Abstract
Background: Halitosis of oral origin is very common in the general population. Due to their antimicrobial properties, chlorhexidine-based products are widely used in the management of this condition, but these are associated with reversible side effects. In this study we evaluated the efficacy of Lacer HaliTM mouthrinse and toothpaste in subjects with intraoral halitosis after several applications under normal conditions of use. Methods: In this randomized clinical trial with mouth rinse and toothpaste, single-center, double-blinded, parallel participants were assigned to an experimental group (Lacer HaliTM,, n = 20), a positive control group (HalitaTM, n = 20), and a placebo group (n = 20). The active duration of the study was 18 days. The clinical follow-up evaluations were performed at five time points (T0, T1, T2, T3, and T4). The intensity of halitosis was evaluated by organoleptic measurement and the portable gas chromatograph OralChromaTM. The data were analyzed using generalized mixed linear models. Results: Sixty patients completed the study. Lacer HaliTM, in comparison with HalitaTM, did not show statistically significant differences at any time during the study except for the levels of hydrogen sulfide and total volatile sulfur compounds at 15 days, where HalitaTM was better. Compared to the placebo treatment, Lacer HaliTM, was significantly more efficient, in terms of both the organoleptic evaluations at 8 days and the levels of hydrogen sulfide. Conclusions: Lacer HaliTM is an alternative to chlorhexidine-based toothpaste and mouthwashes in the management of halitosis.
1. Introduction
Halitosis, or oral malodor, is defined as the set of unpleasant odors emitted from a person’s mouth. Halitosis of oral origin is very common in the general population, representing 31.8% [1]. It lowers quality of life, and is a cause of significant social and psychological disability [2].
Not all halitosis is of oral origin—extraoral halitosis is caused by problems in the respiratory tract, disorders of the gastrointestinal tract, some systemic diseases, or metabolic disorders, and, less frequently, by carcinomas [3,4]. However, approximately 90.0% [5] of all halitosis cases are caused by the accumulation of bacteria and food residues present at the tongue and periodontal locations [6], even in individuals free of periodontal diseases such as gingivitis or periodontitis [7].
As intraoral halitosis is mainly produced by bacterial colonization, it is associated with inflammation and some infectious conditions of soft oral tissues [8,9]. Rarely, halitosis could be a sign of several other diseases such as oral cancer, which require a rapid diagnosis [10].
Treatment can be addressed by mechanical interventions such as brushing, flossing, tongue scraping [11], and chemical antibacterial agents such as chlorhexidine (CHX), phenol, tricolosan, dioxide chlorine, alcohol, and metal ions [12,13].
The most common methods used to diagnose intraoral halitosis are organoleptic evaluation and gas chromatography to quantify volatile sulfur compounds (VSCs).
Organoleptic measurement is considered the gold standard in detecting halitosis despite its subjectivity [14,15]. However, VSC analysis is more reliable to detect halitosis in the case of OralChroma™ (Abimedical, Kawasaki, Japan), because it gives independent values of the three main VSCs, and can also contribute to discriminating between intraoral and extraoral origins [16]. Additionally, the identification and quantification of bacteria responsible for the production of VSCs may be useful in the diagnosis and management of halitosis.
Different mouthrinses try to reduce oral malodors, and a large number are on the market [17]. CHX is the most-used oral antiseptic [18] and as oral bacteria are the major source of intraoral halitosis, CHX is found in most mouthrinses for controlling halitosis [19]. However, as CHX is associated with reversible side effects such as tooth staining or taste alteration, products with lower percentages of CHX have been developed to avoid these disadvantages with similar efficacy, such as Halita™ (Dentaid, Cerdanyiola, Barcelona, Spain) mouthwash, which contains 0.05% CHX, 0.05% cetylpyridinium chloride (CPC), and zinc (0.14%) [20]. The combination of CHX and CPC has a synergistic effect on the reduction of VSC-producing bacteria [21,22,23,24] and plays a role in the decrease in halitosis in the long-term [25]. Moreover, zinc ions can bind to VSCs and transform them into nonodoriferous products [26], inducing short- and long-term neutralising effects [27,28,29].
There are no published studies on the efficacy against halitosis of Lacer Hali™, which consists of octenidine dihydrochloride (0.01%), zinc chloride (0.10%), sodium fluoride (0.55% for the toothpaste and 0.05% for the mouthwash), and xylitol (1%).
The aim of this study was to evaluate the efficacy of Lacer Hali™ (Lacer, Barcelona, Spain) mouthwash and toothpaste in subjects with intraoral halitosis after several applications under normal conditions of use.
2. Materials and Methods
2.1. Experimental Design
This clinical trial, with mouthrinse and toothpaste, was a single-center, randomized, double-blind, parallel trial with three arms, with, for each participant, an active duration of 18 days. This study followed the CONSORT (Consolidated Standards of Reporting Trials) [30] recommendation guidelines. An internal protocol designed by the researchers and Zurko (Zurko Research S.L., Madrid, Spain) was established to undertake the clinical trial. It was approved by the Ethics Committee of Rey Juan Carlos University (internal registration number: 2110202019020).
The study was designed by Zurko, and the tests were carried out under dental control in a dental clinic (Core Odontología y Estética Facial, S.L., Madrid, Spain).
2.2. Patient Population
The inclusion criteria to participate in the study were as follows: age 18–60 years, good health, and intraoral halitosis present at the start of the study. Participants must have had a score equal to or greater than 1 on the organoleptic scale. In addition, they must have had a score greater than 160 ppb for VSCs (obtained by the addition of hydrogen sulfide, methyl mercaptan, and dimethyl sulfide levels) and/or a score greater than 112 ppb for hydrogen sulfide and/or a score greater than 26 for methyl mercaptan. They must also have had one of these two elevated gases (described above), availability for the duration of the test, saliva rating greater than 0.2 mL/min, and have signed the informed consent form. The exclusion criteria were as follows: record of allergies or idiosyncrasies to tooth ingredients; had received treatment with antibiotics during the three weeks prior to the beginning of the study; had used commercialized anti-halitosis products up to 30 days before the test; had used another type of oral hygiene product for halitosis during the test; were pregnant or breastfeeding; were, after anamnesis, exploration, and panoramic radiography in the first clinical examination suspected to have sinus pathology, that could explain extraoral halitosis; and had extraoral halitosis, pseudohalitosis, and/or halitophobia.
Subjects were randomized to the tree arms using a list of random numbers by central telephone allocation through the Zurko Center: group 1 (experimental: Lacer HaliTM mouthwash and toothpaste); group 2 (positive control: HalitaTM mouthwash and toothpaste); and group 3 (placebo: placebo mouthwash and toothpaste). The packaging and interior of the toothpastes and mouthwashes had the same visual appearance. The researcher who provided the materials to the participants was blinded.
The sample size was based on the study by Roldán S. et al. [21], where the effectiveness of Halita™ in two arms of 20 patients each was evaluated. A possible loss of participants of 15% was taken into account.
2.3. Patient Follow-Up
Clinical follow-up evaluations (T0, T1, T2, T3, and T4) were designed to evaluate the treatment efficacy over time and compared to the reference treatment and placebo. For these evaluations, the following analyses were conducted:
Organoleptic evaluation: A subjective assessment was performed according to the following scale [31]: 0 (absence of odor); 1 (barely perceived odor); 2 (slight odor but clearly perceived); 3 (moderate odor); 4 (strong odor); and 5 (extremely strong odor). Two dentists were olfactory-calibrated previously with the Smell Identification TestTM but to reduce the risk of bias, all the organoleptic measurements were carried out by only one investigator (Laiqi Xiang).
The concentrations of VSCs (hydrogen sulfide, methyl mercaptan, and dimethyl sulfide) were determined using Oral Chroma™ (Abimedical, Kawasaki, Japan). We decided to focus on the hydrogen sulfide and methyl mercaptan in our analysis because they are the main contributors to intraoral halitosis [3,16,32].
The portable gas chromatograph Oral Chroma™ model CHM1 was checked and professionally calibrated by the manufacturers prior to the study.
2.3.1. Baseline Visit
In the initial part of the first visit (T0), the patient was subjected to anamnesis, panoramic radiograph, saliva production rating, organoleptic evaluation, and VSCs determination. After 1 h (T1), the organoleptic test and VSCs determination were repeated. The products, instructions, and, follow-up table were given to the participants.
2.3.2. Follow-Up Visits
After 7 (T2) and 15 days (T3) of continuous use of the treatment, the organoleptic evaluation, and VSC determination were repeated. At the last visit, the participants returned their products and they were given a placebo product. The spare products were weighed and the volume was measured to check the participant’s compliance.
2.3.3. Final Visit (T4)
Three days after stopping the experimental treatment, the organoleptic evaluation, and VSCs determination were repeated.
2.4. Procedure and Adverse Events
Each participant was provided with a toothbrush, a tube of toothpaste, and mouthwash. The assigned products were applied twice a day for 15 days after the main meals (lunch and dinner). Participants applied a sufficient amount of toothpaste to cover the brush. They subsequently brushed their teeth and rinsed their mouth with 15 mL of mouthrinse for 1 min without rinsing the mouth later with water or another mouthrinse. All activities were reported in a patient record form.
On the T3 time point appointment, the participants were asked to return their products and new products containing the placebo were given to them. So, from T3 to T4 the participants of all groups were using the placebo. This was performed to test the duration of the therapeutic effect of the interventions once they were suppressed.
Participants were advised not to apply any other products orally during the study or receive any other dental treatments. At least one week before the clinic appointment, participants were advised to take the following precautions: abstain from alcohol in the last 12 h and avoid spicy foods, garlic, or onions two days before.
On the day of the appointment, the participants were asked to come with an empty stomach and to not use perfumed cosmetic products, but they were allowed to drink water up to 3 h before the appointment. As all the measurements were taken in the morning, they were also asked not to perform oral hygiene procedures from the previous day after dinner time No additional instructions or modifications to their routine hygiene techniques or eating habits were given. In cases of adverse reactions or doubt, the participants were advised to immediately suspend the application of the products, and to contact the research center.
2.5. Data Analysis
Descriptive statistics of the organoleptic values and concentrations of VSCs, hydrogen sulfide, and methyl mercaptan were obtained at each of the study times. For each of the diagnostic tests, the percentage of variation relative to the baseline, placebo, and HalitaTM was calculated. Categorical models were adjusted to assess the differences between Lacer HaliTM and placebo or HalitaTM over time. Generalized linear mixed-effect models (GLMM, negative-binomial model) were fitted to the gas concentration data to evaluate differences between Lacer HaliTM and placebo or HalitaTM over time. When analyzing the organoleptic scale, generalized linear mixed models (GLMM, cumulative logit model) were fitted to the ordered categorical data to evaluate the differences between Lacerhali and the placebo or HalitaTM over time. To evaluate the efficacy of the product, the null hypothesis that there was no difference between Lacer HaliTM and placebo or HalitaTM was evaluated by using Wald tests on the model parameters. R version 3.1.3 (R Core Development Team, R Foundation, Vienna, Austria) was used for the statistical analyses. The established significance value was 0.05 (95% confidence interval).
3. Results
Between June 2015 and November 2017, 69 participants were recruited and followed-up (Figure 1). The mean age of the participants was 38.39 (12.33) years, and 61.22% were women and 38.78% were men. The results by treatment groups were: placebo, with mean age of the participants was 38.39 (12.33) years, and 61.11% were women; Lacer HaliTM, with mean age of the participants was 38.91 (11.83) years, and 50.00% were women; HalitaTM, with mean age of the participants was 38.77 (12.01) years, and 70.59% were women. During the study, there were five dropouts due to reasons unrelated to the participants, and in the data analysis, abnormal data were observed in four participants who were excluded. Each arm of the study consisted of 20 participants, so further recruitment was not considered.
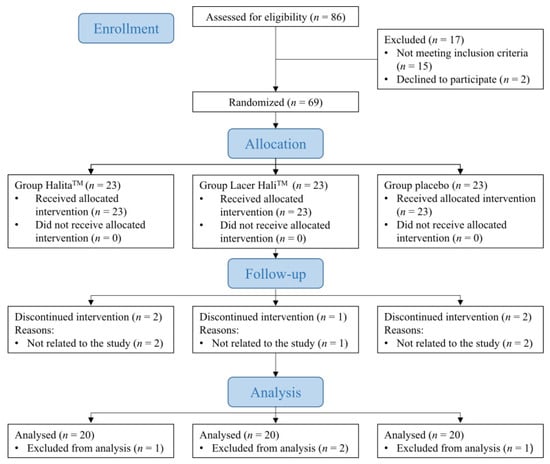
Figure 1.
CONSORT flow chart of the study patients.
3.1. Analysis of the Organoleptic Test
During the study, the most intense oral malodor was found in the placebo group. The average value for the Lacer HaliTM group was always higher than that of the positive control group, (HalitaTM, Figure 2). The mean values and percentage variation of organoleptic scores are represented in Table 1 and Table 2, respectively.
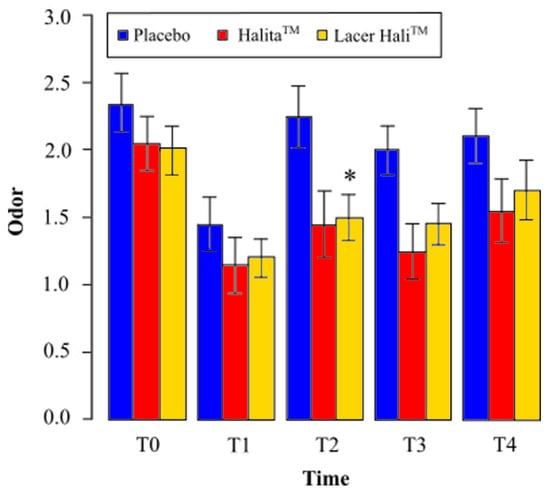
Figure 2.
Mean perceived odor and standard deviation at different times for the organoleptic test. *: Statistically significant results (p < 0.05) between the placebo and Lacer HaliTM groups were found. No significant differences were found between the HalitaTM and Lacer HaliTM groups.

Table 1.
Descriptive statistics of the evaluation of odors and the concentrations of hydrogen sulfide, methyl mercaptan, and VSCs.

Table 2.
Variations in odor intensity and the concentrations of hydrogen sulfide, methyl mercaptan, and total VSCs.
3.1.1. Lacer HaliTM vs. Placebo
The Lacer HaliTM group showed a reduction in the average observed oral malodor at all study times compared to the placebo. After one hour of treatment (T1), there was a 17% reduction in the average oral malodor. This number was 33% at eight days (T2), 28%at 15 days (T3), and 19% after 18 days (T4) (Table 2).
Odor intensity with Lacer HaliTM treatment was significantly lower (p = 0.009) after eight days (T2) of treatment, and close to statistically significant at 15 days (T3) (p = 0.059), compared to placebo treatment. There was a 92% and 83% probability of decrease of oral malodor with Lacer HaliTM treatment compared to placebo treatment, respectively (Table 3).

Table 3.
A cumulative logit mixed model was fitted to evaluate the difference between Lacer HaliTM vs. HalitaTM and Lacer HaliTM vs. placebo treatments at different time points during the organoleptic evaluation. Statistically significant p-value < 0.05.
3.1.2. Lacer HaliTM vs. HalitaTM
There was an increase in the observed average oral malodor at all study times compared to the positive control treatment (HalitaTM). After an hour of treatment (T1), there was a 4% increase in the average oral malodor, which was 3% at eight days (T2), 16% at 15 days (T3), and 10% after 18 days (T4), (Table 2).
With Lacer HaliTM treatment, the odor intensity was not significantly greater or lower than the with HalitaTM treatment at any experimental time point (Table 3).
3.2. Analysis of Gases (VCS)
We analysed the values of hydrogen sulfide, methyl mercaptan, and the addition of the three gases (VCSs). The mean values and the percentages of variation of hydrogen sulfide, methyl mercaptan, and total VCSs are shown in Table 1 and Table 2, respectively. Figure 3 shows that Lacer HaliTM had a tendency to decrease the hydrogen sulfide concentration at one hour (T1) compared with the placebo, with a statistically significant reduction at eight days (T2). However, a lower concentration of hydrogen sulfide was observed with HalitaTM compared with Lacer HaliTM and the placebo at all study times.
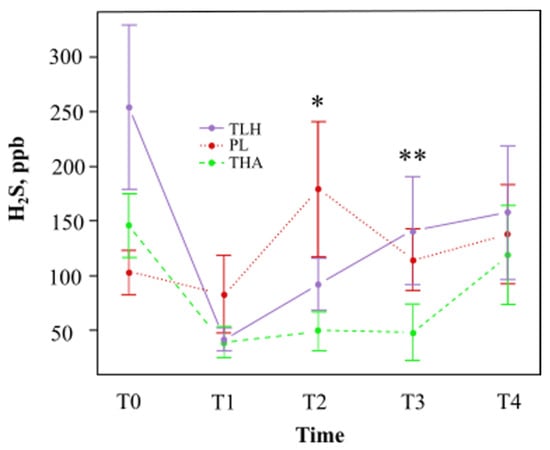
Figure 3.
Mean average hydrogen concentration (parts per billion) and standard deviation at different time points (TLH, Lacer HaliTM treatment; PL, placebo; THA, HalitaTM treatment). *: Statistically significant results (p < 0.05) between the placebo and Lacer HaliTM groups were found. **: Statistically significant results (p < 0.05) between the Lacer HaliTM and HalitaTM groups were found.
As shown in Figure 4, Lacer HaliTM showed a tendency to decrease the concentration of methyl mercaptan at one hour (T1) and at 15 days (T3) compared with placebo treatment. Regarding HalitaTM treatment, the tendency was to decrease the concentration of methyl mercaptan at all study times.
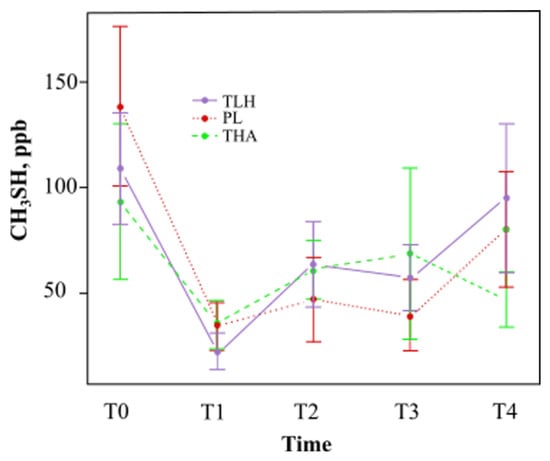
Figure 4.
Mean methyl mercaptan concentration (parts per billion) and standard deviation at different times (TLH, Lacer HaliTM treatment; PL, placebo; THA, HalitaTM treatment). There were no statistically significant results.
3.2.1. Lacer HaliTM vs. Placebo
There was a reduction in the concentration of hydrogen sulfide observed with Lacer HaliTM after one hour (T1) and eight days (T2) of treatment, 51% and 49%, respectively (Table 1). There was a 57% probability on the eighth day (T2) of observing a significant reduction (p = 0.026) in hydrogen sulfide. At 15 (T3) and 18 days (T4) of treatment, the probabilities of finding a significantly higher concentration of hydrogen sulfide were 11% and 4%, respectively, but these results were not statistically significant (Table 4).

Table 4.
Cumulative logit mixed model fitted to evaluate the difference between Lacer HaliTM vs. HalitaTM and Lacer HaliTM vs. placebo at different time points in the evaluation of the concentration of hydrogen sulfide, methyl mercaptan, and VSCs.
There was a reduction in the concentration of methyl mercaptan with Lacer HaliTM after one hour (T1) and 15 days of treatment (T3), 38% and 17%, respectively (Table 1), but the results were not statistically significant. The concentration of methyl mercaptan with Lacer HaliTM treatment was not significantly lower or higher than the concentration of methyl mercaptan with placebo treatment at any of the experimental time points (Table 4).
There was a reduction in the concentration of VSCs observed with Lacer HaliTM after one hour (T1) and 8 days of treatment (T3), 47% and 23%, respectively (Table 2). The VSC concentration with Lacer HaliTM treatment was not significantly lower or higher than the VSC concentration with placebo treatment at any of the experimental time points (Table 4).
3.2.2. Lacer HaliTM vs. HalitaTM
HalitaTM was able to better reduce the concentration of hydrogen sulfide compared to Lacer HaliTM at all experimentation time points (Table 2). At 15 days (T3), the concentration of hydrogen sulfide with HalitaTM was significantly lower than with Lacer HaliTM treatment. HalitaTM showed a 64% probability of decreasing the hydrogen sulfide concentration (p = 0.013). After one hour (T1) of treatment, there was also a 36% decrease in the methyl mercaptan concentration (Table 2), but this result was not statistically significant.
The concentration of methyl mercaptan with Lacer HaliTM treatment was not significantly lower or higher than the concentration of methyl mercaptan with HalitaTM treatment at any of the experimental time points (Table 4).
After only one hour (T1) of HalitaTM treatment, there was a 22% decrease in the concentration of VSCs (Table 1), but this result was not statistically significant. At 15 days (T3), HalitaTM treatment decreased the concentration of VSCs more than Lacer HaliTM treatment. HalitaTM showed a 64% and a 53% probability of decreasing the concentration of hydrogen sulfide (p = 0.013) and VCSs (p = 0.019), respectively (Table 4).
4. Discussion
This clinical trial with mouthrinse and toothpaste was designed to assess the effects of Lacer HaliTM toothpaste and mouthrinse for which there are currently no scientific publications. The findings showed that its efficacy in reducing VSCs seemed to be higher than that of placebo treatment but no better than that of HalitaTM treatment.
Bad breath is a prevalent problem in many people worldwide, although some of those who have it may not be aware of it due to adaptation of their sense of smell [3]. Halitosis negatively affects the social interactions of a person’s daily life, causing discomfort and emotional stress. There are chemical methods, such as mouthrinse, and mechanical methods, such as brushing and flossing, to control oral malodors [24,33,34]. These therapeutic practices are complementary and used together, and represent the most effective approaches against halitosis. Multiple studies have evaluated the efficacy of an antimicrobial products using a single mouthrinse [35,36,37]. Other trial designs, such as ours, used both mechanical and chemical oral hygiene measures [35] in all participants.
CHX is the most common oral antiseptic, and its effectiveness for reducing of VSCs- producing bacteria has been demonstrated [19,22,38], but it is not exempt from side effects such as staining of the tongue and teeth, bad taste, and a reduction in taste perception [23,39]. Because of this, and to improve the antimicrobial effects on VSC-producing bacteria and consequently reduce oral malodors, new formulations based on a lower concentration of CHX associated to other antiseptics, such as cetylpyridinium chloride (CPC), have been developed. The combination of CHX/CPC/Zn (Halita™) appears to be the most effective to control the halitosis [19,21,25,40]. Zn also plays an important role as an odor-neutralizing agent because it is able to act on VSCs and transform them into nonodoriferous complexes [41,42,43,44,45,46,47].
Other therapeutic options should not be underestimated in the inhibition of oral malodors, including essential oils [48,49], triclosan [38], and oxidizing agents such as chlorine dioxide [35,50] and sodium chlorate [51]. Regarding this, octenidine may also be an additional therapeutic alternative, but there are no existing scientific studies about it. In this study, we compared Lacer Hali™, whose main composition is octenidine dihydrochloride, with a placebo group, and Halita™. The results obtained with Halita™ confirmed previous studies showing its greater efficacy against the placebo [52].
The organoleptic score scale was used to measure the intensity of halitosis due to its ease of use and frequent application in clinical trials to assess odor intensity in the oral cavity [20,35]. However, the results are based on an evaluator’s perception and could introduce bias. Therefore, as in other clinical trials [25,36,41,53], we used a portable gas chromatograph. Both HalimeterTM (Interscan Corporation, Chatsworth, CA, USA) and OralChroma™ are available on the market to determine VSC concentrations. Halimeter® measures the total amount of VSCs and only detects intraoral halitosis. OralChroma™ can differentiate between intraoral and extraoral halitosis, and provides separate values for each sulfide compound (hydrogen sulfide, methyl mercaptan, and dimethyl sulfide) [16,36]. In our study, although we used OralChroma™ and measured all three mentioned gases, we decided to focus our analysis on hydrogen sulfide and methyl mercaptan [54,55]. These two gases are the main contributors to intraoral malodor [3,16,32]. Additionally, methyl mercaptan and hydrogen sulfide are associated with bad odor of the tongue and periodontal pockets, respectively [56], whereas dymetil sulfide is more linked to extraoral causes of halitosis, such as respiratory, metabolic, or drug-associated [57], the menstrual cycle [58] or lifestyle factors such as ingestion of odiferous food [59].
The results obtained with OralChroma™ agree with those analyzed using the organoleptic scale. Several studies have demonstrated a high correlation between organoleptic scores and VSCs measurements obtained by a sulfide monitor or a gas chromatograph, showing that both methods are suitable to diagnose halitosis [45,60]. According to this, in our study, Lacer HaliTM was demonstrated to be significantly better than the placebo at T2. Moreover, a decrease in both organoleptic scores and hydrogen sulfide levels was detected. For the rest of the time, there was no statistically significant difference between Lacer HaliTM and the placebo. However, we can appreciate a minor trend in the decrease of the total VSCs measurement in comparison with the organoleptic results. This finding suggests that other odorous nonsulfur compounds may contribute to oral cavity malodor. Thus, indole, skatole, cadaverine, putrescine, and short-chain fatty acids may play a role in the genesis of halitosis [61,62,63]. On the other hand, although we observed that the placebo seems to reduce methyl mercaptan levels, the difference was not statistically significant and we assume it could be due to chance.
In our study, there were hardly any dropouts (9 of 69), and none of the cases of dropout were due to the development of unpleasant sensations or adverse effects. In our clinical trial, a complete follow-up of three arms with 20 patients each was performed based on a clinical trial conducted with HalitaTM [21], where each of the two arms of the study had 20 participants. Other trial designs, have been performed with HalitaTM, where its efficacy was compared against four groups, and each of the arms consisted of 18 patients [25]; therefore, the sample size of our study could be considered adequate.
The tongue is where most of the bacteria in the mouth are located and is mostly responsible for producing the bad smells. Some studies have incorporated tongue cleaning procedures in their designs, with no beneficial effects on the microbiota [46], although they do reduce the organoleptic measurement index and the tongue coating index [64]. On the other hand, several investigations have described that the major source of VCSs, especially hydrogen sulfide, is the lingual dorsum [65,66,67]. Therefore, LacerHali™ seems to be more efficient for hydrogen sulfide control than for methyl mercaptan control. This suggests that Lacer Hali™ could be a good therapeutic option as a complement of mechanical cleaning for the management of intraoral halitosis, whose prevalent origin is the lingual dorsum.
From T3 to T4 time points the participants of all groups used placebo products in order to check the persistence of the therapeutic effects once the use of the products was suspended. As was expected, the levels of organoleptic scores and hydrogen sulfide increased in the three groups in this period. However, whereas the levels of methyl mercaptan rose for the Lacer HaliTM and placebo group, these decreased for the HalitaTM group. These results can suggest that HalitaTM, due to its chlorhexidine content, had a residual antimicrobial effect (substantivity) that could persist over the time [68,69].
Regarding oral hygiene measures, in some designs [21], the participants were asked to modify their daily cleaning behaviors. In this clinical trial, they were recommended to continue with their usual brushing technique and usual behavior to minimize the risk of bias.
It would have been interesting to include an analysis to quantify odoriferous microorganisms using a polymerase chain reaction (PCR) technique [70,71,72]. However, we consider that combined analysis using organoleptic measurements and VSC analysis is sufficient to test the overall efficacy of Lacer Hali™.
Concerning the study population selection, in our trial we decided to establish the cut-off point at ≥1 and ≥160 ppb for the organoleptic score and VSC concentrations, respectively. Similar values were used in previous studies [21,25,40]. In relation to this, there is a strong heterogeneity in the literature about the threshold of inclusion criteria for the organoleptic score and VSC concentration, being from 1 to 4 and 80 to 300 ppb, respectively [19,24].
It would have been rewarding for our study to have performed additional measurements of nasal breath air, blood analysis, and metabolic and digestive system tests in order to completely discard extraoral halitosis pathologies, but performing these tests exceeded the resources of this study.
Our main goal was to investigate the effects of our intervention in terms of reduction or increase of intraoral halitosis, although a complete elimination of oral malodor was not possible independently of its specific oral origin. Regarding this, from a clinical point of view, it is important for the success of halitosis treatments to identify the source of oral malodor to establish the suitable intervention. Intraoral halitosis caused by periodontitis, gingivitis, lingual dorsum, or other intraoral conditions will require specific additional treatments. It would be interesting for future studies to investigate the effect of this mouthrinse and toothpaste in volunteers with intraoral halitosis classified according to its prevalent origin (e.g., lingual dorsum, gingivitis, or periodontitis). Likewise, it would be clinically useful to determine the effectiveness of this novel mouthrinse depending on the severity of halitosis. Additionally, long-term studies that incorporate identification and quantification techniques for microorganisms are needed to assess the effect of octenidine on microbiota levels in tongue and periodontal localizations, as well as its correlation with VSC levels and organoleptic scores.
In conclusion, although more studies are needed, the results of this clinical trial show that the combination of Lacer Hali™ toothpaste and mouthrinse could be considered an alternative to CHX-based products in the management of halitosis.
Author Contributions
Conceptualization, L.X.; data curation, L.X.; formal analysis, R.R.; investigation, L.X.; methodology, L.X.; supervision, J.C.P.-F.; validation, R.R. and J.C.P.-F.; visualization, R.R.; writing—original draft, L.X. and R.R.; writing—review and editing, J.C.P.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was promoted by Zurko Research, Spain.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Rey Juan Carlos University (internal registration number: 2110202019020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors gratefully thank José I. Herrera, Luz Herrera, and Gema Cidoncha from CORE Dental Clinic, and Fernando Vivancos, for their contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, M.F.; Leite, F.R.M.; Ferreira, L.B.; Pola, N.M.; Scannapieco, F.A.; Demarco, F.F.; Nascimento, G.G. Estimated prevalence of halitosis: A systematic review and meta-regression analysis. Clin. Oral Investig. 2018, 22, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Mongardini, C.; van Steenberghe, D. The effect of a 1-stage full-mouth disinfection on oral malodor and microbial colonization of the tongue in periodontitis. A pilot study. J. Periodontol. 1998, 69, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Tangerman, A. Halitosis in medicine: A review. Int. Dent. J. 2002, 52 (Suppl. 3), 201–206. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). Br. Med. J. 2006, 333, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluijs, E.; Slot, D.E.; Bakker, E.W.P.; Van der Weijden, G.A. The effect of water on morning bad breath: A randomized clinical trial. Int. J. Dent. Hyg. 2016, 14, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Van Steenberghe, D. Breath malodor. Curr. Opin. Periodontol. 1997, 4, 137–143. [Google Scholar] [PubMed]
- Bosy, A.; Kulkarni, G.V.; Rosenberg, M.; McCulloch, C.A.G. Relationship of Oral Malodor to Periodontitis: Evidence of Independence in Discrete Subpopulations. J. Periodontol. 1994, 65, 37–46. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral piercing and oral diseases: A short time retrospective study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral infection by Staphylococcus aureus in patients affected by White Sponge Nevus: A description of two cases occurred in the same family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Non-Hodgkin lymphoma affecting the tongue: Unusual intra-oral location. Head Neck Oncol. 2011, 3, 1. [Google Scholar] [CrossRef]
- Quirynen, M.; Zhao, H.; van Steenberghe, D. Review of the treatment strategies for oral malodour. Clin. Oral Investig. 2002, 6, 1–10. [Google Scholar] [CrossRef]
- Carvalho, M.D.; Tabchoury, C.M.; Cury, J.A.; Toledo, S.; Nogueira-Filho, G.R. Impact of mouthrinses on morning bad breath in healthy subjects. J. Clin. Periodontol. 2004, 31, 85–90. [Google Scholar] [CrossRef]
- Farrell, S.; Baker, R.A.; Somogyi-Mann, M.; Witt, J.J.; Gerlach, R.W. Oral malodor reduction by a combination of chemotherapeutical and mechanical treatments. Clin. Oral Investig. 2006, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Duffield, J.; Spencer, P.; Rosenberg, M.; Corry, D.; Saad, S.; Lenton, P.; Majerus, G.; Nachnani, S.; El-Maaytah, M. Study on the organoleptic intensity scale for measuring oral malodor. J. Dent. Res. 2004, 83, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-J.; Lee, J.-Y.; Kho, H.-S.; Chung, J.-W.; Park, H.-K.; Kim, Y.-K. A New Organoleptic Testing Method for Evaluating Halitosis. J. Periodontol. 2009, 80, 93–97. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. The portable gas chromatograph OralChromaTM: A method of choice to detect oral and extra-oral halitosis. J. Breath Res. 2008, 2. [Google Scholar] [CrossRef]
- Rösing, C.K.; Jonski, G.; Rølla, G. Comparative analysis of some mouthrinses on the production of volatile sulfur-containing compounds. Acta Odontol. Scand. 2002, 60, 10–12. [Google Scholar] [CrossRef]
- Addy, M.; Moran, J.; Wade, W. Chemical Plaque Control in the Prevention of Gingivitis and Periodontitis. In Proceedings of the 1st European Workshop in Periodontology, Thurgau, Switzerland, 1–4 February 1993; Quintessence: London, UK, 1994. [Google Scholar]
- Blom, T.; Slot, D.E.; Quirynen, M.; Van der Weijden, G.A. The effect of mouthrinses on oral malodor: A systematic review. Int. J. Dent. Hyg. 2012, 10, 209–222. [Google Scholar] [CrossRef]
- Jamali, Z.; Alipour, M.; Ebrahimi, S.; Aghazadeh, M. Effect of Halita mouthwash on oral halitosis treatment: A randomized triple-blind clinical trial. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 31–35. [Google Scholar] [CrossRef]
- Roldán, S.; Winkel, E.G.; Herrera, D.; Sanz, M.; Van Winkelhoff, A.J. The effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc lactate on the microflora of oral halitosis patients: A dual-centre, double-blind placebo-controlled study. J. Clin. Periodontol. 2003, 30, 427–434. [Google Scholar] [CrossRef]
- Roldán, S.; Herrera, D.; Santa-Cruz, I.; O’Connor, A.; González, I.; Sanz, M. Comparative effects of different chlorhexidine mouth-rinse formulations on volatile sulphur compounds and salivary bacterial counts. J. Clin. Periodontol. 2004, 31, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, Z.; Aljufairi, H.; Nasser, M.; Outhouse, T.L.; Pedrazzi, V. Mouthrinses for the treatment of halitosis. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Slot, D.E.; De Geest, S.; Van Der Weijden, F.A.; Quirynen, M. Treatment of oral malodour. Medium-term efficacy of mechanical and/or chemical agents: A systematic review. J. Clin. Periodontol. 2015, 42, S303–S316. [Google Scholar] [CrossRef] [PubMed]
- Dadamio, J.; Tournout, M.; Teughels, W.; Dekeyser, C.; Coucke, W.; Quirynen, M. Efficacy of different mouthrinse formulations in reducing oral malodour: A randomized clinical trial. J. Clin. Periodontol. 2013, 40, 505–513. [Google Scholar] [CrossRef]
- Young, A.; Jonski, G.; Rölla, G.; Wåler, S.M. Effects of metal salts on the oral production of volatile sulfur-containing compounds (VSC). J. Clin. Periodontol. 2001, 28, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Navada, R.; Kumari, H.; Le, S.; Zhang, J. Oral malodor reduction from a zinc-containing toothpaste. J. Clin. Dent. 2008, 19, 69–73. [Google Scholar]
- Newby, E.E.; Hickling, J.M.; Hughes, F.J.; Proskin, H.M.; Bosma, M.P. Control of oral malodour by dentifrices measured by gas chromatography. Arch. Oral Biol. 2008, 53 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef]
- Young, A.; Jonski, G. Effect of a single brushing with two Zn-containing toothpastes on VSC in morning breath: A 12 h, randomized, double-blind, cross-over clinical study. J. Breath Res. 2011, 5, 046012. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; McCulloch, C.A.G. Measurement of Oral Malodor: Current Methods and Future Prospects. J. Periodontol. 1992, 63, 776–782. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Intra- and extra-oral halitosis: Finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J. Clin. Periodontol. 2007, 34, 748–755. [Google Scholar] [CrossRef]
- Aung, E.E.; Ueno, M.; Zaitsu, T.; Furukawa, S.; Kawaguchi, Y. Effectiveness of three oral hygiene regimens on oral malodor reduction: A randomized clinical trial. Trials 2015, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Khurana, C.; Tandon, S.; Chinmaya, B.R. A crossover clinical trial to assess the effectiveness of different oral hygiene regimens on the reduction of morning bad breath in healthy young adults. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2018, 29, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Suprono, M.S.; Stephens, J.; Withers, S.A.; Li, Y. Efficacy of stabilized chlorine dioxide-based unflavored mouthwash in reducing oral malodor: An 8-week randomized controlled study. Am. J. Dent. 2018, 31, 309–312. [Google Scholar]
- Van der Sluijs, E.; Van der Weijden, G.A.; Hennequin-Hoenderdos, N.L.; Slot, D.E. The effect of a tooth/tongue gel and mouthwash regimen on morning oral malodour: A 3-week single-blind randomized clinical trial. Int. J. Dent. Hyg. 2018, 16, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Faveri, M.; Guerra, M.C.; Mateo, L.R.; Stewart, B.; Williams, M.; Panagakos, F. The efficacy of two oral hygiene regimens in reducing oral malodour: A randomised clinical trial. Int. Dent. J. 2015, 65, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.; Coimbra, J.; Pereira, A.L.; Resende, M.; Pinto, M.G. Comparative effect of a new mouthrinse containing chlorhexidine, triclosan and zinc on volatile sulphur compounds: A randomized, crossover, double-blind study. Int. J. Dent. Hyg. 2016, 14, 202–208. [Google Scholar] [CrossRef]
- Yadav, S.R.; Kini, V.V.; Padhye, A. Inhibition of Tongue Coat and Dental Plaque Formation by Stabilized Chlorine Dioxide Vs Chlorhexidine Mouthrinse: A Randomized, Triple Blinded Study. J. Clin. Diagn. Res. 2015, 9, ZC69. [Google Scholar] [CrossRef]
- Winkel, E.G.; Roldán, S.; Van Winkelhoff, A.J.; Herrera, D.; Sanz, M. Clinical effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc-lactate on oral halitosis. A dual-center, double-blind placebo-controlled study. J. Clin. Periodontol. 2003, 30, 300–306. [Google Scholar] [CrossRef]
- Erovic Ademovski, S.; Mårtensson, C.; Persson, G.R.; Renvert, S. The long-term effect of a zinc acetate and chlorhexidine diacetate containing mouth rinse on intra-oral halitosis-A randomized clinical trial. J. Clin. Periodontol. 2017, 44, 1010–1019. [Google Scholar] [CrossRef]
- Young, A.; Jonski, G.; Rölla, G. Inhibition of orally produced volatile sulfur compounds by zinc, chlorhexidine or cetylpyridinium chloride--effect of concentration. Eur. J. Oral Sci. 2003, 111, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Jonski, G.; Rölla, G. Combined effect of zinc ions and cationic antibacterial agents on intraoral volatile sulphur compounds (VSC). Int. Dent. J. 2003, 53, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Thrane, P.S.; Young, A.; Jonski, G.; Rölla, G. A new mouthrinse combining zinc and chlorhexidine in low concentrations provides superior efficacy against halitosis compared to existing formulations: A double-blind clinical study. J. Clin. Dent. 2007, 18, 82–86. [Google Scholar] [PubMed]
- Saad, S.; Greenman, J.; Shaw, H. Comparative effects of various commercially available mouthrinse formulations on oral malodor. Oral Dis. 2011, 17, 180–186. [Google Scholar] [CrossRef]
- Ademovski, S.E.; Persson, G.R.; Winkel, E.; Tangerman, A.; Lingström, P.; Renvert, S. The short-term treatment effects on the microbiota at the dorsum of the tongue in intra-oral halitosis patients—A randomized clinical trial. Clin. Oral Investig. 2013, 17, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Jervøe-Storm, P.-M.; Schulze, H.; Jepsen, S. A randomized cross-over short-term study on the short-term effects of a zinc-lactate containing mouthwash against oral malodour. J. Breath Res. 2019, 13, 26005. [Google Scholar] [CrossRef]
- Chung, S.-H. The effects of an essential oil mouthrinse on oral health in the community indwelling elderly. Taehan Kanho Hakhoe Chi 2006, 36, 84–93. [Google Scholar] [CrossRef]
- Satthanakul, P.; Taweechaisupapong, S.; Paphangkorakit, J.; Pesee, M.; Timabut, P.; Khunkitti, W. Antimicrobial effect of lemongrass oil against oral malodour micro-organisms and the pilot study of safety and efficacy of lemongrass mouthrinse on oral malodour. J. Appl. Microbiol. 2015, 118, 11–17. [Google Scholar] [CrossRef]
- Frascella, J.; Gilbert, R.D.; Fernandez, P.; Hendler, J. Efficacy of a chlorine dioxide-containing mouthrinse in oral malodor. Compend. Contin. Educ. Dent. 2000, 21, 241–244, 246, 248 passim; quiz 256. [Google Scholar]
- Kim, J.-S.; Park, J.-W.; Kim, D.-J.; Kim, Y.-K.; Lee, J.-Y. Direct effect of chlorine dioxide, zinc chloride and chlorhexidine solution on the gaseous volatile sulfur compounds. Acta Odontol. Scand. 2014, 72, 645–650. [Google Scholar] [CrossRef]
- Alsaffar, D.; Alzoman, H. Efficacy of antioxidant mouthwash in the reduction of halitosis: A randomized, double blind, controlled crossover clinical trial. J. Dent. Sci. 2021, 16, 621–627. [Google Scholar] [CrossRef]
- Erovic Ademovski, S.; Lingström, P.; Renvert, S. The effect of different mouth rinse products on intra-oral halitosis. Int. J. Dent. Hyg. 2016, 14, 117–123. [Google Scholar] [CrossRef]
- Wilhelm, D.; Himmelmann, A.; Krause, C.; Wilhelm, K.-P. Short term clinical efficacy of new meridol HALITOSIS tooth & tongue gel in combination with a tongue cleaner to reduce oral malodor. J. Clin. Dent. 2013, 24, 12–19. [Google Scholar] [PubMed]
- Wilhelm, D.; Gysen, K.; Himmelmann, A.; Krause, C.; Wilhelm, K.-P. Short-term effect of a new mouthrinse formulation on oral malodour after single use in vivo: A comparative, randomized, single-blind, parallel-group clinical study. J. Breath Res. 2010, 4, 36002. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; MacHigashira, M.; Yamashita, D.; Kozono, S.; Nakajima, Y.; Miyamoto, M.; Takeuchi, N.; Setoguchi, T.; Noguchi, K. The association of periodontal disease with oral malodour in a Japanese population. Oral Dis. 2010, 16, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Fujiyama, Y.; Yamaga, T.; Miyazaki, H. Breath malodor in an asthmatic patient caused by side-effects of medication: A case report and review of the literature. Oral Dis. 2003, 9, 273–276. [Google Scholar] [CrossRef]
- Suarez, F.; Springfield, J.; Furne, J.; Levitt, M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am. J. Physiol. 1999, 276, G425–G430. [Google Scholar] [CrossRef]
- Kawamoto, A.; Sugano, N.; Motohashi, M.; Matsumoto, S.; Ito, K. Relationship between oral malodor and the menstrual cycle. J. Periodontal Res. 2010, 45, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; El-Maaytah, M.; Duffield, J.; Spencer, P.; Rosenberg, M.; Corry, D.; Saad, S.; Lenton, P.; Majerus, G.; Nachnani, S. Assessing the relationship between concentrations of malodor compounds and odor scores from judges. J. Am. Dent. Assoc. 2005, 136, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Kozlovsky, A.; Gordon, D.; Gelernter, I.; Sintov, A.; Rosenberg, M. Cadaverine as a putative component of oral malodor. J. Dent. Res. 1994, 73, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Kazor, C. Microbiology and treatment of halitosis. Periodontol. 2000 2002, 28, 256–279. [Google Scholar] [CrossRef]
- Kazor, C.E.; Mitchell, P.M.; Lee, A.M.; Stokes, L.N.; Loesche, W.J.; Dewhirst, F.E.; Paster, B.J. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 2003, 41, 558–563. [Google Scholar] [CrossRef]
- Oliveira-Neto, J.M.; Sato, S.; Pedrazzi, V. How to deal with morning bad breath: A randomized, crossover clinical trial. J. Indian Soc. Periodontol. 2013, 17, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Washio, J.; Sato, T.; Koseki, T.; Takahashi, N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J. Med. Microbiol. 2005, 54, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhang, Y.; He, M.; Zhu, C.; Feng, X.-P. Relationship of tongue coating microbiome on volatile sulfur compounds in healthy and halitosis adults. J. Breath Res. 2019, 14, 16005. [Google Scholar] [CrossRef] [PubMed]
- Seerangaiyan, K.; Jüch, F.; Winkel, E.G. Tongue coating: Its characteristics and role in intra-oral halitosis and general health—A review. J. Breath Res. 2018, 12, 34001. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontol. 2000 1997, 15, 55–62. [Google Scholar] [CrossRef]
- García-Caballero, L.; Quintas, V.; Prada-López, I.; Seoane, J.; Donos, N.; Tomás, I. Chlorhexidine substantivity on salivary flora and plaque-like biofilm: An in situ model. PLoS ONE 2013, 8, e83522. [Google Scholar] [CrossRef]
- Adedapo, A.H.; Kolude, B.; Dada-Adegbola, H.O.; Lawoyin, J.O.; Adeola, H.A. Targeted polymerase chain reaction-based expression of putative halitogenic bacteria and volatile sulphur compound analysis among halitosis patients at a tertiary hospital in Nigeria. Odontology 2020, 108, 450–461. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ohmori, M.; Sato, S. The relationship between physiologic halitosis and periodontopathic bacteria of the tongue and gingival sulcus. Odontology 2010, 98, 44–51. [Google Scholar] [CrossRef]
- Kamaraj, D.; Bhushan, K.S. An evaluation of microbial profile in halitosis with tongue coating using PCR (polymerase chain reaction)—A clinical and microbiological study. J. Clin. Diagn. Res. 2014, 8, 263. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).