Bleb Compressive Sutures in the Management of Hypotony Maculopathy after Glaucoma Surgery
Abstract
1. Introduction
2. Materials and Methods
- Age at glaucoma diagnosis of over 18 years;
- At least 6 months after the antiglaucoma procedure;
- Clinically significant hypotony: intraocular pressure (IOP) lower than 6 mmHg, associated with the BCVA decreased by at least 2 lines on Snellen charts in comparison to pre-trabeculectomy results;
- Features of hypotony maculopathy with macular folds;
- No progression of the cataract;
- No leakage from the bleb;
- No kissing choroidal effusion.
- IOP over 6 mmHg;
- BCVA improvement of at least 2 lines on a Snellen chart.
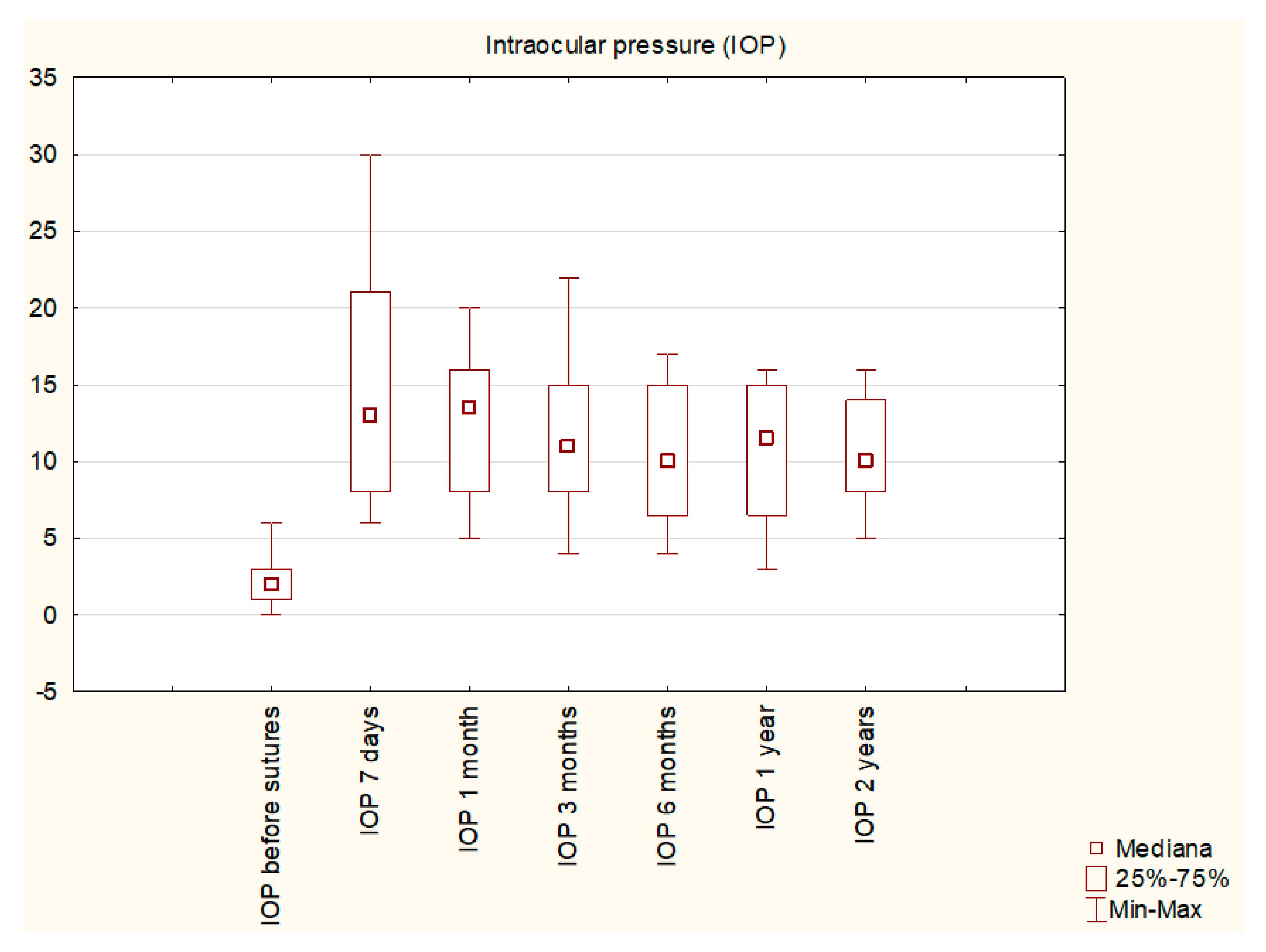
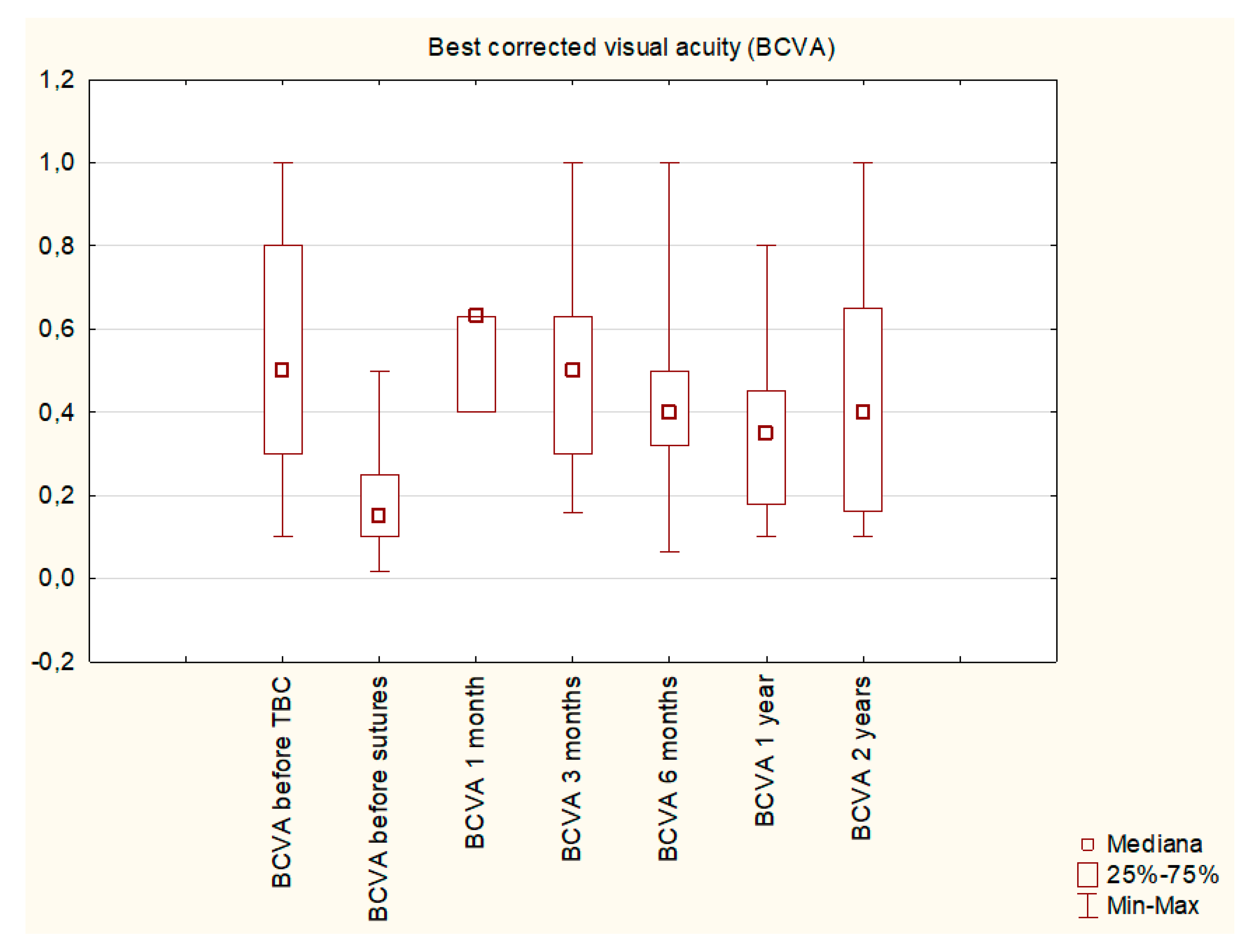
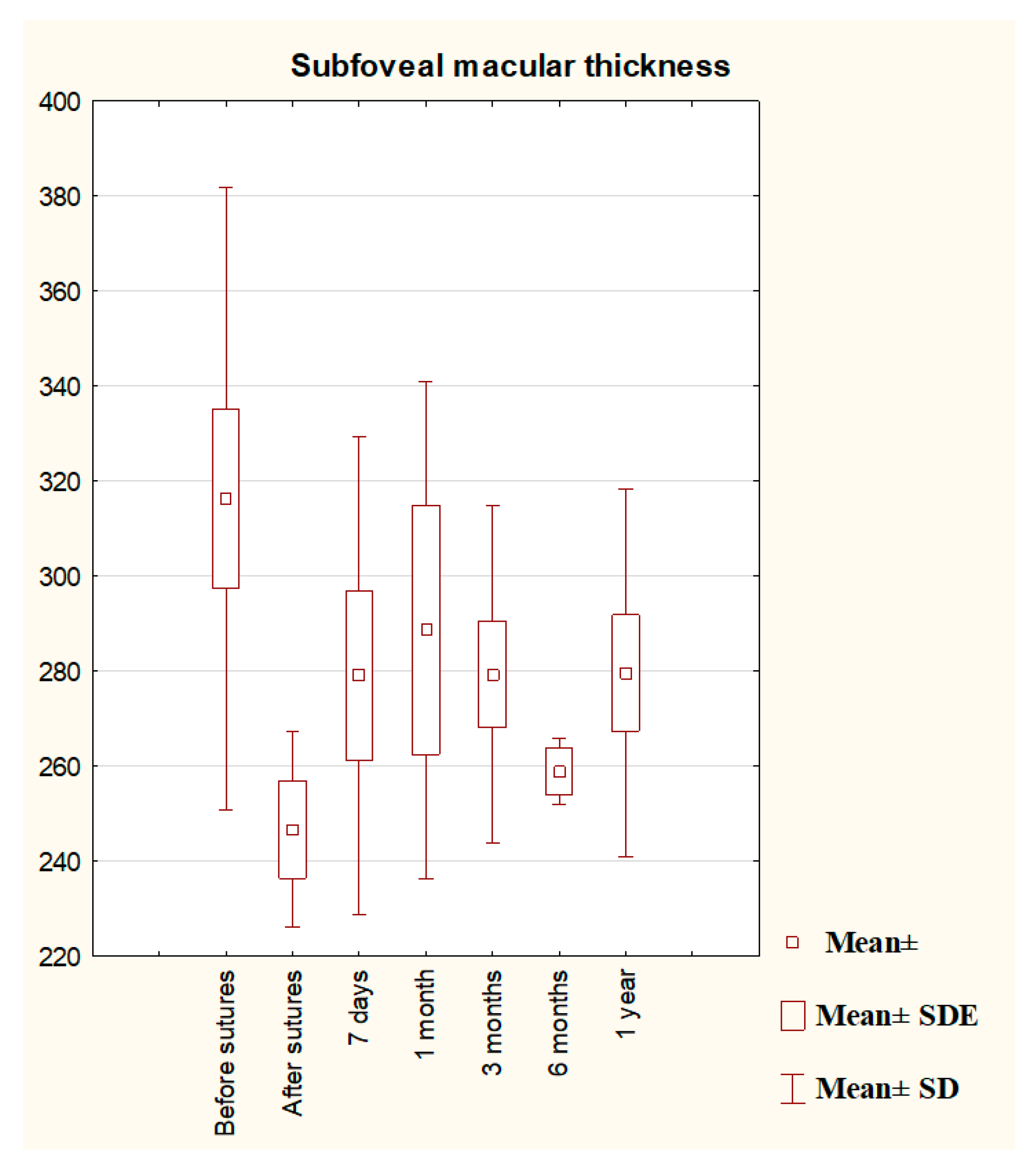
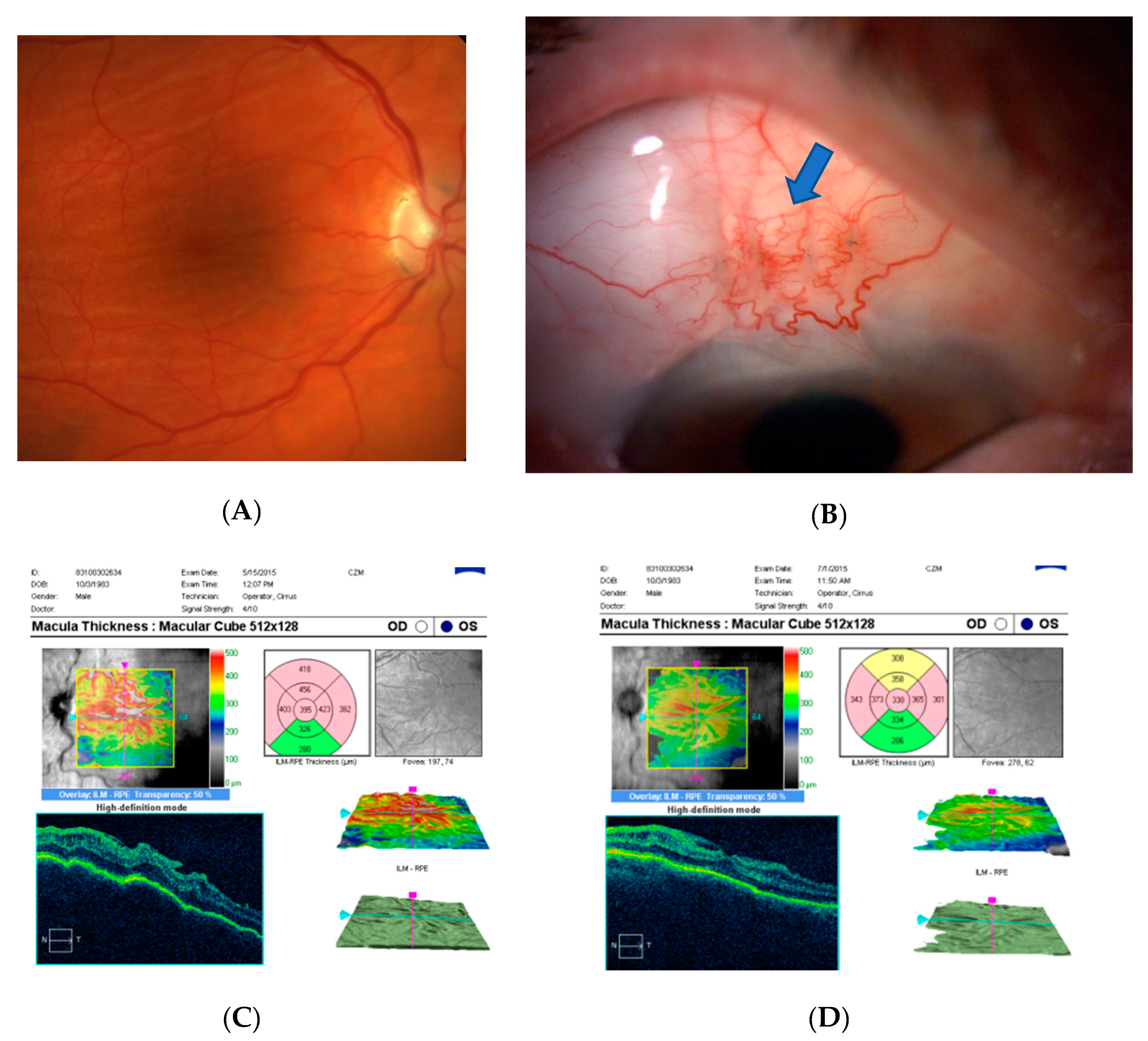
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 14, 1901–1911. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 22, 1711–1720. [Google Scholar] [CrossRef]
- Khaw, P.T.; Chiang, M.; Shah, P.; Sii, F.; Lockwood, A.; Khalili, A. Enhanced Trabeculectomy: The Moorfields Safer Surgery System. Dev. Ophthalmol. 2017, 59, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.P.; Miller, M.H.; Hitchings, R.A. Wound healing as a barrier to successful filtration surgery. Eye 1988, 2, S113–S123. [Google Scholar] [CrossRef]
- Costa, V.P.; Arcieri, E.S. Hypotony maculopathy. Acta Ophthalmol. Scand. 2007, 85, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Seah, S.K.; Prata, J.A., Jr.; Minckler, D.S.; Baerveldt, G.; Lee, P.P.; Heuer, D.K. Hypotony following trabeculectomy. J. Glaucoma 1995, 4, 73–79. [Google Scholar] [CrossRef]
- Pederson, J.E. Ocular Hypotony. In The Glaucomas; Ritch, R., Krupin, T., Shields, M.B., Eds.; Mosby: St. Louis, MO, USA, 1996; pp. 385–395. [Google Scholar]
- Rahman, A.; Mendonca, M.; Simmons, R.B.; Simmons, R.J. Hypotony after glaucoma filtration surgery. Int. Ophthalmol. Clin. 2000, 40, 127–136. [Google Scholar] [CrossRef]
- Edmunds, B.; Thompson, J.R.; Salmon, J.F.; Wormald, R.P. The National Survey of Trabeculectomy. III. Early and late complications. Eye 2002, 16, 297–303. [Google Scholar] [CrossRef]
- Saeedi, O.J.; Jefferys, J.L.; Solus, J.F.; Jampel, H.D.; Quigley, H.A. Risk factors for adverse consequences of low intraocular pressure after trabeculectomy. J. Glaucoma 2014, 23, e60–e68. [Google Scholar] [CrossRef]
- Francis, B.A.; Hong, B.; Winarko, J.; Kawji, S.; Dustin, L.; Chopra, V. Vision loss and recovery after trabeculectomy: Risk and associated risk factors. Arch. Ophthalmol. 2011, 129, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Dellaporta, A. Creasing of retina in hypotonia. Klin. Mon. Augenheilkd. Augenarztl. Fortbild. 1954, 125, 872–878. [Google Scholar]
- Gass, J.D. Hypotony Maculopathy. In Contemporary Ophthalmology: Honoring Sir Stewart Duke-Elder; Bellows, J.G., Ed.; Williams and Wilkins: Baltimore, MD, USA, 1972; pp. 336–343. [Google Scholar]
- Oyakhire, J.O.; Moroi, S.E. Clinical and anatomical reversal of long-term hypotony maculopathy. Am. J. Ophthalmol. 2004, 137, 953–995. [Google Scholar] [CrossRef] [PubMed]
- Bitrian, E.; Song, B.J.; Caprioli, J. Bleb revision for resolution of hypotony maculopathy following primary trabeculectomy. Am. J. Ophthalmol. 2014, 158, 597–604.e1. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Tomidokoro, A.; Tomita, G.; Araie, M. Topical application of autologous serum for the treatment of lateonset aqueous oozing or point-leak through filtering bleb. Eye 2005, 19, 23–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, M.F.; Magauran, R.G., 3rd; Betchkal, J.; Doyle, J.W. Treatment of postfiltration bleb leaks with autologous blood. Ophthalmology 1995, 102, 868–871. [Google Scholar] [CrossRef]
- Higashide, T.; Tagawa, S.; Sugiyama, K. Intraoperative Healon5 injection into blebs for small conjunctival breaks created during trabeculectomy. J. Cataract Refract. Surg. 2005, 31, 1279–1282. [Google Scholar] [CrossRef]
- Hosoda, S.; Yuki, K.; Ono, T.; Tsubota, K. Ophthalmic viscoelastic device injection for the treatment of flat anterior chamber after trabeculectomy: A case series study. Clin. Ophthalmol. 2013, 7, 1781–1785. [Google Scholar]
- Kurtz, S.; Leibovitch, I. Combined perfluoropropane gas and viscoelastic material injection for anterior chamber reformation following trabeculectomy. Br. J. Ophthalmol. 2002, 86, 1225–1227. [Google Scholar] [CrossRef]
- Eha, J.; Hoffmann, E.M.; Wahl, J.; Pfeiffer, N. Flap suture—A simple technique for the revision of hypotony maculopathy following trabeculectomy with mitomycin C. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 869–874. [Google Scholar] [CrossRef]
- Shirato, S.; Maruyama, K.; Haneda, M. Resuturing the sclera flap through conjunctiva for treatment of excess filtration. Am. J. Ophthalmol. 2004, 137, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.L.; Alward, W.L. Rapid visual recovery and longterm intraocular pressure control after donor scleral patch grafting for trabeculectomy-induced hypotony maculopathy. J. Glaucoma 1995, 4, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Harizman, N.; Ben-Cnaan, R.; Goldenfeld, M.; Levkovitch-Verbin, H.; Melamed, S. Donor scleral patch for treating hypotony due to leaking and/or overfiltering blebs. J. Glaucoma 2005, 14, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, F.; Kaufmann, C.; Kipfer, A.; Thiel, M.A. Corneal patch graft for the repair of late-onset hypotony or filtering bleb leak after trabeculectomy: A new surgical technique. J. Glaucoma 2014, 23, e76–e80. [Google Scholar] [CrossRef] [PubMed]
- Panday, M.; Shantha, B.; George, R.; Boda, S.; Vijaya, L. Outcomes of bleb excision with free autologous conjunctival patch grafting for bleb leak and hypotony after glaucoma filtering surgery. J. Glaucoma 2011, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, T.S.; Lappas, A.; Rosentreter, A. Secondary subconjunctival implantation of a biodegradable collagen-glycosaminoglycan matrix to treat ocular hypotony following trabeculectomy with mitomycin C. Br. J. Ophthalmol. 2013, 97, 985–988. [Google Scholar] [CrossRef]
- Laspas, P.; Wahl, J.; Peters, H.; Prokosch-Willing, V.; Chronopoulos, P.; Grehn, F.; Pfeiffer, N.; Hoffmann, E.M. Outcome of Bleb Revision With Autologous Conjunctival Graft Alone or Combined With Donor Scleral Graft for Late-onset Bleb Leakage with Hypotony after Standard Trabeculectomy with Mitomycin C. J. Glaucoma 2021, 1, 175–179. [Google Scholar] [CrossRef]
- Maruyama, K.; Shirato, S. Efficacy and safety of transconjunctival scleral flap resuturing for hypotony after glaucoma filtering surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1751–1756. [Google Scholar] [CrossRef]
- Jampel, H.D.; Pasquale, L.R.; Dibernardo, C. Hypotony maculopathy following trabeculectomy with mitomycin C. Arch. Ophthalmol. 1992, 110, 1049–1050. [Google Scholar] [CrossRef]
- Thomas, M.; Vajaranant, T.S.; Aref, A.A. Hypotony maculopathy: Clinical presentation and therapeutic methods. Ophthalmol. Ther. 2015, 4, 79–88. [Google Scholar] [CrossRef]
- Higashide, T.; Ohkubo, S.; Sugimoto, Y.; Kiuchi, Y.; Sugiyama, K. Persistent hypotony after trabeculectomy: Incidence and associated factors in the Collaborative Bleb-Related Infection Incidence and Treatment Study. Jpn. J. Ophthalmol. 2016, 60, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Chua, B.; Clement, C.I. Does Chronic Hypotony following Trabeculectomy Represent Treatment Failure? J. Curr. Glaucoma Pract. 2015, 9, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Flynn, H.W., Jr.; Palmberg, P.F.; Gass, J.D.; Grajewski, A.L.; Parrish, R.K., 2nd. Treatment of hypotony maculopathy after trabeculectomy. Ophthalmic Surg. Lasers 1995, 26, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Scoralick, A.L.B.; Almeida, I.; Ushida, M.; Dias, D.T.; Dorairaj, S.; Prata, T.S.; Kanadani, F.N. Hypotony Management through Transconjunctival Scleral Flap Resuturing: Analysis of Surgical Outcomes and Success Predictors. J. Curr. Glaucoma Pract. 2017, 11, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Stamper, R. Bilateral chronic hypotony following trabeculectomy with mitomycin C. J. Glaucoma 2001, 10, 325–328. [Google Scholar] [CrossRef]
- Silva, R.A.; Doshi, A.; Law, S.K.; Singh, K. Postfiltration hypotony maculopathy in young chinese myopic women with glaucomatous appearing optic neuropathy. J. Glaucoma 2010, 19, 105–110. [Google Scholar] [CrossRef]
- Singh, K.; Byrd, S.; Egbert, P.R.; Budenz, D. Risk of hypotony after primary trabeculectomy with antifibrotic agents in a black West African population. J. Glaucoma 1998, 7, 82–85. [Google Scholar] [CrossRef]
- Fannin, L.A.; Schiffman, J.C.; Budenz, D.L. Risk factors for hypotony maculopathy. Ophthalmology 2003, 110, 1185–1191. [Google Scholar] [CrossRef]
- Curtin, B.J.; Iwamoto, T.; Renaldo, D.P. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch. Ophthalmol. 1979, 97, 912–915. [Google Scholar] [CrossRef]
- Nicolela, M.T.; Carrillo, M.M.; Yan, D.B.; Rafuse, P.E. Relationship between central corneal thickness and hypotony maculo-pathy after trabeculectomy. Ophthalmology 2007, 114, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Letartre, L.; Basheikh, A.; Anctil, J.-L.; Des Marchais, B.; Goyette, A.; Kasner, O.P.; Lajoie, C. Transconjunctival suturing of the scleral flap for overfiltration with hypotony maculopathy after trabeculectomy. Can. J. Ophthalmol. 2009, 44, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Eha, J.; Hoffmann, E.M.; Pfeiffer, N. Long-term results after transconjunctival resuturing of the scleral flap in hypotony following trabeculectomy. Am. J. Ophthalmol. 2013, 155, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Tunç, Y.; Tetikoglu, M.; Kara, N.; Sagdık, H.M.; Özarpaci, S.; Elçioğlu, M.N. Management of hypotony and flat anterior chamber associated with glaucoma filtration surgery. Int. J. Ophthalmol. 2015, 8, 950–953. [Google Scholar]
- Cashwell, L.F.; Martin, C.A. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology 1999, 106, 2307–2311. [Google Scholar] [CrossRef]
- Camp, A.S.; Weinreb, R.N. Hypotony Keratopathy Following Trabeculectomy. J. Glaucoma 2020, 29, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Saito, H.; Takao, M.; Araie, M. Frequency of hypotonic maculopathy observed by spectral domain optical coherence tomography in post glaucoma filtration surgery eyes. Am. J. Ophthalmol. Case Rep. 2020, 18, 100786. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, O.; Pillar, A.; Jefferys, J.; Arora, K.; Friedman, D.; Quigley, H. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br. J. Ophthalmol. 2014, 98, 976–979. [Google Scholar] [CrossRef]

| Feature | n/Mean Value ± SD |
|---|---|
| Number of patients | 17 |
| Gender | 8F/10 M |
| Age | 60.5 ± 20.5 |
| Diagnosis | POAG: 7 cases; PDSG: 4 cases: PEXG: 4 cases; PACG: 1 case; traumatic glaucoma: 1 case. |
| Primary procedure | Trabeculectomy: 9 cases; Phacotrabeculectomy: 4 cases; Needle revision: 3 cases; Deep sclerectomy: 1 case. |
| Mean IOP before primary procedure | 33.6 ± 11.6 mmHg |
| Mean IOP before sutures | 2.3 ± 1.57 mmHg |
| BCVA before primary procedure | 0.55 ± 0.31 |
| BCVA before sutures | 0.18 ± 0.13 |
| Mean MD | 16.21 ± 7.45 dB |
| Mean time between primary procedure and compression sutures | 3.08 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosior-Jarecka, E.; Wróbel-Dudzińska, D.; Święch, A.; Żarnowski, T. Bleb Compressive Sutures in the Management of Hypotony Maculopathy after Glaucoma Surgery. J. Clin. Med. 2021, 10, 2223. https://doi.org/10.3390/jcm10112223
Kosior-Jarecka E, Wróbel-Dudzińska D, Święch A, Żarnowski T. Bleb Compressive Sutures in the Management of Hypotony Maculopathy after Glaucoma Surgery. Journal of Clinical Medicine. 2021; 10(11):2223. https://doi.org/10.3390/jcm10112223
Chicago/Turabian StyleKosior-Jarecka, Ewa, Dominika Wróbel-Dudzińska, Anna Święch, and Tomasz Żarnowski. 2021. "Bleb Compressive Sutures in the Management of Hypotony Maculopathy after Glaucoma Surgery" Journal of Clinical Medicine 10, no. 11: 2223. https://doi.org/10.3390/jcm10112223
APA StyleKosior-Jarecka, E., Wróbel-Dudzińska, D., Święch, A., & Żarnowski, T. (2021). Bleb Compressive Sutures in the Management of Hypotony Maculopathy after Glaucoma Surgery. Journal of Clinical Medicine, 10(11), 2223. https://doi.org/10.3390/jcm10112223







