.; Wang, Q.-T.; Rao, G.-T.; Yang, B. Modulation of miR-10a- mediated TGF-b1/Smads signaling affects atrial fibrillation-induced cardiac fibro- sis and cardiac fibroblast proliferation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- De Sensi, F.; Costantino, S.; Limbruno, U.; Paneni, F. Atrial fibrillation in the cardiometabolic patient. Minerva. Med. 2019, 110, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef]
- Pandit, S.V.; Anumonwo, J.; Jalife, J. Atrial Fibrillation Susceptibility in Obesity: An excess adiposity and Fibrosis complicity? Circ. Res. 2016, 118, 1468–1471. [Google Scholar] [CrossRef]
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A myocardial Nox2 containing Nad(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 2005, 97, 629–636. [Google Scholar] [CrossRef]
- Guler, N.; Ozkara, C.; Dulger, H.; Kutay, V.; Sahin, M.; Erbilen, E.; Gumrukcuoglu, H.A. Do cardiac neuropeptides play a role in the occurrence of atrial fibrillation after coronary bypass surgery? Ann. Thorac. Surg. 2007, 83, 532–537. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. JACC Coll. Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef]
- Vyas, V.; Hunter, R.J.; Longhi, P.; Finlay, M.C. Inflammation and adiposity: New frontiers in atrial fibrillation. Europace 2020, 22, 1609–1618. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Yanaka, S.; Nakajima, H.; Atsumi, A.; Yamamoto, M.; Kawamura, R.; Koshino, Y.; Machino, T.; et al. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circ. J. 2013, 77, 1695–1704. [Google Scholar] [CrossRef]
- Gal, P.; Pacchia, C.; Morris, A.; Kholmovski, E.G. Ablation scar recovery is significantly stronger in atrial fibrillation free patients (abstr P258). Europace 2015, 17, iii20–iii29. [Google Scholar] [CrossRef]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef]
- Malagoli, A.; Rossi, L.; Bursi, F.; Zanni, A.; Sticozzi, C.; Piepoli, M.F.; Villani, G.Q. Left Atrial Function Predicts Cardiovascular Events in Patients With Chronic Heart Failure With Reduced Ejection Fraction. J. Am. Soc. Echocardiogr. 2019, 32, 248–256. [Google Scholar] [CrossRef]
- Daccarett, M.; Badger, T.J.; Akoum, N.; Burgon, N.S.; Mahnkopf, C.; Vergara, G.; Kholmovski, E.; McGann, C.J.; Parker, D.; Brachmann, J.; et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2011, 57, 831–838. [Google Scholar] [CrossRef]
- Akoum, N.; Fernandez, G.; Wilson, B.; McGann, C.; Kholmovski, E.; Marrouche, N. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J. Cardiovasc. Electr. 2013, 24, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Vuissoz, P.A.; Odille, F.; Fernandez, B.; Lohezic, M.; Benhadid, A.; Mandry, D.; Felblinger, J. Free-Breathing Imaging of the Heart Using 2D Cine-GRICS (Generalized Reconstruction by Inversion of Coupled Systems) With Assessment of Ventricular Volumes and Function. J. Magn. Reson. Imaging 2012, 35, 340–351. [Google Scholar] [CrossRef]
- Kholmovski, E.G.; Damal, K.; Burgon, N.S.; Vijayakumar, S.; Tek, C.; Mihaylova, A.G.; Marrouche, N.F. A multi-center trial of LGE-MRI of the left atrium. J. Cardiovasc. Magn. Reson. 2013, 15, 1–2. [Google Scholar] [CrossRef]
- Cochet, H.; Mouries, A.; Nivet, H.; Sacher, F.; Derval, N.; Denis, A.; Merle, M.; Relan, J.; Hocini, M.; Haïssaguerre, M.; et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J. Cardiovasc. Electrophysiol. 2015, 26, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Khurram, I.M.; Beinart, R.; Zipunnikov, V.; Dewire, J.; Yarmohammadi, H.; Sasaki, T.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; Halperin, H.R.; et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm 2014, 11, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bisbal, F.; Guiu, E.; Cabanas-Grandío, P.; Berruezo, A.; Prat-Gonzalez, S.; Vidal, B.; Garrido, C.; Andreu, D.; Fernandez-Armenta, J.; Tolosana, J.M.; et al. CMR-guided approach to localize and ablate gaps in repeat AF ablation procedure. JACC Cardiovasc. Imaging 2014, 7, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Mahnkopf, C.; Badger, T.J.; Burgon, N.S.; Daccarett, M.; Haslam, T.S.; Badger, C.T.; McGann, C.J.; Akoum, N.; Kholmovski, E.; Macleod, R.S.; et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed- enhanced MRI: Implications for disease progression and response to catheter ablation. Heart Rhythm 2010, 7, 1475–1481. [Google Scholar] [CrossRef]
- Kuppahally, S.S.; Akoum, N.; Badger, T.J.; Burgon, N.S.; Haslam, T.; Kholmovski, E.; Macleod, R.; McGann, C.; Marrouche, N.F. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am. Heart J. 2010, 160, 877–884. [Google Scholar] [CrossRef]
- Akoum, N.; Wilber, D.; Hindricks, G.; Jais, P.; Cates, J.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. MRI assessment of ablation-induced scarring in atrial fibrillation: Analysis from the DECAAF study. J. Cardiovasc. Electrophysiol. 2015, 26, 473–480. [Google Scholar] [CrossRef]
- Kühl, J.T.; Lønborg, J.; Fuchs, A.; Andersen, M.J.; Vejlstrup, N.; Kelbæk, H.; Engstrøm, T.; Møller, J.E.; Kofoed, K.F. Assessment of left atrial volume and function: A comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int. J. Cardiovasc. Imaging 2012, 28, 1061–1071. [Google Scholar] [CrossRef]
- Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; Pump, A.; Brantes, M.C.; Gallinghouse, J.; et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar]
- Kistler, P.M.; Ho, S.Y.; Rajappan, K.; Morper, M.; Harris, S.; Abrams, D.; Sporton, S.; Schilling, R. Electrophysiologic and anatomic characterization of sites resistant to electrical isolation during circumferential pulmonary vein ablation for atrial fibrillation: A prospective study. J. Cardiovasc. Electrophysiol. 2007, 18, 1282–1288. [Google Scholar] [CrossRef]
- Inoue, J.; Skanes, A.C.; Gula, L.J.; Drangova, M. Effect of Left Atrial Wall Thickness on Radiofrequency Ablation Success. J. Cardiovasc. Electrophysiol. 2016, 27, 1298–1303. [Google Scholar] [CrossRef]
- Suenari, K.; Nakano, Y.; Hirai, Y.; Ogi, H.; Oda, N.; Makita, Y.; Ueda, S.; Kajihara, K.; Tokuyama, T.; Motoda, C.; et al. Left atrial thickness under the catheter ablation lines in patients with paroxysmal atrial fibrillation: Insights from 64-slice multidetector computed tomography. Heart Vessels 2013, 28, 360–368. [Google Scholar] [CrossRef]
- Teres, C.; Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Ordoñez, B.; Chauca, A.; Huguet, M.; Ramírez, C.; Oller, G.; Jornet, A.; et al. Left atrial wall thickness of the pulmonary vein reconnection sites during atrial fibrillation redo procedures. Pacing Clin. Electrophysiol. 2021, 20. [Google Scholar] [CrossRef]
- Boyd, A.C.; Richards, D.A.; Marwick, T.; Thomas, L. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart 2011, 97, 1513–1519. [Google Scholar] [CrossRef]
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Köhncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef]
- Tops, L.F.; Delgado, V.; Bertini, M.; Marsan, N.A.; Den Uijl, D.W.; Trines, S.A.; Zeppenfeld, K.; Holman, E.; Schalij, M.J.; Bax, J.J. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 2011, 57, 324–331. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Oh, I.Y.; Kim, S.-A.; Park, K.-H.; Kim, S.H.; Park, J.-H.; Kim, J.-E.; Lee, S.-P.; Kim, H.-K.; Kim, Y.-J.; et al. Echocardiographic predictors of progression to persistent or permanent atrial fibrillation in patients with paroxysmal atrial fibrillation (E6P Study). J. Am. Soc. Echocardiogr. 2015, 28, 709–717. [Google Scholar] [CrossRef]
- Yasuda, R.; Murata, M.; Roberts, R.; Tokuda, H.; Minakata, Y.; Suzuki, K.; Tsuruta, H.; Kimura, T.; Nishiyama, N.; Fukumoto, K.; et al. Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: Study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1008–1014. [Google Scholar] [CrossRef]
- Sade, L.E.; Keskin, S.; Can, U.; Çolak, A.; Yüce, D.; Çiftçi, O.; Özin, B.; Müderrisoğlu, H. Left atrial mechanics for secondary prevention from embolic stroke of undetermined source. Eur. Heart J. Cardiovasc. Imaging 2020, 18, 311. [Google Scholar] [CrossRef]
- Potpara, T.S.; Lip, G.Y.H.; Blomstrom-Lundqvist, C.; Boriani, G.; Van Gelder, I.C.; Heidbuchel, H.; Hindricks, G.; Camm, A.J. The 4S-AF Scheme (Stroke Risk; Symptoms; Severity of Burden; Substrate): A Novel Approach to In-Depth Characterization (Rather than Classification) of Atrial Fibrillation. Thromb Haemost 2021, 121, 270–278. [Google Scholar]
- Proietti, M.; Lip, G.Y.H.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.A.; Kalarus, Z.; Tavazzi, L.; et al. ESC-EORP Atrial Fibrillation General Long-Term Registry Investigators Group. Relation of outcomes to ABC (Atrial Fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace 2021, 23, 174–183. [Google Scholar]
- Yoon, M.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Sung, J.H.; Pak, H.N.; Lee, M.H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb Haemost 2019, 119, 1695–1703. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, J.; Shi, X.; Yao, Y.; Sun, Y.; Xia, Y.; Yu, B.; Liu, T.; Chen, Y.; Lip, G.Y.H. mAF-App II Trial investigators. Mobile health technology-supported atrial fibrillation screening and integrated care: A report from the mAFA-II trial Long-term Extension Cohort. Eur. J. Intern. Med. 2020, 82, 105–111. [Google Scholar] [CrossRef]
- Luongo, G.; Azzolin, L.; Schuler, S.; Rivolta, M.W.; Almeida, T.P.; Martinez, J.P.; Soriano, D.C.; Luik, A.; Muller-Edenborn, B.; Jadidi, A.; et al. Machine learning enables noninvasive prediction of atrial fibrillation driver location and acute pulmonary vein ablation success using the 12-lead ECG. Cardiovasc. Digit. Health, J. 2021, 2, 126–136. [Google Scholar] [CrossRef]
- Ni, L.; Scott, L., Jr.; Campbell, H.M.; Pan, X.; Alsina, K.M.; Reynolds, J.; Philippen, L.E.; Hulsurkar, M.; Lagor, W.R.; Li, N.; et al. Atrial-specific gene delivery using an adeno-associated viral vector. Circ. Res. 2019, 124, 256–262. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).