Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non–ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials ant Methods
2.1. Study Population
2.2. Angiographic Analysis
2.3. Nutritional Status Measurement Tools
2.4. Study Endpoint
2.5. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Characteristics of Malnourished Patients
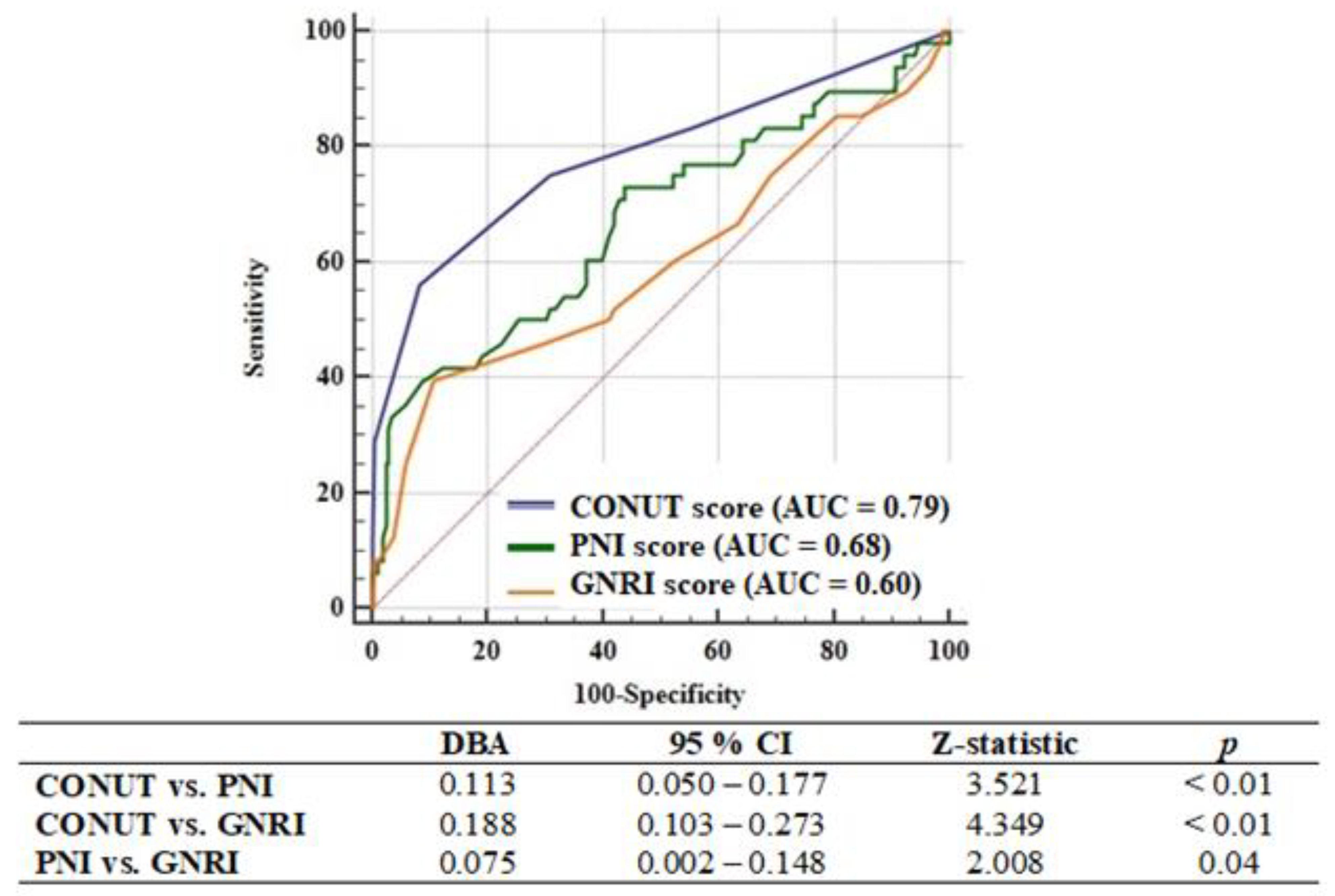
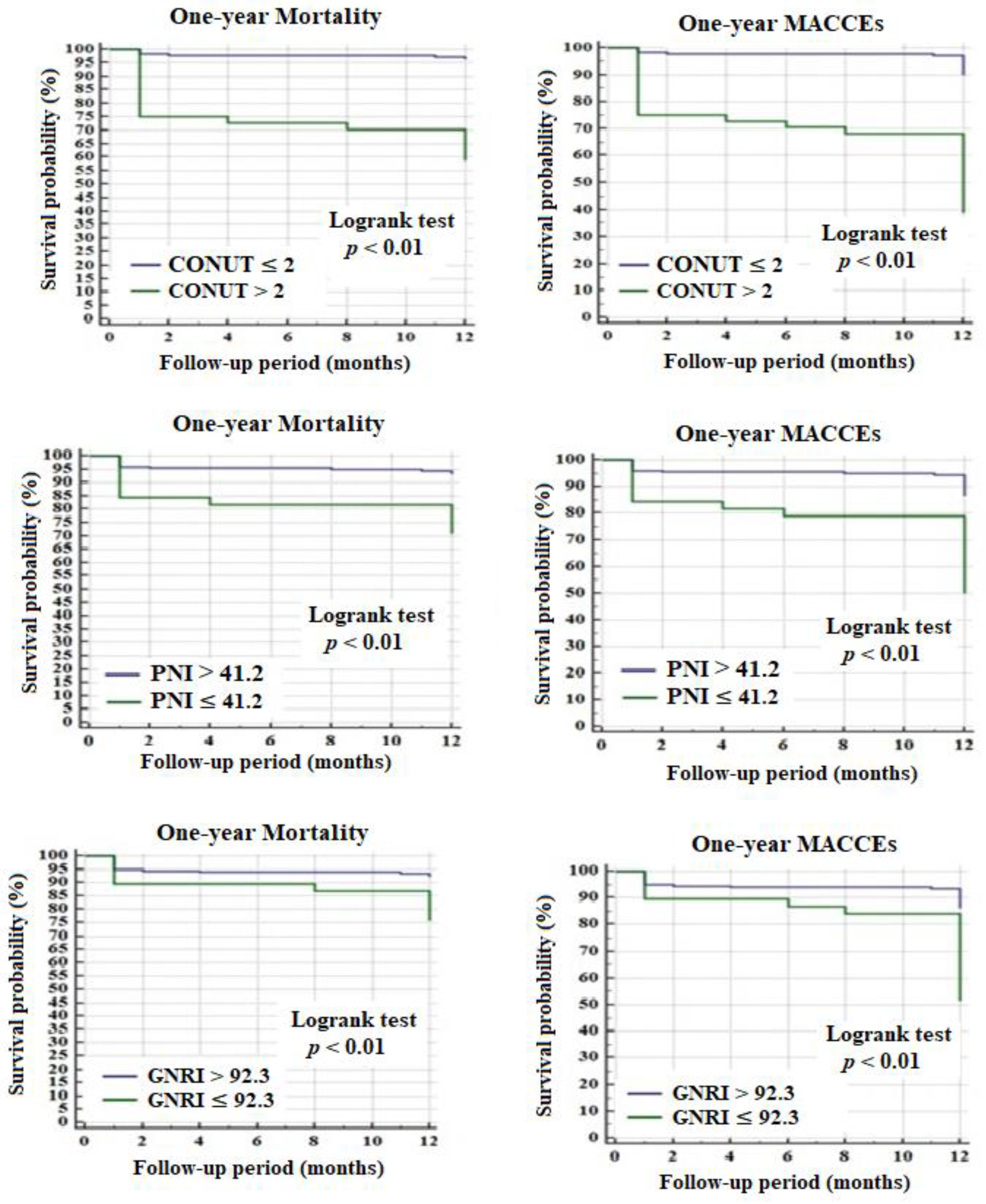
3.2. Factors Associated with One-Year Major Adverse Cardiac and Cerebrovascular Events
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, X.; Busby-Whitehead, J.; Alexander, K.P. Acute coronary syndrome in the older adults. J. Geriatr. Cardiol. 2016, 13, 101–108. [Google Scholar] [CrossRef]
- Reaño, J.D.P.; Shiu, L.A.B.; Miralles, K.V.; Dimalala, M.G.C.; Pestaño, N.S.; Punzalan, F.E.R.; Tumanan-Mendoza, B.; Reyes, M.J.T.; Castillo, R.R. A systematic review and meta-analysis on the effectiveness of an invasive strategy compared to a conservative approach in patients > 65 years old with non-ST elevation acute coronary syndrome. PLoS ONE 2020, 15, e0229491. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Pichard, C.; Lochs, H.; Matthias, P. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.S.; Magno, C.P.; Lane, K.T.; Chan, T.; Hoyt, D.B.; Greenfield, S. Nutrition impacts the prevalence of peripheral arterial disease in the United States. J. Vasc. Surg. 2008, 48, 897–904. [Google Scholar] [CrossRef]
- Kato, T.; Yaku, H.; Morimoto, T.; Inuzuka, Y.; Tamaki, Y.; Yamamoto, E.; Yoshikawa, Y.; Kitai, T.; Taniguchi, R.; Iguchi, M.; et al. Association with Controlling Nutritional Status (CONUT) Score and In-hospital Mortality and Infection in Acute Heart Failure. Sci. Rep. 2020, 10, 3320. [Google Scholar] [CrossRef]
- Honda, Y.; Nagai, T.; Iwakami, N.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Usefulness of Geriatric Nutritional Risk Index for Assessing Nutritional Status and Its Prognostic Impact in Patients Aged ≥65 Years with Acute Heart Failure. Am. J. Cardiol. 2016, 118, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Luo, L.; Zhao, X.; Ye, P. Controlling Nutritional Status (CONUT) score as a predictor of all-cause mortality in elderly hypertensive patients: A prospective follow-up study. BMJ Open 2017, 187, e015649. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ahn, J.M.; Kang, D.Y.; Ko, E.; Kwon, O.; Lee, P.H.; Lee, S.W.; Kim, D.H.; Kim, H.J.; Kim, J.B.; et al. Nutritional status and risk of all-cause mortality in patients undergoing transcatheter aortic valve replacement assessment using the geriatric nutritional risk index and the controlling nutritional status score. Clin. Res. Cardiol. 2020, 109, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras-Roubin, S.; Abu-Assi, E.; Paz, R.C.; Rosselló, X.; Barreiro Pardal, C.; Piñón Esteban, M.; Pascual, C.R.; García Comesaña, J.; González-Carrero López, A.; Caneiro-Queija, B.; et al. Impact of malnutrition in the embolic haemorrhagic trade-off of elderly patients with atrial fibrillation. Europace 2020, 22, 878–887. [Google Scholar] [CrossRef]
- Basta, G.; Chatzianagnostou, K.; Paradossi, U.; Botto, N.; Del Turco, S.; Taddei, A.; Berti, S.; Mazzone, A. The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int. J. Cardiol. 2016, 221, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras Roubín, S.; Abu Assi, E.; Cespón Fernandez, M.; Barreiro Pardal, C.; Lizancos Castro, A.; Parada, J.A.; Pérez, D.D.; Blanco Prieto, S.; Rossello, X.; Ibanez, B.; et al. Prevalence and Prognostic Significance of Malnutrition in Patients with Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 76, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, ehaa575. [Google Scholar] [CrossRef]
- Liu, G.Y.; Meng, X.X.; Zhang, Z. Triglyceride to HDL-cholesterol ratio as an independent risk factor for the poor development of coronary collateral circulation in elderly patients with ST-segment elevation myocardial infarction and acute total occlusion. Medicine 2018, 97, e12587. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.A.; van Es, G.A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Novotny, N.L. Clinical Prediction Model of Medical Inpatients at Risk of Early Readmission: Development and Validation; University of Illinois at Chicago, Health Sciences Center: Chicago, IL, USA, 2008; pp. 82–83. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Hickson, M. Malnutrition and ageing. Postgrad. Med. J. 2006, 82, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.T.; Peng, Y.; Liu, W.; Zhang, C.; Chai, H.; Huang, F.Y.; Zuo, Z.L.; Liao, Y.B.; Xia, T.L.; Chen, M. Nutritional State Predicts All-Cause Death Independent of Comorbidities in Geriatric Patients with Coronary Artery Disease. J. Nutr. Health Aging 2016, 20, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, T.Y.; Cheng, Y.J.; Ma, Y.; Xu, Y.K.; Yang, J.Q.; Zhou, Y.J. Impacts of geriatric nutritional risk index on prognosis of patients with non-ST-segment elevation acute coronary syndrome: Results from an observational cohort study in China. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1685–1696. [Google Scholar] [CrossRef]
- Chen, Q.J.; Qu, H.J.; Li, D.Z.; Li, X.M.; Zhu, J.J.; Xiang, Y.; Li, L.; Ma, Y.T.; Yang, Y.N. Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci. Rep. 2017, 7, 3285. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Li, D.; Liang, L.; Jia, Y.; Zou, L.; Li, F.; Zhu, X.; Qian, H.; He, N.; et al. Prognostic nutritional index may not be a good prognostic indicator for acute myocardial infarction. Sci. Rep. 2019, 9, 14717. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncuoglu, M.; Durmus, G. Relationship between C-reactive protein-to-albumin ratio and the extent of coronary artery disease in patients with non-ST-elevated myocardial infarction. Coron. Artery Dis. 2020, 31, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncuoglu, M.; Biter, H.I.; Ozturk, S.; Belen, E.; Can, M.M. Predictive accuracy of lymphocyte-to-monocyte ratio and monocyte-to-high-density-lipoprotein-cholesterol ratio in determining the slow flow/no-reflow phenomenon in patients with non-ST-elevated myocardial infarction. Coron. Artery Dis. 2020, 31, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Berton, G.; Cordiano, R.; Mahmoud, H.T.; Palmieri, R.; Cavuto, F.; Pasquinucci, M. Baseline plasma lipid levels in patients with acute coronary syndrome: Association with 20-year mortality. The ABC-5a Study on Heart Disease. Int. J. Clin. Pract. 2020, 74, e13492. [Google Scholar] [CrossRef]
- Wang, T.Y.; Newby, L.K.; Chen, A.Y.; Mulgund, J.; Roe, M.T.; Sonel, A.F.; Bhatt, D.L.; DeLong, E.R.; Ohman, E.M.; Gibler, W.B.; et al. Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome. Clin. Cardiol. 2009, 32, E22–E28. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Takahashi, P.Y.; Major, B.; Ahmmad, E.; Al-Zubi, H.; Peters, J.; Doyle, T.; Jensen, K.; Al Ward, R.Y.; Sharma, U.; et al. Association between hyperlipidemia and mortality after incident acute myocardial infarction or acute decompensated heart failure: A propensity score matched cohort study and a meta-analysis. BMJ Open 2019, 9, e028638. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Fatyga, P.; Pac, A.; Fedyk-Łukasik, M.; Grodzicki, T.; Skalska, A. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur. Geriatr. Med. 2020, 11, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sueta, D.; Hokimoto, S.; Sakamoto, K.; Akasaka, T.; Tabata, N.; Kaikita, K.; Honda, O.; Naruse, M.; Ogawa, H. Multi-center Study of Hemodialysis Patients Undergoing Invasive Cardiovascular Procedures Study Investigators. Validation of the high mortality rate of Malnutrition-Inflammation-Atherosclerosis syndrome: Community-based observational study. Int. J. Cardiol. 2017, 230, 97–102. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Normal | Light | Moderate | Severe |
|---|---|---|---|---|
| Serum Albumin (g/dL) | 3.5–4.5 | 3.0–3.49 | 2.5–2.9 | <2.5 |
| Score | 0 | 2 | 4 | 6 |
| Total Lymphocytes (109/L) | >1.60 | 1.20–1.59 | 0.80–1.19 | <0.80 |
| Score | 0 | 1 | 2 | 3 |
| Total Cholesterol (mg/dL) | >180 | 140–180 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Total Score | 0–1 | 2–4 | 5−8 | 9–12 |
| Variables | All Population (n = 253) | Non-Malnourished (n = 155) | Malnourished (n = 98) | p-Value |
|---|---|---|---|---|
| Male gender, n (%) | 181 (71.5) | 112 (72.3) | 69 (70.4) | 0.75 |
| Age, years, ± SD | 68.5 ± 6.9 | 66.9 ± 6.1 | 70.9 ± 7.3 | <0.01 |
| BMI, kg/m2, ± SD | 28 ± 2.8 | 28.5 ± 2.6 | 27.2 ± 3.0 | <0.01 |
| Hypertension, n (%) | 136 (53.8) | 80 (51.6) | 56 (57.1) | 0.39 |
| Diabetes mellitus, n (%) | 74 (29.2) | 41 (26.5) | 33 (33.7) | 0.22 |
| Dyslipidemia, n (%) | 156 (61.7) | 91 (58.7) | 65 (66.3) | 0.23 |
| Smoking, n (%) | 94 (37.2) | 62 (40) | 32 (32.7) | 0.24 |
| Family history, n (%) | 93 (36.8) | 52 (33.5) | 41 (41.8) | 0.18 |
| CAD history, n (%) | 77 (37.4) | 41 (26.5) | 36 (36.7) | 0.08 |
| CHF history, n (%) | 38 (15) | 17 (11) | 21 (22.4) | 0.02 |
| Killip III-IV, n (%) | 34 (13.4) | 10 (6.5) | 24 (24.5) | <0.01 |
| LVEF,%, ± SD | 49.2 ± 7.6 | 50.9 ± 6.4 | 46.5 ± 8.4 | <0.01 |
| Grace risk score, ± SD | 118.8 ± 18.5 | 113.6 ± 14.1 | 127 ± 21.4 | <0.01 |
| Syntax Score I, (IQR) | 12 (8–18) | 10 (7–15) | 15 (11–24) | <0.01 |
| Syntax Score II for PCI, (IQR) | 29 (24–37) | 27 (23–33) | 34 (28–43) | <0.01 |
| 30-day Mortality, n (%) | 15 (5.9) | 1 (0.6) | 14 (14.3) | <0.01 |
| One-year Mortality, n (%) | 26 (10.3) | 4 (2.6) | 22 (22.4) | <0.01 |
| One-year MACCEs, n (%) | 48 (19) | 12 (7.7) | 36 (36.7) | <0.01 |
| Medications, n (%) | ||||
| Acetylsalicyclic acid | 90 (35.6) | 51 (32.9) | 39 (39.8) | 0.27 |
| ADP receptor antagonists | 14 (5.5) | 7 (4.5) | 7 (7.1) | 0.37 |
| Anticoagulant | 21 (8.3) | 11 (7.1) | 10 (10.2) | 0.39 |
| Beta-blockers | 82 (32.4) | 45 (29) | 37 (37.8) | 0.15 |
| ACEI | 67 (26.5) | 38 (24.5) | 29 (29.6) | 0.37 |
| ARB | 59 (23.3) | 33 (21.3) | 26 (26.5) | 0.34 |
| CCBs | 54 (21.3) | 31 (20) | 23 (23.5) | 0.51 |
| Anti-anginal agents | 24 (9.5) | 11(7.1) | 13 (13.3) | 0.10 |
| Statin | 57 (22.5) | 31 (20) | 25 (25.5) | 0.30 |
| Fibrats | 26 (10.3) | 17 (11) | 9 (9.2) | 0.65 |
| OADs | 71 (28.1) | 40 (25.8) | 31 (31.6) | 0.32 |
| Insulin | 27 (10.7) | 14 (9) | 13 (13.3) | 0.29 |
| Variables | All Population (n = 253) | Non-Malnourished (n = 155) | Malnourished (n = 98) | p-Value |
|---|---|---|---|---|
| FBG, mg/dL, (IQR) | 123 (102–169) | 119 (102–166) | 127 (103–178) | 0.29 |
| eGFR, mL/min/1.73 m2, ±SD | 79 ± 20 | 82 ± 18 | 74 ± 21 | <0.01 |
| Total cholesterol, mg/dL, ±SD | 207 ± 42 | 217 ± 38 | 192 ± 44 | <0.01 |
| LDL-C, mg/dL, ±SD | 135 ± 35 | 142 ± 33 | 124 ± 35 | <0.01 |
| HDL-C, mg/dL, ±SD | 43 ± 10 | 44 ± 10 | 41 ± 11 | 0.02 |
| Triglyceride, mg/dL, (IQR) | 145 (104–195) | 149 (112–208) | 135 (98–183) | <0.01 |
| Albumin, g/L, ±SD | 37.4 ± 3.5 | 38.7 ± 3.0 | 35.3 ± 3.2 | <0.01 |
| Haemoglobin, g/dL, ±SD | 13.0 ± 1.9 | 13.6 ± 1.6 | 12.6 ± 2.2 | <0.01 |
| Neutrophil, 103/μL, (IQR) | 6.0 (4.4–8.1) | 5.7 (4.3–7.9) | 6.2 (4.7–8.6) | 0.01 |
| Lymphocyte, 109/L, (IQR) | 1.9 (1.3–2.4) | 2.1 (1.7–2.5) | 1.2 (1.0–1.8) | <0.01 |
| Platelet, 109/L, ±SD | 234 ± 71 | 235 ± 74 | 233 ± 66 | 0.81 |
| CRP, mg/dL, (IQR) | 6.9 (4.0–13) | 6.4 (3.8–11.9) | 9.3 (4.1–18.1) | 0.55 |
| PNI score, ±SD | 46.9 ± 5.9 | 49.6 ± 4.5 | 42.7 ± 4.0 | <0.01 |
| GNRI score, ±SD | 97.5 ± 5.2 | 99.4 ± 4.5 | 94.5 ± 4.8 | <0.01 |
| Univariate | Model 1 Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| Age | 1.055 (1.015–1.097) | <0.01 | 1.028 (0.986–1.072) | 0.19 |
| BMI | 0.886 (0.798–0.985) | 0.03 | 1.009 (0.899–1.134) | 0.88 |
| Diabetes mellitus | 2.172 (1.231–3.834) | <0.01 | 1.995 (1.115–3.570) | 0.02 |
| LVEF | 0.888 (0.857–0.919) | <0.01 | 0.890 (0.851–0.931) | <0.01 |
| eGFR | 0.979 (0.966–0.992) | <0.01 | 0.996 (0.980–1.012) | 0.61 |
| Total cholesterol | 0.992 (0.985–0.999) | <0.01 | 0.998 (0.991–1.004) | 0.46 |
| Lymphocyte | 0.473 (0.301–0.744) | 0.03 | 0.624 (0.402–0.968) | 0.04 |
| Albumin | 0.907 (0.830–0.990) | <0.01 | 1.066 (0.966–1.177) | 0.20 |
| CONUT Score | 1.731 (1.503–1.993) | 0.03 | - | - |
| PNI score | 0.918 (0.868–0.970) | <0.01 | - | - |
| GNRI score | 0.951 (0.896–1.009) | 0.09 | - | - |
| Variables | Model 2 Multivariate | Model 3 Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Age | 1.005 (0.960–1.052) | 0.82 | 1.027 (0.984–1.073) | 0.22 |
| BMI | 1.007 (0.897–1.131) | 0.91 | 1.004 (0.896–1.125) | 0.94 |
| Diabetes mellitus | 1.852 (1.034–3.315) | 0.04 | 2.072 (1.161–3.698) | 0.01 |
| eGFR | 0.999 (0.983–1.015) | 0.91 | 0.995 (0.979–1.011) | 0.53 |
| LVEF | 0.919 (0.879–0.961) | <0.01 | 0.897 (0.861–0.934) | <0.01 |
| CONUT score | 1.434 (1.194–1.723) | <0.01 | - | - |
| PNI score | - | - | 0.979 (0.928–1.032) | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalyoncuoğlu, M.; Katkat, F.; Biter, H.I.; Cakal, S.; Tosu, A.R.; Can, M.M. Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non–ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2021, 10, 2247. https://doi.org/10.3390/jcm10112247
Kalyoncuoğlu M, Katkat F, Biter HI, Cakal S, Tosu AR, Can MM. Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non–ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2021; 10(11):2247. https://doi.org/10.3390/jcm10112247
Chicago/Turabian StyleKalyoncuoğlu, Muhsin, Fahrettin Katkat, Halil Ibrahim Biter, Sinem Cakal, Aydin Rodi Tosu, and Mehmet Mustafa Can. 2021. "Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non–ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention" Journal of Clinical Medicine 10, no. 11: 2247. https://doi.org/10.3390/jcm10112247
APA StyleKalyoncuoğlu, M., Katkat, F., Biter, H. I., Cakal, S., Tosu, A. R., & Can, M. M. (2021). Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non–ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine, 10(11), 2247. https://doi.org/10.3390/jcm10112247







