Risk Stratification after an Acute Coronary Syndrome: Significance of Antithrombotic Therapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with St-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with St-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent St-Segment Elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent St-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Neumann, J.F.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided De-Escalation of Antiplatelet Treatment in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention (Tropical-Acs): A Randomised, Open-Label, Multicentre Trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Averkova, O.A.; Brazhnik, V.A.; Koroleva, O.S.; Zubova, E.A.; Hazarov, N.R.; Chichkov, Y.M.; Chichkova, M.A.; Kozlova, O.S.; Kovalenko, N.V.; Kosmacheva, E.D.; et al. Acute Coronary Syndrome in Young Patients with Familial Hypercholesterolemia Based on the Results of ORACLE II Observation Trial. Med. News North Cauc. 2017, 12, 5–8. [Google Scholar]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Zanchin, T.; Temperli, F.; Karagiannis, A.; Zanchin, C.; Rasanen, M.; Koskinas, K.C.; Stortecky, S.; Hunziker, L.; Praz, F.; Blochlinger, S.; et al. Frequency, Reasons, and Impact of Premature Ticagrelor Discontinuation in Patients Undergoing Coronary Revascularization in Routine Clinical Practice: Results from the Bern Percutaneous Coronary Intervention Registry. Circ. Cardiovasc. Interv. 2018, 11, e006132. [Google Scholar] [CrossRef] [PubMed]
- Angeras, O.; Hasvold, P.; Thuresson, M.; Deleskog, A.; Braun, O.O. Treatment Pattern of Contemporary Dual Antiplatelet Therapies after Acute Coronary Syndrome: A Swedish Nationwide Population-Based Cohort Study. Scand. Cardiovasc. J. 2016, 50, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.M.; Peterson, E.D.; McCoy, L.A.; Effron, M.B.; Anstrom, K.J.; Henry, T.D.; Baker, B.A.; Messenger, J.C.; Cohen, D.J.; Wang, T.Y.; et al. Switching of Adenosine Diphosphate Receptor Inhibitor after Hospital Discharge among Myocardial Infarction Patients: Insights from the Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (Translate-Acs) Observational Study. Am. Heart J. 2017, 183, 62–68. [Google Scholar] [PubMed]
- Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Knot, J.; Varvarovsky, I.; Dusek, J.; Jarkovsky, J.; Miklik, R.; Rokyta, R.; et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated with Prasugrel Versus Ticagrelor. J. Am. Coll. Cardiol. 2018, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Aleman, L.; Marin, F.; Rivera-Caravaca, J.M.; Vicente-Ibarra, N.; Candela-Sanchez, E.; Esteve-Pastor, M.A.; Lozano, T.; Sandin-Rollan, M.; Pernias-Escrig, V.; Macias, M.; et al. Switching of Oral P2y12 Inhibitor Treatment in Patients with Acute Coronary Syndrome: Prevalence, Predictors, and Prognosis. Clin. Drug Investig. 2019, 39, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.; Kim, J.H.; Tan, S.Y.D.; Leong, W.Q.; Syed, S.I.; Leow, K.L.; Wee, W.L.E. Switching from Ticagrelor to Clopidogrel in Asian Patients with St-Elevated Myocardial Infarction—A Time Dependent Analysis Study. Acta Cardiol. Sin. 2020, 36, 8–15. [Google Scholar] [PubMed]
- Wang, C.; Zheng, W.; Shaqdan, A.; Wang, C.; Qin, X.; Zhao, X.; Wang, X.; Yuan, L.; Nie, S.; Liu, R. Efficacy and Safety of Switching from Ticagrelor to Clopidogrel During the Early and Late Phase in Acute Coronary Syndrome Patients after Percutaneous Coronary Intervention. Platelets 2020, 31, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Deharo, P.; Quilici, J.; Johnson, T.W.; Deffarges, S.; Bassez, C.; Bonnet, G.; Fourcade, L.; Mouret, J.P.; Lambert, M.; et al. of Switching Dual Antiplatelet Therapy after Acute Coronary Syndrome: The Topic (Timing of Platelet Inhibition after Acute Coronary Syndrome) Randomized Study. Eur. Heart J. 2017, 38, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, M.; Lv, Y.H.; Zhang, M.B.; Wang, Z.L. De-Escalation Versus Standard Dual Antiplatelet Therapy in Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Platelets 2020, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Coons, J.C.; Iasella, C.J.; Chanas, T.; Wang, N.; Williams, K.; Boyd, A.; Lyons, J.; Eckardt, J.; Rihtarchik, L.; Merkel, A.; et al. Comparative Effectiveness and Safety Analysis of Dual Antiplatelet Therapies within an Integrated Delivery System. Ann. Pharmacother. 2017, 51, 649–655. [Google Scholar] [CrossRef] [PubMed]
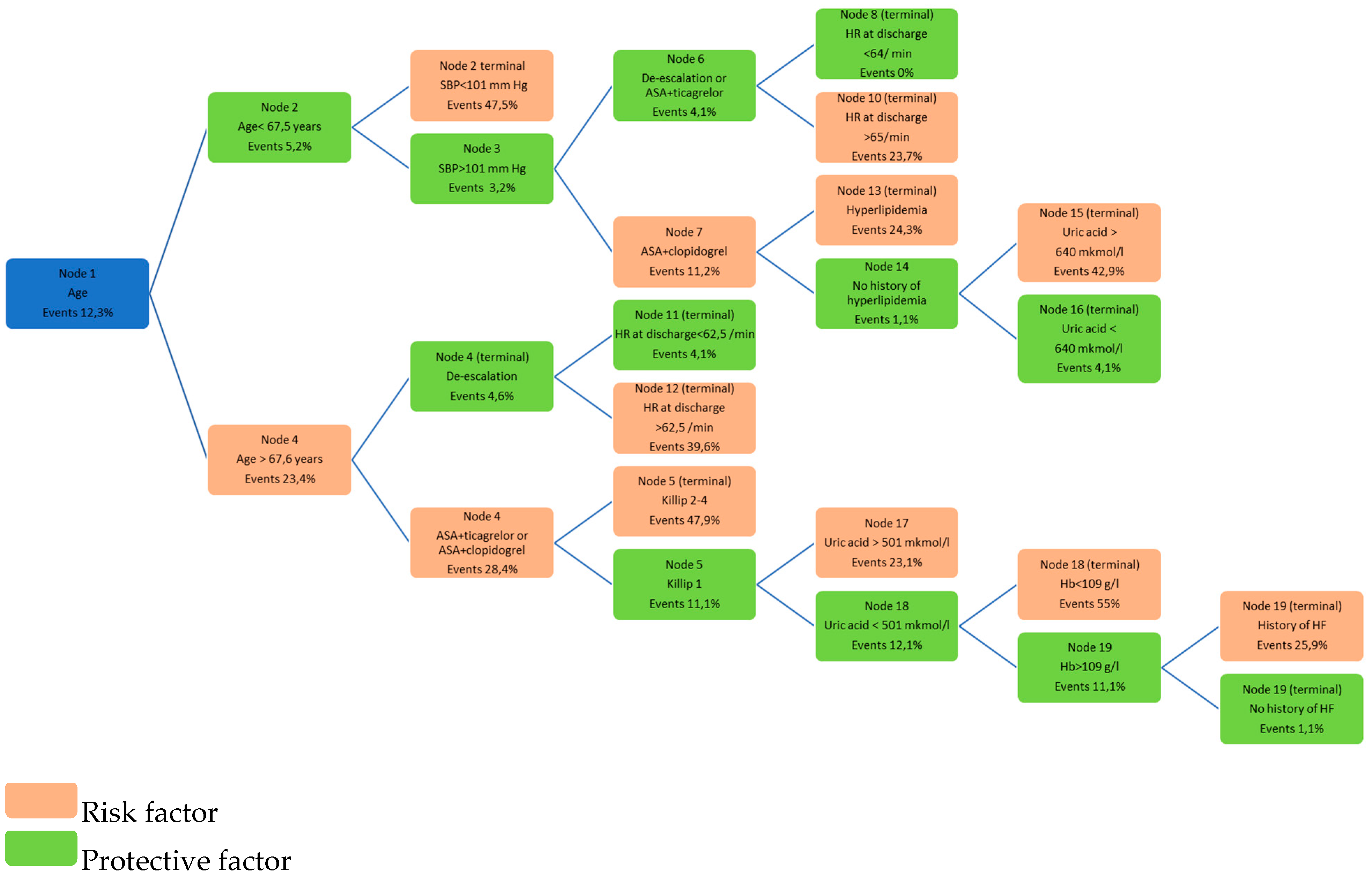
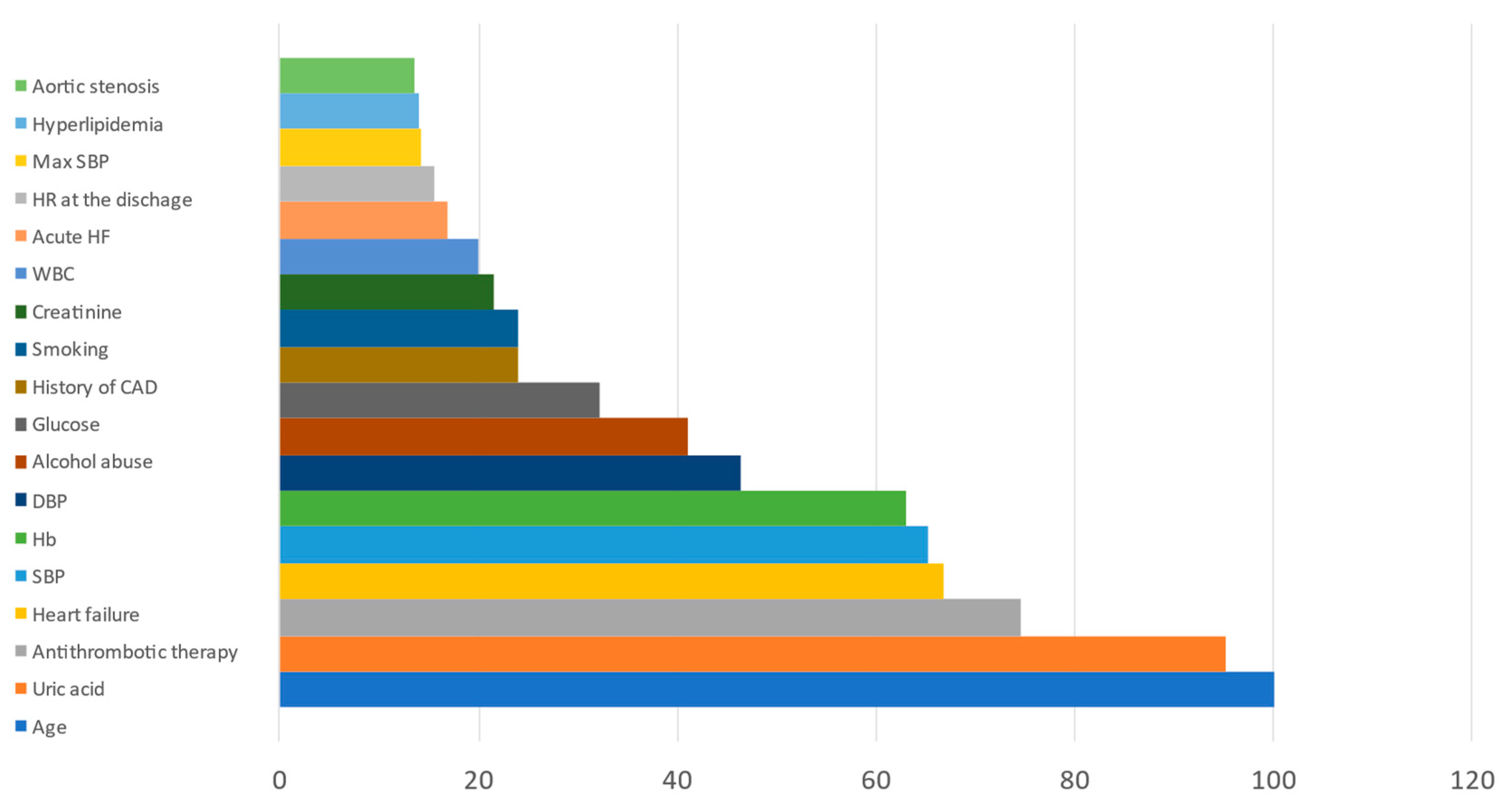
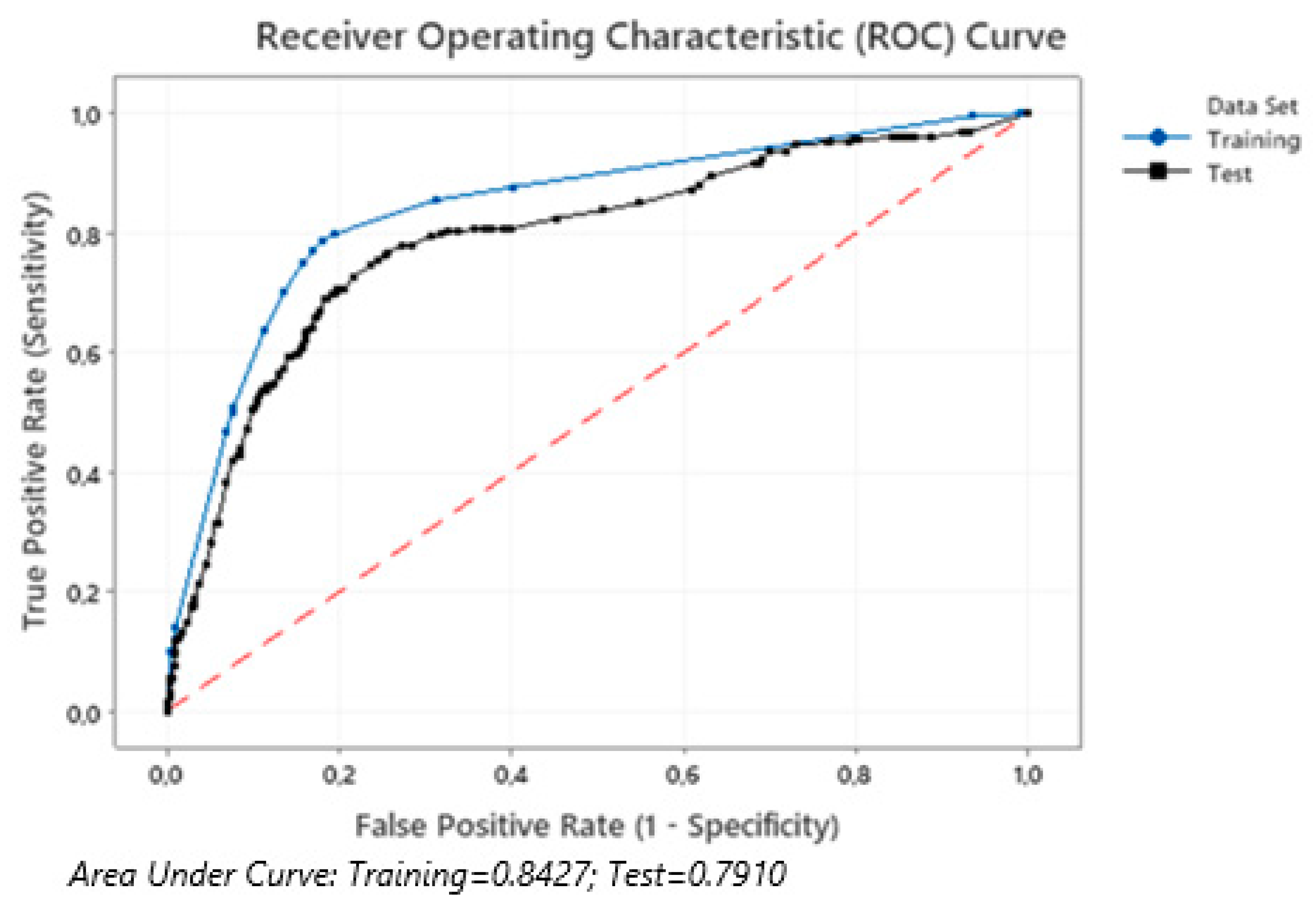
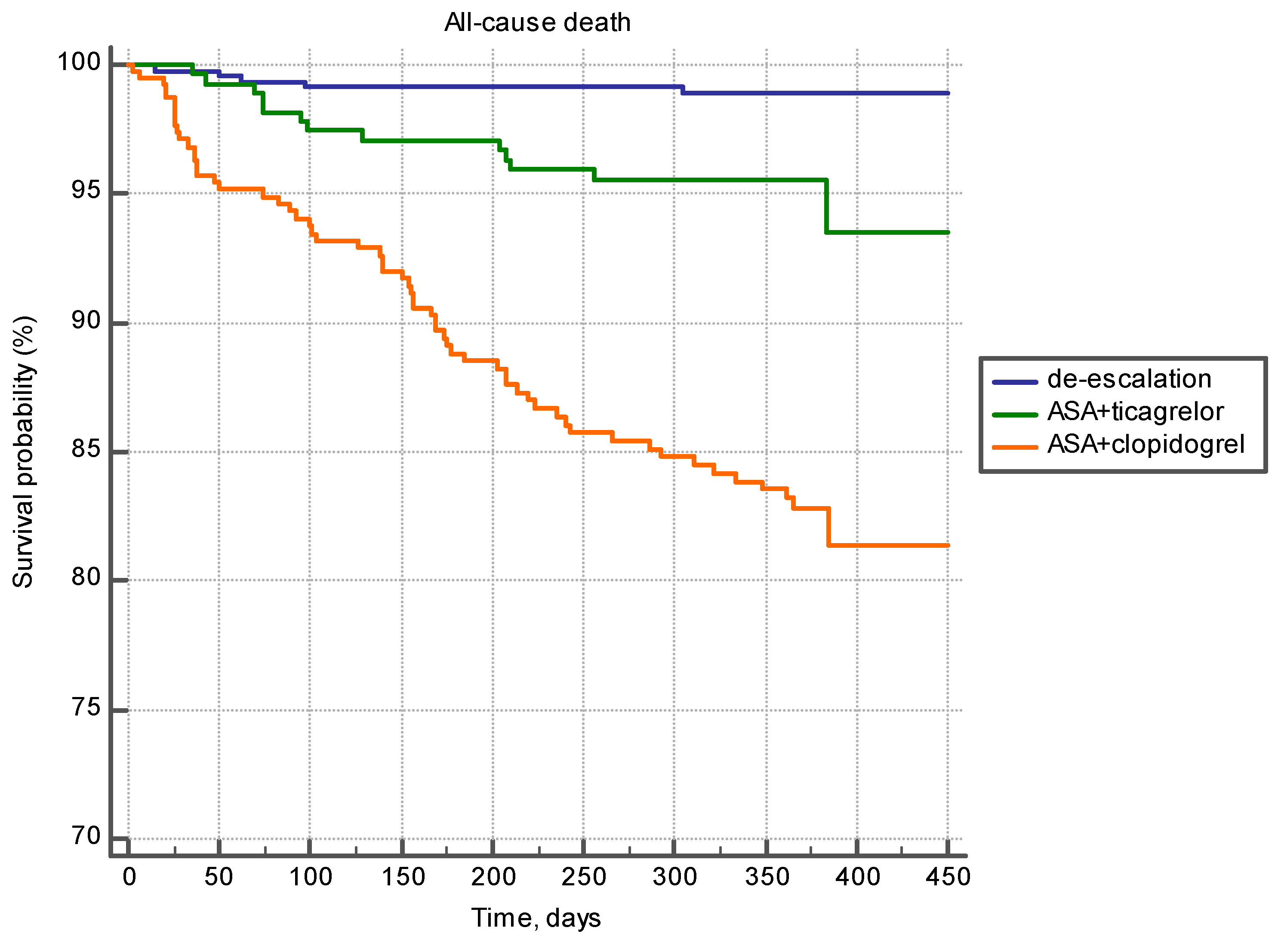

| Visit/Reason | Inpatient Care (Primary Hospital Admission) | 25 Days | 90 Days | 180 Days | 360 Days |
|---|---|---|---|---|---|
| All-cause mortality, including: | 104 | 30 | 29 | 30 | 35 |
| - coronary | 90 | 19 | 9 | 17 | 9 |
| - stroke | 2 | 2 | 4 | 1 | 4 |
| - pulmonary embolism (PE) | 2 | 1 | 1 | 2 | |
| - chronic heart failure (CHF) | 4 | 5 | 5 | 5 | 6 |
| - other | 6 | – | 7 | 5 | 12 |
| - unspecified | – | 3 | 3 | – | 4 |
| Therapy | At Discharge | At Last Known Follow-Up Visit | Under 67.5 Years of Age | Over 67.5 Years of Age |
|---|---|---|---|---|
| Without APT | 12 | 92 | 39 | 53 |
| Monotherapy (ASA or clopidogrel) | 127 | 254 | 103 | 151 |
| ASA + clopidogrel | 739 | 434 | 210 | 224 |
| ASA + ticagrelor | 640 | 283 | 175 | 108 |
| De-escalation (changing from ticagrelor to clopidogrel) | – | 357 | 193 | 164 |
| No data or indication of different therapy at all visits (low adherence) | – | 98 | 31 | 67 |
| Variable | ASA + Ticagrelor (n = 283) | ASA + Clopidogrel (n = 434) | De-Escalation (n = 357) | p |
|---|---|---|---|---|
| Age in years, (M ± m) | 61.83 ± 12.419 | 65.58 ± 12.550 | 61.82 ± 11.816 | 0.001 |
| Gender, male, n (%) | 188 (66.4%) | 255 (58.8%) | 245 (68.5%) | 0.040 |
| ACS with ST-segment elevation, n (%) | 120 (42.4%) | 138 (31.8%) | 164 (46.0%) | 0.005 |
| Increased markers of myocardial damage at the index event, n (%) | 277 (97.8%) | 360 (83.0%) | 336 (94.1%) | 0.001 |
| Killip II–IV at the index event, n (%) | 51 (18.1%) | 74 (17.0%) | 91 (25.6%) | 0.012 |
| History of ischemic heart disease, n (%) | 191 (67.4%) | 302 (69.6%) | 255 (71.4%) | 0.556 |
| Arterial hypertension, n (%) | 244 (86.1%) | 382 (88.0%) | 308 (86.3%) | 0.756 |
| Type II diabetes mellitus, n (%) | 62 (21.9%) | 122 (28.2%) | 70 (19.5%) | 0.035 |
| Heart failure, n (%) | 118 (41.7%) | 198 (45.6%) | 171 (47.9%) | 0.182 |
| Aortic stenosis, n (%) | 13 (4.6%) | 42 (9.6%) | 13 (3.7%) | 0.002 |
| Peripheral atherosclerosis, n (%) | 52 (18.4%) | 97 (22.4%) | 95 (26.5%) | 0.088 |
| Gastrointestinal erosive and ulcerative lesions, n (%) | 35 (12.4%) | 61 (14.0%) | 36 (10.1%) | 0.476 |
| History of gastrointestinal bleeding, n (%) | 16 (5.7%) | 23 (5.2%) | 16 (4.5%) | 0.883 |
| Hemoglobin on admission, g/l | 139.64 ± 17.839 | 133.54 ± 21.685 | 134.60 ± 18.249 | 0.043 |
| Creatinine on admission, µmol/l | 97.65 ± 27.916 | 101.98 ± 51.571 | 97.59 ± 27.458 | 0.088 |
| Glomerular filtration rate (GFR), ml/min | 82.64 ± 27.850 | 76.66 ± 33.845 | 82.11 ± 37.537 | 0.001 |
| GFR < 60 mL/min/1.73 m2 | 60 (21.1%) | 158 (36.4%) | 81 (22.8%) | 0.001 |
| PCI during hospitalization, n (%) | 237 (83.7%) | 181 (41.8%) | 215 (60.2%) | 0.0001 |
| Adverse outcomes, n (%) | ||||
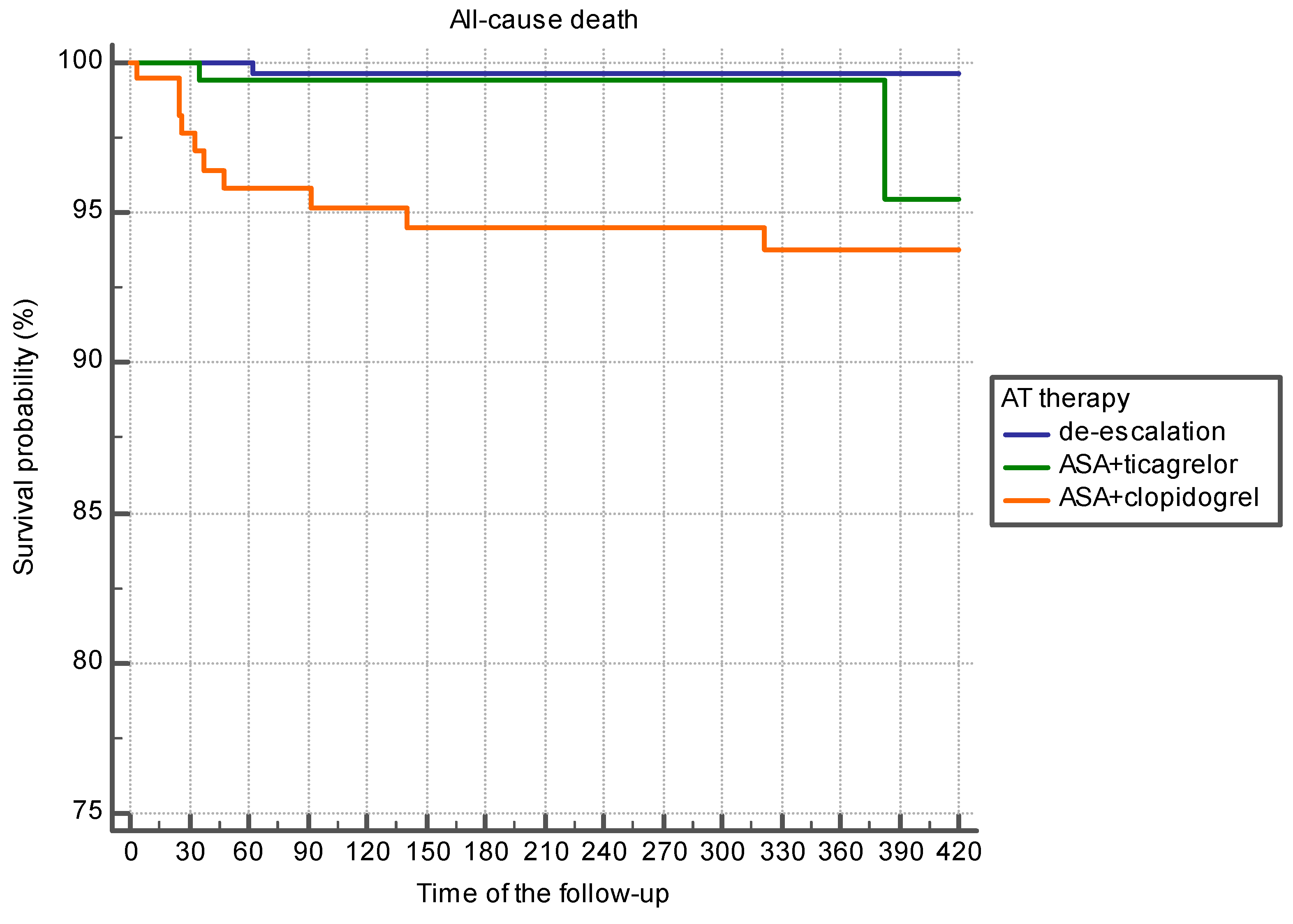
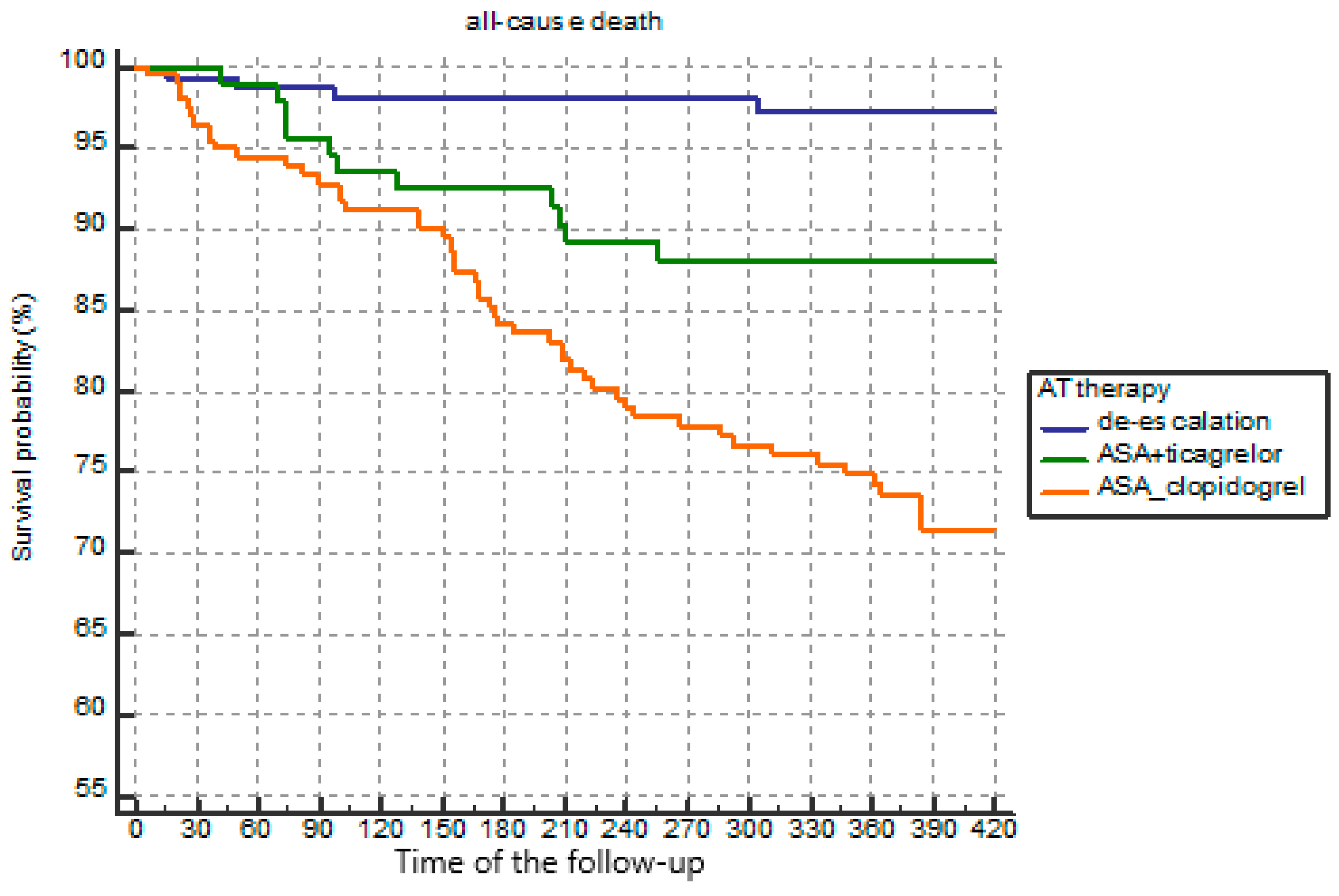
| All-cause death, n (%) | 15 (5.3%) | 34 (7.8%) | 5 (1.4%) | 0.0001 |
| Coronary death, n (%) | 5 (1.8%) | 42 (9.7%) | 2 (0.6%) | 0.001 |
| Recurrent coronary events | 43 (15.1%) | 62 (14.2%) | 41 (11.6%) | 0.354 |
| Bleeding of any kind, n (%) | 54 (19.1%) | 27(6.2%) | 34 (9.4%) | 0.001 |
| Clinically significant bleeding (2–5 BARC), n (%) | 15 (5.3%) | 15 (3.5%) | 9 (2.6%) | 0.144 |
| Major bleeding (3–5 BARC), n (%) | 2 (0.7%) | 10 (2.3%) | 4 (1.1%) | 0.283 |
| Variable | Mean Follow-Up Time (before All-Cause Death or Last Known Visit) | p | Mean Expected Survival Time by Kaplan–Meier Analysis | p | Mean Follow-Up Time, Excluding Deaths | |
|---|---|---|---|---|---|---|
| Observation data in real practice | ||||||
| De-escalation | 372.4 ± 4.47 | <0.0001 | 911.1 ± 3.97 | <0.0001 | 375.4 ± 4.31 | 0.878 |
| ASA + ticagrelor | 368.4 ± 7.88 | 757.7 ± 18.254 | 378.3 ± 7.56 | |||
| ASA+ clopidogrel | 292.3 ± 9.3737 | 706.9 ± 18.72 | 373.8 ± 10.12 | |||
| Groups formed as a result of pseudorandomization | ||||||
| De-escalation | 377.2 ± 8.08 | <0.0001 | 846.8 ± 5.15 | 0.001 | 379.2 ± 7.86 | 0.921 |
| ASA + ticagrelor | 368.4 ± 12.003 | 784.1 ± 12.54 | 379.9 ± 11.69 | |||
| ASA + clopidogrel | 303.6 ± 15.95 | 752.1 ± 26.77 | 376.7 ± 16.76 | |||
| Variable | ASA + Ticagrelor (n = 149) | ASA + Clopidogrel (n = 149) | De-Escalation (n = 149) | p |
|---|---|---|---|---|
| Age in years, (M ± m) | 63.83 ± 10.64 | 64.51 ± 11.051 | 64.32 ± 11.049 | 0.898 |
| Gender, male, n (%) | 91(61.1%) | 87 (58.4%) | 83 (55.7%) | 0.643 |
| ACS with ST-segment elevation, n (%) | 48 (32.2%) | 46 (30.9%) | 51 (34.2%) | 0.824 |
| Increased markers of myocardial damage at the index event, n (%) | 114 (76.5%) | 114 (76.5%) | 115 (77.2%) | 0.988 |
| Killip II–IV at an index event, n (%) | 26 (17.4%) | 17 (11.4%) | 24 (16.1%) | 0.309 |
| History of ischemic heart disease, n (%) | 112 (75.2%) | 111 (74.5%) | 106 (71.1%) | 0.700 |
| Arterial hypertension, n (%) | 131 (87.9%) | 131 (87.9%) | 130 (84.4%) | 0.980 |
| Type II diabetes mellitus, n (%) | 34 (22.8%) | 35 (23.5%) | 29 (19.5%) | 0.667 |
| Heart failure, n (%) | 66 (44.3%) | 73 (49.0%) | 76 (51.0%) | 0.493 |
| Aortic stenosis, n (%) | 2 (1.3%) | 2 (1.3%) | 2 (1.3%) | 0.999 |
| Peripheral atherosclerosis, n (%) | 28 (18.8%) | 28 (18.8%) | 26 (17.4%) | 0.988 |
| Gastrointestinal erosive and ulcerative lesions, n (%) | 21 (14.1%) | 20 (13.4%) | 22 (14.7%) | 0.884 |
| History of gastrointestinal bleeding, n (%) | 10 (6.7%) | 12 (8.1%) | 12 (8.1%) | 0.886 |
| Hemoglobin on admission, g/l | 138.6 ± 17.21 | 139.7 ± 16.87 | 138.81 ± 18.863 | 0.567 |
| Creatinine on admission, µmol/l | 97.12 ± 22.782 | 99.26 ± 21.421 | 98.01 ± 17.185 | 0.788 |
| Glomerular filtration rate (GFR), ml/min | 81.15 ± 31.031 | 77.62 ± 25.076 | 80.12 ± 27.454 | 0.564 |
| GFR < 60 mL/min/1.73 m2 | 38 (25.5%) | 37 (24.4%) | 39 (26.2%) | 0.886 |
| PCI during hospitalization, n (%) | 115 (77.2%) | 115 (77.2%) | 115 (77.2%) | 0.999 |
| Adverse outcomes, n (%) | ||||
| All-cause death, n (%) | 7 (4.7%) | 13 (8.7%) | 2 (1.3%) *,¥ | 0.013 |
| Coronary death, n (%) | 3 (2.0%) | 7 (4.7%) | 1 (0.7%) **,χ | 0.074 |
| Recurrent nonfatal coronary events, n (%) | 22 (14.8%) | 20 (13.4%) | 22 (14.8%) | 0.930 |
| Bleeding of any kind, n (%) | 23 (15.4%) | 22(14.8%) | 18 (11.7%) | 0.679 |
| Clinically significant bleeding (2–5 BARC), n (%) | 8 (5.4%) | 11(7.4%) | 7 (4.5%) | 0.589 |
| Major bleeding (3–5 BARC), n (%) | 1 (0.7%) | 4 (2.7%) | 1 (0.7%) | 0.219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazhnik, V.A.; Minushkina, L.O.; Boeva, O.I.; Khasanov, N.R.; Kosmacheva, E.D.; Chichkova, M.A.; Zateyshchikov, D.A. Risk Stratification after an Acute Coronary Syndrome: Significance of Antithrombotic Therapy. J. Clin. Med. 2021, 10, 1572. https://doi.org/10.3390/jcm10081572
Brazhnik VA, Minushkina LO, Boeva OI, Khasanov NR, Kosmacheva ED, Chichkova MA, Zateyshchikov DA. Risk Stratification after an Acute Coronary Syndrome: Significance of Antithrombotic Therapy. Journal of Clinical Medicine. 2021; 10(8):1572. https://doi.org/10.3390/jcm10081572
Chicago/Turabian StyleBrazhnik, Victoria A., Larisa O. Minushkina, Olga I. Boeva, Niyaz R. Khasanov, Elena D. Kosmacheva, Marina A. Chichkova, and Dmitry A. Zateyshchikov. 2021. "Risk Stratification after an Acute Coronary Syndrome: Significance of Antithrombotic Therapy" Journal of Clinical Medicine 10, no. 8: 1572. https://doi.org/10.3390/jcm10081572
APA StyleBrazhnik, V. A., Minushkina, L. O., Boeva, O. I., Khasanov, N. R., Kosmacheva, E. D., Chichkova, M. A., & Zateyshchikov, D. A. (2021). Risk Stratification after an Acute Coronary Syndrome: Significance of Antithrombotic Therapy. Journal of Clinical Medicine, 10(8), 1572. https://doi.org/10.3390/jcm10081572







