Abstract
Non-alcoholic fatty liver disease (NAFLD), including non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), represents the hepatic manifestation of obesity and metabolic syndrome. Due to the spread of the obesity epidemic, NAFLD is becoming the most common chronic liver disease and one of the principal indications for liver transplantation. However, no pharmacological treatment is currently approved to prevent the outbreak of NASH, which leads to fibrosis and cirrhosis. Preclinical research is required to improve our knowledge of NAFLD physiopathology and to identify new therapeutic targets. In the present review, we summarize advances in NAFLD preclinical models from cellular models, including new bioengineered platforms, to in vivo models, with a particular focus on genetic and dietary mouse models. We aim to discuss the advantages and limits of these different models.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) comprises two histological forms: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH) [1]. NAFL is defined as the presence of hepatic steatosis without hepatocellular necrosis and no or minimal inflammation, while NASH is characterized by the association of steatosis, liver inflammation and hepatocyte ballooning, with or without fibrosis. The progression of inflammation and fibrosis in NASH underlies cirrhosis and hepatocellular carcinoma (HCC) [1]. NAFLD is the hepatic manifestation of obesity and metabolic syndrome, affecting up to one-third of the adult population in the western world [2,3]. Thus, NAFLD is rapidly becoming a worldwide public health problem with an exponential growth prediction within the 10 next years due to high caloric intake combined with a sedentary lifestyle [4]. NASH is also predicted to become the most common indication of liver transplantation in the near future [5,6]. Molecular mechanisms leading to NASH and its evolution to liver fibrosis and cirrhosis remain partially unknown. A “two-hit” hypothesis has been postulated [7]. The first hit is defined as fat accumulation in the liver, which triggers an inflammatory process that results in steatohepatitis and fibrosis. The nature of the second hit is not fully understood, but it may involve oxidation of fatty acids and subsequent free-radical-induced liver damage. Although widely accepted, this theory is now questioned, as dysfunction in metabolic pathways such as lipid transport and insulin signaling, as well as gut microbiota and genetic polymorphisms, are important pathogenic drivers contributing to disease heterogeneity [7,8]. Even if NAFLD is the most common chronic liver disease, no pharmacological treatment is currently approved for this disease. Therefore, basic and preclinical research remains essential to improve our understanding of NAFLD pathophysiology and for the development of pharmacotherapies. The biological systems used in this research range in complexity and scale from monolayer cell culture to complex three-dimensional (3D) organoids and model organisms. Here, we review advances in the cellular and in vivo models currently used to study NAFLD.
2. Materials and Methods
We used NCBI PubMed and ScienceDirect databases for literature research. Publications in English were searched using the terms: NAFLD, NASH, liver steatosis, mouse models of NAFLD, non-rodent models of NAFLD, in vitro cell culture models of NAFLD, spheroids, organoids, liver-on-a-chip. Articles were screened to identify potentially relevant studies. Reference lists of retrieved literature were also screened.
3. In Vitro Cell Culture Models of Non-Alcoholic Fatty Liver Disease
3.1. Hepatic Cell Sources
Cell lines are widely used in research and drug development. They display a higher replicative capacity than primary cells and a stable phenotype so that they can be used for a long period-of-time. They are also inexpensive. Primary cells are directly issued from tissues. In contrast to cell lines, they have a limited life and expansion capacity, but they maintain the morphological and functional characteristics of their origin for a short period of time. Here, we review the cell culture models and discuss their advantages and limitations to study the development and progression of NAFLD (Figure 1).
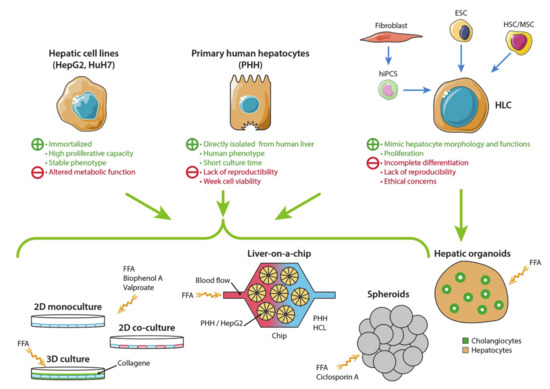
Figure 1.
Hepatic cell sources and in vitro models of non-alcoholic fatty liver disease (NAFLD). Different cell sources are now available to build in vitro models of NAFLD. Primary human hepatocytes (PHH) display the closest phenotype to the human liver. A short culture time and low availability of PHHs are alleviated by using immortalized cell lines (HepG2, HuH7 or HepaRG) and hepatocyte-like cells (HLC) derived from stem cells (as human-induced pluripotent cell—hIPSC, embryonic stem cells—ESC or hepatic stem cells—HSC, or mesenchymal stem cells—MSC). However, altered metabolic functions and incomplete differentiation of HLC are limitations of the model. Several models have been developed to mimic NALFD in vitro. NAFLD can be studied in 2- or 3-dimensional cell cultures by adding free fat acid (FFA), Bisphenol A or Valproate in the culture medium. New 3D models have been recently developed: spheroids are derived from different hepatic cell sources; hepatic organoids are produced by stem cell differentiation in parenchymal and non-parenchymal liver cells. Livers-on-a-chip are devices designed to mimic the physiological environment of the liver lobule: hepatocytes are placed inside a micro-scaffold, and a fluidic flow passes through the chip to reproduce blood circulation.
3.1.1. Human Hepatic Cell Lines
Human cell lines are immortalized cells obtained from resected tumor tissue (e.g., HepG2, HepaRG or HuH7 hepatoma cell lines) or generated by the genetic manipulation of primary liver cells (e.g., SV40 Large T, hTERT) [9,10,11]. The principal advantages of immortalized cell lines are their unlimited growth potential and a stable phenotype throughout culture time [12]. Thus, using these models facilitates standardized protocols and reproducible studies. However, due to their tumor phenotype or the immortalization process, hepatic cell lines are generally altered in certain metabolic functions that limit a direct comparison to the human situation, especially when studying NAFLD. The available immortalized human hepatic cell lines and their in vitro application other than NAFLD have been reviewed here [13,14].
3.1.2. Primary Human Hepatocytes
Primary human hepatocytes (PHHs) are cells isolated from resected liver tissue using a two-step collagenase perfusion or magnetic cell separation technique and maintained in culture for a few days [15,16]. Due to direct isolation from tissue, PHHs are the closest model to mimic the phenotype of a hepatocyte in vivo; notably, their plasma membrane maintains active uptake/secretion mechanisms and metabolism [9,17]. However, PHHs show phenotypic instability, and their accessibility and culture time are limited [18,19]. Moreover, human sample collection requires ethical authorization, and non-pathological tissues are necessary and difficult to obtain in clinical practice. Cell viability is highly dependent on the surgical and transport conditions of tissue collection, and genetic variability between donors can introduce a bias leading to a lack of reproducibility between studies and experiments [20,21]. Therefore, PHHs appear particularly suitable for drug metabolism studies that do not require a long period of culture time, and to a lesser extent for modeling specific liver diseases such as NAFLD [17].
3.1.3. Hepatocyte-Like Cells
Hepatocyte-like cells (HLCs) are cells differentiated from human stem cells (hSCs), including liver stem cells and embryonic pluripotent stem cells [22]. Human SCs can be differentiated into HLCs in vitro using combined exposure to growth factors and nutrients [23,24]. Furthermore, techniques now exist for the production of human-induced pluripotent stem cells (hiPSCs) from somatic cells such as fibroblastic cells, which represent an unlimited source of stem cells that can secondarily differentiate into HLCs [25,26,27]. HLCs display morphological and functional characteristics that are similar to those of primary hepatocytes but without culture time limitation [28]. Nevertheless, HLCs also raise issues regarding the ethical use of embryonic stem cells, the incomplete hepatic differentiation and the lack of standardized differentiation protocol, which limits studies reproducibility [20].
3.2. Two-Dimensional Cell Culture Models
3.2.1. Monoculture
The most commonly used type of cell culture is the two-dimensional (2D) model. To mimic NAFLD in 2D cultures of hepatocytes of different sources as outlined in Section 3.1, steatosis can be induced by adding free fatty acids (FFA) to the cell culture medium, especially oleic and/or palmitic acids [15,29]. The induction of hepatocytes with FFA leads to triglyceride accumulation in their cytoplasm, which ultimately causes endoplasmic reticulum (ER) stress, inflammation and cell death, which are all key hallmarks of NASH [30,31]. Steatosis can also be induced in cultured cells using drugs such as Bisphenol A, which increases lipid accumulation due to SREBP1 upregulation, or Valproate, which enhances fatty acid uptake and triglyceride synthesis [32,33]. Additionally, triglyceride accumulation can be enhanced in the presence of an inducer of ER-stress [34]. Hepatocyte 2D monoculture allows the study of all major metabolic pathways, namely carbohydrates, lipids and amino acids [11,35,36]. It is also suitable for preclinical drug testing to evaluate drug efficacy and cellular tolerance [17,37]. However, 2D human monocellular models fall short of expectations due to the absence of critical hepatocyte–non-parenchymal-cell (NPC) interactions, which are important for the initiation of inflammatory processes leading to fibrogenesis in NAFLD.
3.2.2. Co-Culture
2D co-culture models are used to study the mutual interaction between hepatocytes and NPCs in vitro. Different cell types are grown together in the same environment, and steatosis can be induced like in monoculture by adding FFA to the culture medium. One model of particular interest is the co-culture of hepatocytes with hepatic stellate cells (HSCs) that are key effectors of fibrosis in NAFLD [38]. This interplay has been studied by Barbero-Becerra et al., who developed a model of co-culture of HuH7 (human hepatocyte cell line) and LX-2 (Human HSC line) cells [39]. They demonstrated that FFA exposure induced the expression of α-SMA in LX-2 cells only when they were simultaneously co-cultured with Huh-7 and that HSC activation was independent of FFA accumulation but required cell-to-cell interaction with hepatocytes. Co-culture of primary hepatocytes with Kupffer- or endothelial-cells provides a powerful tool to assess the mechanisms whereby FFA triggers inflammatory processes in vitro [40,41]. However, co-culture is quite difficult to implement, as, for example, growth medium optimization is required to select the medium that best sustains the different cell populations. Human cells are also poorly available, adding another difficulty to the co-culture models [42,43]. For all these reasons, these models are rare in the literature compared to monoculture and animal models.
3.3. Three-Dimensional Cell Culture Models
In the past decade, the development of three-dimensional (3D) culture models has dramatically increased the knowledge of cellular and molecular mechanisms in the pathogenesis of liver diseases, especially NAFLD, and offers new opportunities for drug testing in this context.
3.3.1. 3D Cell Culture in a Collagen Gel Sandwich
In this model, hepatocytes are placed between two layers of collagen gel, allowing them to reconstruct the cellular polarity normally found in the liver. In this configuration, sandwiched hepatocytes maintain the secretion of albumin, transferrin, fibrinogen, bile acids and urea for at least 6 weeks, whereas hepatocytes cultured on a single layer of collagen gel cease such secretion between 1 and 2 weeks [44]. This secretion can be restored when a second layer of collagen is applied to the monolayer of collagen gel culture [44]. Re-establishment of bi-polarity allows one to prolong survival and to maintain hepatic metabolism in PHHs [19,45]. Recently, a more complex 3D model has been developed where hepatocytes are co-cultivated with liver sinusoidal endothelial cells (LSECs) inside collagen gels to mimic liver lobular architecture [40]. Hepatocytes and LSECs account for approximately 80% of the liver mass where the LSECs line the sinusoidal walls, and act as a barrier between hepatocytes and blood. This co-culture configuration provides an environment wherein hepatocyte and LSECs, through cell–cell contacts and/or soluble factors, lead to enhanced cell function and survival [40]. However, cell–cell interactions can be hidden or blocked due to collagen thickness in this model [46]. Thus, the sandwich configuration has been rarely used as an NAFLD model.
3.3.2. Hepatic Spheroids and Organoids
Spheroids or 3D cell aggregates are now the most commonly used 3D culture model. Hepatic spheroids are aggregates of hepatocytes usually derived from liver progenitor cells but also consisting of PHHs. The spheroid formation is initiated by spontaneous self-aggregation of the hepatocytes and does not require extracellular matrix to develop. Several techniques have been developed based on non-adherent surfaces and gravitational adherence [47,48]. PHHs in spheroids remain differentiated for at least 3 to 4 weeks, and hepatic steatosis can be induced by supplementing the culture medium with pathophysiological concentrations of FFA, carbohydrates and insulin [49,50]. In another model of PHH spheroids, hepatic steatosis can be induced by Cyclosporine A [51]. Hepatic spheroids can be built together with NPCs such as stellate cells and endothelial cells to develop more complex liver-like structures [50,52,53,54]. However, in standard non-adhesive plates, it is difficult to maintain uniform spheroid size, and clusters of spheroids can emerge that limit nutrient and oxygen absorption to the distal spheroids, leading to cell mortality [55].
Organoids are new research tools, which are defined as artificially grown masses of cells that resemble miniature organs. Liver organoids are obtained through isolation and expansion of stem and progenitor cells from hepatic stem cell niches to form small self-organizing 3D structures that simulate many of the functions of a native liver [56,57]. Successful organoid formation requires a careful orchestration of spatiotemporal signals from growth factors to supportive matrices in order to stimulate the different cell niches. To create human liver organoids (HLOs), human pluripotent stem cells are co-differentiated into epithelial and mesenchymal lineages to form spheroids. These spheroids are then embedded in Matrigel and cultured with retinoic acid, and hepatocyte differentiation is achieved using a specific maturation medium. Ouchi et al. have shown that the resulting HLOs are self-organized clusters of cells comprising liver epithelial cells (cholangiocytes and hepatocytes) and NPC (stellate-, biliary stem- and Kupffer-cells) [58]. When these HLOs are exposed to FFA, hepatocytes developed steatosis and ballooning, Kupffer cells released pro-inflammatory cytokines, and stellate cells produced collagen, three key processes in NASH [59]. In addition, increased ROS levels and overexpressed lipid- and carbohydrate-related genes were observed [60]. More recently, Collin de l’Hortet et al. developed HLOs based on the control of the expression of SIRT1 (silent information regulator 1), known to exert protective functions in hepatocytes and macrophages by modulating metabolic and inflammatory pathways, respectively [61,62]. In these HLOs, the downregulation of SIRT1 induces a rapid accumulation of lipid droplets in hepatocytes accompanied by a pro-inflammatory response of the neighboring cells. Moreover, the majority of the metabolic pathways seen in livers of NAFLD patients were also upregulated in these HLOs [61,62]. Thus, HLOs appear to be promising tools for pathophysiological studies in NAFLD. As compared to 2D cell culture, the 3D nature of organoids can more closely mimic natural physiological processes, including stem cell differentiation, cellular movement and cell–cell interactions. In addition, organoids can undergo extensive expansion and culture and maintain their genomics stability, making long-term storage and high-throughput screening possible [63]. As compared with animal models, organoids reduce animal experiments and are easily accessible to live imaging techniques, and in some instances, they can provide a more accurate model of human development and disease than animal models do [63]. However, cell differentiation remains sometimes incomplete, and cell organization is random, which leads to a lack of reproducibility. Methods and techniques still need further improvement to reach a complete differentiation and a standardized architecture of the organoids.
3.3.3. Liver-On-A-Chip Technology
Liver-on-a-chip is based on microstructures and microfluidic devices aimed to establish microscale functional liver constructs on a chip. Basically, the microarchitecture of the liver is mimicked by a polymeric scaffold that consists of hundreds of small channels [64]. The dimensions are such that when hepatocytes are seeded into these channels, they form a long donut-shaped arrangement that closely resembles the hepatic lobule that a microfluid containing various nutrients and oxygen can pass through to simulate blood flow [65]. A more complex system can be engineered by integrating NPCs in a vascular layer comprising endothelial cells and macrophages to a hepatic layer comprising stellate cells co-cultured with hepatocytes [66]. The 3D culture is then embedded in a microfluidically perfused biochip that enables sufficient nutrition supply and reproduces the morphological aspects of the human liver sinusoid [66]. In this model, the perfusion chamber provides a fluidic shear stress and mimics the microenvironment of native liver [66].
Liver-on-a-chip has been extensively studied to create in vitro NAFLD models. Gori et al. have cultured HepG2 cells under free fatty acid (FFA) supplementation in a microfluidically perfused device, which mimics the endothelial–parenchymal interface of a liver sinusoid and allows the diffusion of nutrients and removal of waste products similar to that in liver microcirculation [67]. In this chip, the microfluidic dynamic allows gradual and lower intracellular lipid accumulation, higher hepatic cell viability and minimal oxidative stress compared with static cultures. It closely recapitulates the chronic conditions linked to the fat accumulation that occurs in NAFLD patients [67]. Likewise, Kostrzewski et al. cultured PHHs in a 3D perfused platform and showed that fat accumulation in the cultured cells was gradual over a long period of time [59]. Moreover, the metabolic activities (e.g., cytochrome P activity) of PHHs were reduced gradually across culture time in line with what has been observed in the liver of NAFLD patients [59]. Lee et al. also built a “gut-liver-on-a-chip” to study the gut–liver axis in the context of NAFLD [68]. In this microfluidic chip, FFAs were absorbed through a gut layer, and the subsequent secretion of chylomicrons was observed and coincided with fat accumulation in hepatocytes.
Liver-on-a-chip models dramatically increase the possibility to model NAFLD in vitro, including interplay between liver and other organs, which is an essential way to understand metabolic disorders. Compared to organoids, the chip technology is more reproducible because of standardized protocols and bioengineering fabrication. However, due to high complexity and cost, it is not widely spread in laboratories.
3.4. Human Precision-Cut Liver Slices Model
Human precision-cut liver slices (hPCLS) represent a robust ex vivo model in which multi-cellular histoarchitecture of the hepatic environment remains functional for at least 5 to 21 days in culture, although viability usually decreases afterward [69,70,71,72]. Inflammation is a key feature to distinguish steatohepatitis from simple steatosis. Thus, hPCLS that retain liver-infiltrating immune cells such as lymphocytes and macrophages allow one to study the changes observed during the different stages of NAFLD and to test the therapeutic efficacy of new compounds [71,73,74]. Hepatic steatosis can be induced by adding FFA to the culture medium of hPCLS, which results in the activation of inflammation and fibrogenesis in NPCs [71,75]. This model is also well suited to understand whether intrahepatic lipid accumulation can be modulated through epigenetic manipulation [71].
Taken together, co-culture or 3D models that mimic the architecture of liver tissue are powerful tools to elucidate the cellular mechanisms of fatty liver disease in order to study cell interactions in the progression of the disease from simple steatosis to steatohepatitis. However, further research is needed to standardize the culture conditions and to increase the availability of last-generation technologies.
4. Mouse Models of Non-Alcoholic Fatty Liver Disease
4.1. Dietary Mouse Models of NAFLD
4.1.1. High-Fat Diet Feeding
Many studies have demonstrated that a diet rich in fat represents a major risk factor for the development of obesity and associated metabolic disorders such as NAFLD [76]. Accordingly, diets rich in fat, ranging from 45% to 75% of total calories, referred to as high-fat diets (HFDs), and derived from saturated fatty acids, polysaturated fatty acids and various combinations thereof, have been engineered to induce obesity in mice [77,78]. The source of fatty acids may vary from one to another supplier, making it difficult to standardize the diet from lab to lab [79]. Nevertheless, mice fed a “conventional” high-fat diet (i.e., with fat accounting for 45% of total calories) develop obesity, glucose intolerance, insulin resistance and hepatic steatosis, all key features of obesity and associated metabolic syndrome in humans [77,80,81]. While 3 months of HFD feeding is not sufficient to induce inflammation and fibrosis in mouse livers, a longer period of feeding, at least 6 months, may promote the development of these features [82]. Importantly, inconsistency in the development of NAFLD has been reported depending on the genetic background of the mouse strains [83]. The C57BL/6 strain seems to be more sensitive to HFD than the BALB/c strain, which exhibits a reduced hepatic lipid uptake and develops less inflammation than other strains [84]. Furthermore, C57BL/6J mice from the Jackson Laboratories, but not those from the National Institute of Health, Taconic Labs or Charles River, carry a spontaneous mutation in the Nnt (nicotinamide nucleotide transhydrogenase) gene, which is involved in mitochondrial redox functions contributing to NAFLD [85]. Gender differences in obesity-induced metabolic syndrome have also been reported in mice [86]. In summary, although widely used, HFD is not the best option to study NAFLD due to variations related to dietary compositions (source and nature of fatty acids), mouse strains, gender and duration of feeding. For all these reasons, during the past few years, other feeding regimens have emerged tending to reduce variability and to cover the entire spectrum of NAFLD (Figure 2).
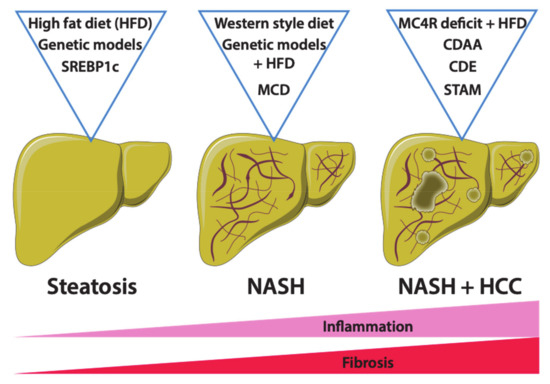
Figure 2.
Mouse models of NAFLD. Several mouse models have been described to mimic NAFLD in vivo. Both genetic and diet models are widely used to create experimental conditions of NAFLD and for preclinical drug testing. However, each model is specific as mice do no present all the typical features of NAFLD, from non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC). High-fat diet (HFD) and Western diet-fed mice, as well as genetic ob/ob (leptin deficiency) and db/db (leptin receptor mutation) mouse models, display metabolic syndrome and severe steatosis but no liver inflammation or fibrosis. However, the Methionine- and Choline-deficient diet (MCD) and its derivate diets such as Choline Deficient Ethionine-supplemented (CDE) and Choline-deficient L-amino acid-defined (CDAA) diets promote liver inflammation and fibrosis. The stelic animal model (STAM) associates a toxic injection of streptozotocin with an HFD, resulting in HCC development. Other genetic models, such as Melanocortin-4 receptor (MC4R) deficiency or Sterol regulatory element-binding proteins (SREBP1c) overexpression, predispose to metabolic syndrome and obesity but need a second hit (such as HFD) to trigger NASH.
4.1.2. Western-Style or Fast-Food Diet Feeding
The Western diet represents a modern dietary pattern found in industrialized countries and characterized by high intakes of fat and sugar. It is referred to as “fast food” diet, shown to be the major risk factor for the development of obesity [87]. By analogy to human nutrition, a dietary model based on the combination of both fat and fructose and sometimes cholesterol (referred to as Western-style or fast-food diet) has been developed to induce NAFLD in mice [88,89,90]. After 6 months of fast-food diet feeding, C57BL/6 mice develop obesity, insulin resistance as well as steatohepatitis [89]. These mice also have gene expression signatures of increased fibrosis, inflammation, endoplasmic reticulum stress and lipoapoptosis, all key features found in humans with NASH [89]. Furthermore, Tsuchiya et al. have shown that feeding a fat- and fructose-rich diet for up to 16 weeks leads to hepatic iron overload, a characteristic often found in humans with NAFLD [91]. This fast-food-diet-induced obesity appears to be a more robust model for human obesity in comparison with HFD, but the lack of a standardized diet interferes with reproducibility.
4.1.3. Methionine- and Choline-Deficient Diet and Derivative Feeding
Methionine- and choline-deficient (MCD) diet is a classical dietary model of NASH comprising high sucrose (40%) and fat (10%) but lacking the nutrients methionine and choline, which are essential components in animal and human nutrition [92]. Choline is the precursor of phosphatidylcholine, which is essential for very low-density lipoproteins (VLDL) production [93]. VLDL is an extracellular lipoprotein complex allowing the transport of triglycerides (TG) from the liver to the adipose tissue and muscles [94]. As for methionine, it is needed for glutathione synthesis, a major anti-oxidant protein [95]. Therefore, mice fed an MCD diet rapidly develop hepatic steatosis as a consequence of increased fatty acid uptake and decreased VLDL secretion. After two weeks, the development of steatosis is followed by cell death, inflammation and pericellular fibrosis [96,97,98]. In addition, oxidative stress and a rise in cytokines and adipokines occur in mice fed an MCD diet, which contributes to hepatic damage in this dietary model [99,100]. The MCD diet is easy to obtain and use, and it induces a more severe form of steatohepatitis than other dietary models, but it also has marked limitations. Notably, the main risk factors for the development of NAFLD in humans, namely overweight and insulin resistance, are lacking, and mice under the MCD diet lose weight progressively across feeding by up to 40% in 8 weeks [101,102]. Furthermore, several studies pointed out that the responsiveness of different mouse strains to the MCD diet varies considerably [103,104]. For all these reasons, caution is needed when using this dietary model, and the additional use of diet-induced obesity (DIO) models is desirable to examine the metabolic profile of the disease.
Choline-deficient, ethionine-supplemented (CDE) diet is derived from the MCD diet. Ethionine is a non-proteinogenic amino acid, structurally derived from methionine (i.e., an ethyl group instead of a methyl group), with the property of inducing steatohepatitis in a relatively short period-of-time [105]. Ethionine also exhibits hepatocarcinogen properties (i.e., DNA methylation interference), making CDE feeding of particular interest to study steatohepatitis-related hepatocellular carcinoma (HCC) [106]. However, this dietary model is associated with weight loss and a high rate of mortality, reaching 60% after 4 months of feeding [106,107]. To limit mortality, some studies alternate the use of a CDE diet with a normal chow diet [105]. This alternative process prevents the high rate of morbidity, whereas steatosis, inflammation and hepatocarcinogenesis are maintained [105].
The choline-deficient L-amino acid-defined (CDAA) diet is a choline-deficient diet in which proteins are replaced with an equimolar mixture of L-amino acids [82,86,108]. Like the MCD diet, CDAA inhibits fatty acid oxidation in hepatocytes, and it increases lipid synthesis, oxidative stress and inflammation, resulting in liver fibrosis, but these histological changes take longer to occur (from 3 weeks for steatosis and lobular inflammation to approximately 21 weeks for moderate fibrosis and 44 weeks for HCC) [86,109,110]. Mice fed a CDAA diet lose significantly less weight than mice under MCD or CDE diets but still do not display hepatic insulin resistance, weight gain or changes in peripheral insulin sensitivity [86,111,112]. Nevertheless, as reported by Miura et al., insulin resistance may develop upon CDAA feeding [113]. It might depend on the dietary compositions (e.g., percentage of fat) and the duration of the feeding. Hence, due to low reproducibility between the different sources of CDAA, this diet should not be used either to examine the metabolic profile of the disease.
4.1.4. STAM Model
The STAM model associates a single injection of streptozotocin (STZ) as a first hit and an HFD diet as a second hit. STZ is an antibiotic produced by Streptomyces achromogens that acts as a DNA alkylating agent, initially used as a type I diabetes inducer due to severe pancreatic islet inflammation and destruction [114]. This model results in steatohepatitis at 8 weeks and liver fibrosis at 12 weeks and leads to HCC in virtually 100% of male mice at 20 weeks [109,115]. The hepatic lipidomic profile of STAM mice was found to be very similar to that of humans with NASH despite the chemical intervention and the absence of obesity in this model [116].
4.2. Genetic Models of NAFLD
Here, we only review genetic models that most closely replicate the disease spectrum of NAFLD, including obesity and metabolic syndrome. Other genetic mouse models of NAFLD have been reviewed elsewhere [86,117].
4.2.1. ob/ob Mice
Genetically modified mice based on leptin deficiency have been developed to better understand NAFLD. Leptin, also named the “satiety hormone”, is a peptide secreted predominantly by the white adipose tissue to negatively regulate food intake and to increase energy expenditure [118]. Its anorexic effect is mediated by its hypothalamic receptor, which is responsible for the transmission of the satiety signal [119]. ob/ob mice harbor a homozygous point mutation for the gene encoding leptin. They are hyperphagic and inactive and develop severe obesity, hyperlipidemia, hyperglycemia, hyperinsulinemia and insulin resistance [120]. Although hyperphagia contributes to obesity, leptin deficiency is not an important contributor to NAFLD [121]. Indeed, serum leptin levels are normal or elevated in NAFLD [122,123,124]. In ob/ob mice, fat accumulation in the liver induces steatosis and hepato-lipotoxicity but rarely progress to steatohepatitis and fibrosis [125]. Hence, a second hit is needed to trigger fibrosis, such as hepatotoxic agents (e.g., CCl4) or MCD diet, and steatohepatitis can be studied using high-caloric diets in these animals [126,127].
4.2.2. db/db Mice
In opposition to ob/ob mice, db/db mice carry a spontaneous mutation in the gene encoding the leptin receptor. Although these mice have increased levels of leptin, they develop leptin resistance conferred by the mutation of its receptor. In general, the liver histology is quite similar to that in ob/ob mice, and db/db mice are also obese, hyperphagic, insulin-resistant, hyperglycemic and hyperinsulinemic and develop hepatic steatosis [109]. However, like ob/ob mice, db/db mice do not display the whole spectrum of human NASH histopathology, and secondary stimuli are necessary to induce steatohepatitis and fibrosis [128]. Thus, the observations derived from monogenic models such as db/db and ob/ob mice may differ from the human population in which obesity is known to be a multifactorial disease.
4.2.3. The Mc4r-Deficient Mice
The binding of leptin on pro-opiomelanocortin (POMC) neurons leads to alpha-melanocyte-stimulating hormone (α-MSH) secretion [129]. This hormone mediates the anorectic signal after binding on the hypothalamic Melanocortin-4 receptor (MC4R) [129,130]. In human pathology, MC4R deficiency is responsible for 6% of monogenic obesity [131,132]. Hence, genetically modified mice with Mc4r deficiency have been developed to better understand NAFLD. MC4R-deficient mice are characterized by early onset of obesity associated with hyperphagia, hyperinsulinemia and hyperglycemia under a regular chow diet [133,134]. Moreover, when fed an HFD, MC4R-deficient mice develop steatohepatitis and fibrosis, and the incidence of HCC is increased after a long period of feeding [135]. Thus, MC4R-deficient mice appear suitable to examine the effects of drugs on the development of steatohepatitis when fed an HFD.
4.2.4. The Srebp1c-Overexpressing Mice
Sterol regulatory element-binding proteins (SREBPs) are a family of transcription factors that upregulate genes involved in FFA and cholesterol synthesis [136,137]. For instance, the overexpression of SREBP1c in hepatocytes results in liver triglyceride accumulation and ER stress induction [138,139]. Moreover, these mice have increased serum FFA and triglycerides that correlate with increased visceral adipose tissue, demonstrating that pathological dysfunctions of the liver contribute to visceral adipogenicity [136,140,141,142]. However, hepatic SREBP1c overexpression is not sufficient to induce inflammation and fibrosis [141]. Hence, fast food diet feeding is secondarily needed to trigger steatohepatitis and fibrosis in these mice [143,144].
4.2.5. The FATZO Mouse Model
The FATZO mouse model, also known as the MS-NASH model, was developed by crossing C57BL/6J and AKR/J mice, two strains that have a strong propensity to develop obesity when fed a high-fat diet, followed by selective inbreeding [145,146]. The crossing of these two strains and the selective inbreeding of the subsequent generations resulted in obesity, metabolic syndrome and insulin resistance predisposition. Sun et al. have shown that FATZO mice fed a fast-food diet supplemented with 5% of fructose display evidence of NASH, including hepatic steatosis, lobular inflammation, ballooning and fibrosis. The FATZO mice also had hypercholesterolemia and progressive elevation of ALT and AST compared with FATZO mice fed a normal chow diet [147]. Unlike monogenic leptin-deficient ob/ob and db/db mouse models, the FATZO mouse model is a polygenic inheritance of predisposition to obesity and diabetes, with an intact leptin pathway, thereby making it more translatable to the human disease [147]. However, selective inbreeding can contribute to a significant decrease in the genetic variability that may introduce a bias in preclinical drug testing.
Taken together, as compared with DIO, genetic models show a generally more severe disease phenotype within a shorter time frame when a diet is used to induce steatohepatitis and fibrosis. However, genetic mutations associated with these models do not reflect NAFLD etiology in humans, which limits interpretation of the results.
5. Other Animal Models of Non-Alcoholic Fatty Liver Disease
5.1. Rat Models
Most of the dietary mouse models of NAFLD, including DIO such as HFD and Western diet, but also non-obesogenic diets such as MCD and CDAA, have been widely used in rats [148,149,150]. Rats are typically more susceptible to diet-induced NAFLD than mice, and they often progress spontaneously towards steatohepatitis and fibrosis [86,151,152]. Gender and genetic background of the different rat strains may explain their susceptibility to developing diet-induced features of NAFLD [153]. Similar to db/db mice, Zucker fatty (fa/fa) rats have a mutated leptin receptor that decreases their affinity for leptin [142,154]. These rats develop severe obesity that leads to insulin resistance and hepatic steatosis [142,155]. Like in db/db mice, the spontaneous progression of steatosis towards steatohepatitis is rare, and a second hit, such as a DIO, is needed [155,156]. Globally, the same variabilities seen in mice apply to the rat model of NAFLD, and dietary compositions (source and nature of fatty acids), strains, gender and duration of feeding have to be rigorously defined before experiments.
5.2. Non-Rodent Models
Different minipig species such as Ossabaw and Göttingen have been studied to model NAFLD [157,158]. Due to a longer lifetime than rodents, minipigs develop obesity and metabolic syndrome that is are similar to humans, which allow long-term studies [157,159]. Therefore, minipigs drug toxicity studies appear more manageable and predictive to humans than rodents [160]. However, DIO does not lead to hepatic steatosis in minipigs, most likely because of the absence of liver de novo lipogenesis [161,162]. Therefore, alternative non-obesogenic feedings have to be used, and for instance, Pedersen et al. have shown that steatohepatitis can be induced within 8 weeks in minipigs fed a CDAA diet [157]. Although minipig physiology is closer to humans than rodents, liver metabolic pathways are somehow divergent, which could limit the interpretation of the results. Furthermore, cost-effectiveness ratio and ethical and legal concerns limit the use of minipigs.
Primates, especially rhesus monkeys, have been proposed to study NAFLD because of their genetic proximity with humans [163]. However, for ethical reasons and due to the high cost of studies, they are rarely used.
6. Conclusions
This review has explored the different in vivo and in vitro models used to study the pathophysiology of human NAFLD. Recent technological advances in cell culture methods (i.e., organoids, printing on a chip) promise to reduce the use of animal models for toxicological studies. Indeed, liver-on-a-chip may closely reproduce the lobular architecture of the liver, and therefore appears to be efficient to study cell-cell interactions that are essential to understand the development of NAFLD. Although the complexity of in vitro models, which better mimic cell interactions of a native liver, has dramatically increased in the past few years, these cell culture models remain imperfect and need further technological improvements and reduced cost. Therefore, in vivo models, notably murine models, are still widely used to identify metabolic pathways that lead to NASH and to investigate new therapeutics targets. Nevertheless, the extension of preclinical findings to humans are quite disappointing in many cases. This interspecies difference, which is now a challenge for research in NAFLD, could be explained for example by the difference in microbiome composition, which seems to play an important role in NAFLD pathogenesis [164]. Disease course is also different between rodents and humans, especially regarding fibrosis progression. Thus, combining NAFLD in vitro and in vivo models could be a solution to accumulate sufficient preclinical evidences to consider a clinical development of a drug.
Author Contributions
Conceptualization, P.-A.S., J.M. and J.G.; writing—original draft preparation, P.-A.S., J.M. and J.G.; writing—review and editing, P.-A.S., C.H. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
J.G. is supported by grants from the Mairie de Paris (Emergences), the Société Francophone du Diabète (SFD), the Institute of Cardiometabolism and Nutrition (ICAN), and the Fondation pour la Recherche Médicale (FRM grant numbers ARF20170938613 & EQU202003010517). P-A.S. is supported by Société National Française de Gastro-Entérologie (SNFGE). C.H. is supported by the FRM (EQU202003010517) and the microbiome foundation.
Acknowledgments
The authors would like to thank Yves Chrétien for expert artwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-Alcoholic Fatty Liver Disease—A Global Public Health Perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Barritt, A.S.; Calmus, Y.; Scatton, O.; Runge, T.; Lebray, P.; Poynard, T.; Ratziu, V.; Conti, F. NAFLD and Liver Transplantation: Current Burden and Expected Challenges. J. Hepatol. 2016, 65, 1245–1257. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of Primary Human Hepatocytes and Hepatoma Cell Line Hepg2 with Regard to Their Biotransformation Properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef]
- Andersson, T.B.; Kanebratt, K.P.; Kenna, J.G. The HepaRG Cell Line: A Unique in Vitro Tool for Understanding Drug Metabolism and Toxicology in Human. Expert Opin. Drug Metab. Toxicol. 2012, 8, 909–920. [Google Scholar] [CrossRef]
- Samanez, C.H.; Caron, S.; Briand, O.; Dehondt, H.; Duplan, I.; Kuipers, F.; Hennuyer, N.; Clavey, V.; Staels, B. The Human Hepatocyte Cell Lines IHH and HepaRG: Models to Study Glucose, Lipid and Lipoprotein Metabolism. Arch. Physiol. Biochem. 2012, 118, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ramboer, E.; De Craene, B.; De Kock, J.; Vanhaecke, T.; Berx, G.; Rogiers, V.; Vinken, M. Strategies for Immortalization of Primary Hepatocytes. J. Hepatol. 2014, 61, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Guguen-Guillouzo, C.; Guillouzo, A. General Review on In Vitro Hepatocyte Models and Their Applications. In Hepatocytes: Methods and Protocols; Maurel, P., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 1–40. ISBN 978-1-60761-688-7. [Google Scholar]
- Ramboer, E.; Vanhaecke, T.; Rogiers, V.; Vinken, M. Immortalized Human Hepatic Cell Lines for In Vitro Testing and Research Purposes. Methods Mol. Biol. Clifton NJ 2015, 1250, 53–76. [Google Scholar] [CrossRef]
- Aoudjehane, L.; Gautheron, J.; Goff, W.L.; Goumard, C.; Gilaizeau, J.; Nget, C.S.; Savier, E.; Atif, M.; Lesnik, P.; Morichon, R.; et al. Novel Defatting Strategies Reduce Lipid Accumulation in Primary Human Culture Models of Liver Steatosis. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Podevin, P.; Carpentier, A.; Pène, V.; Aoudjehane, L.; Carrière, M.; Zaïdi, S.; Hernandez, C.; Calle, V.; Méritet, J.-F.; Scatton, O.; et al. Production of Infectious Hepatitis C Virus in Primary Cultures of Human Adult Hepatocytes. Gastroenterology 2010, 139, 1355–1364. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Donato, M.T.; Castell, J.V.; Jover, R. Human Hepatocytes as a Tool for Studying Toxicity and Drug Metabolism. Curr. Drug Metab. 2003, 4, 292–312. [Google Scholar] [CrossRef]
- Godoy, P.; Hengstler, J.G.; Ilkavets, I.; Meyer, C.; Bachmann, A.; Müller, A.; Tuschl, G.; Mueller, S.O.; Dooley, S. Extracellular Matrix Modulates Sensitivity of Hepatocytes to Fibroblastoid Dedifferentiation and Transforming Growth Factor β–Induced Apoptosis. Hepatology 2009, 49, 2031–2043. [Google Scholar] [CrossRef]
- Schyschka, L.; Sánchez, J.J.M.; Wang, Z.; Burkhardt, B.; Müller-Vieira, U.; Zeilinger, K.; Bachmann, A.; Nadalin, S.; Damm, G.; Nussler, A.K. Hepatic 3D Cultures but Not 2D Cultures Preserve Specific Transporter Activity for Acetaminophen-Induced Hepatotoxicity. Arch. Toxicol. 2013, 87, 1581–1593. [Google Scholar] [CrossRef]
- Zeilinger, K.; Freyer, N.; Damm, G.; Seehofer, D.; Knöspel, F. Cell Sources for in Vitro Human Liver Cell Culture Models. Exp. Biol. Med. 2016, 241, 1684–1698. [Google Scholar] [CrossRef]
- Richert, L.; Alexandre, E.; Lloyd, T.; Orr, S.; Viollon-Abadie, C.; Patel, R.; Kingston, S.; Berry, D.; Dennison, A.; Heyd, B.; et al. Tissue Collection, Transport and Isolation Procedures Required to Optimize Human Hepatocyte Isolation from Waste Liver Surgical Resections. A Multilaboratory Study. Liver Int. 2004, 24, 371–378. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. In Vitro Culture of Isolated Primary Hepatocytes and Stem Cell-Derived Hepatocyte-like Cells for Liver Regeneration. Protein Cell 2015, 6, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Reyes, M.; Koodie, L.; Jiang, Y.; Blackstad, M.; Lund, T.; Lenvik, T.; Johnson, S.; Hu, W.-S.; Verfaillie, C.M. Multipotent Adult Progenitor Cells from Bone Marrow Differentiate into Functional Hepatocyte-like Cells. J. Clin. Investig. 2002, 109, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Touboul, T.; Hannan, N.R.F.; Corbineau, S.; Martinez, A.; Martinet, C.; Branchereau, S.; Mainot, S.; Strick-Marchand, H.; Pedersen, R.; Di Santo, J.; et al. Generation of Functional Hepatocytes from Human Embryonic Stem Cells under Chemically Defined Conditions That Recapitulate Liver Development. Hepatology 2010, 51, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Robinton, D.A.; Daley, G.Q. The Promise of Induced Pluripotent Stem Cells in Research and Therapy. Nature 2012, 481, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.R.F.; Segeritz, C.-P.; Touboul, T.; Vallier, L. Production of Hepatocyte-like Cells from Human Pluripotent Stem Cells. Nat. Protoc. 2013, 8, 430–437. [Google Scholar] [CrossRef]
- Lu, J.; Einhorn, S.; Venkatarangan, L.; Miller, M.; Mann, D.A.; Watkins, P.B.; LeCluyse, E. Morphological and Functional Characterization and Assessment of IPSC-Derived Hepatocytes for In Vitro Toxicity Testing. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 147, 39–54. [Google Scholar] [CrossRef]
- Wobser, H.; Dorn, C.; Weiss, T.S.; Amann, T.; Bollheimer, C.; Büttner, R.; Schölmerich, J.; Hellerbrand, C. Lipid Accumulation in Hepatocytes Induces Fibrogenic Activation of Hepatic Stellate Cells. Cell Res. 2009, 19, 996–1005. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Ning, C.; Lei, D.; Ren, J. Endoplasmic Reticulum Stress Related Molecular Mechanisms in Nonalcoholic Fatty Liver Disease (NAFLD). Curr. Drug Targets 2018, 19, 1087–1094. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic Reticulum Stress Signalling and the Pathogenesis of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Bai, X.; Hong, W.; Cai, P.; Chen, Y.; Xu, C.; Cao, D.; Yu, W.; Zhao, Z.; Huang, M.; Jin, J. Valproate Induced Hepatic Steatosis by Enhanced Fatty Acid Uptake and Triglyceride Synthesis. Toxicol. Appl. Pharmacol. 2017, 324, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ding, D.; Huang, Q.; Liu, Q.; Lu, H.; Lu, Y.; Chi, Y.; Sun, X.; Ye, G.; Zhu, H.; et al. Downregulation of MiR-192 Causes Hepatic Steatosis and Lipid Accumulation by Inducing SREBF1: Novel Mechanism for Bisphenol A-Triggered Non-Alcoholic Fatty Liver Disease. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2017, 1862, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Kirby, R.J.; Khorasanizadeh, S.; Rastinejad, F.; Malany, S. A Nonalcoholic Fatty Liver Disease Model in Human Induced Pluripotent Stem Cell-Derived Hepatocytes, Created by Endoplasmic Reticulum Stress-Induced Steatosis. Dis. Model. Mech. 2018, 11, dmm033530. [Google Scholar] [CrossRef]
- Ling, J.; Lewis, J.; Douglas, D.; Kneteman, N.M.; Vance, D.E. Characterization of Lipid and Lipoprotein Metabolism in Primary Human Hepatocytes. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Johnson, D.; Amin, H.D.; Sivathondan, P.; Silva, M.A.; Wang, L.M.; Stevanato, L.; McNeil, C.A.; Miljan, E.A.; Sinden, J.D.; et al. Characterization of Lipid Metabolism in a Novel Immortalized Human Hepatocyte Cell Line. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E511–E522. [Google Scholar] [CrossRef]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, R.M. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2020, 9, 37. [Google Scholar] [CrossRef]
- Giraudi, P.J.; Barbero Becerra, V.J.; Marin, V.; Chavez-Tapia, N.C.; Tiribelli, C.; Rosso, N. The Importance of the Interaction between Hepatocyte and Hepatic Stellate Cells in Fibrogenesis Induced by Fatty Accumulation. Exp. Mol. Pathol. 2015, 98, 85–92. [Google Scholar] [CrossRef]
- Barbero-Becerra, V.J.; Giraudi, P.J.; Chávez-Tapia, N.C.; Uribe, M.; Tiribelli, C.; Rosso, N. The Interplay between Hepatic Stellate Cells and Hepatocytes in an in Vitro Model of NASH. Toxicol. Vitr. 2015, 29, 1753–1758. [Google Scholar] [CrossRef]
- Bale, S.S.; Golberg, I.; Jindal, R.; McCarty, W.J.; Luitje, M.; Hegde, M.; Bhushan, A.; Usta, O.B.; Yarmush, M.L. Long-Term Coculture Strategies for Primary Hepatocytes and Liver Sinusoidal Endothelial Cells. Tissue Eng. Part C Methods 2014, 21, 413–422. [Google Scholar] [CrossRef]
- Suurmond, C.-A.E.; Lasli, S.; van den Dolder, F.W.; Ung, A.; Kim, H.-J.; Bandaru, P.; Lee, K.; Cho, H.-J.; Ahadian, S.; Ashammakhi, N.; et al. In Vitro Human Liver Model of Nonalcoholic Steatohepatitis by Coculturing Hepatocytes, Endothelial Cells, and Kupffer Cells. Adv. Healthc. Mater. 2019, 8, 1901379. [Google Scholar] [CrossRef]
- Müller, F.A.; Sturla, S.J. Human in Vitro Models of Nonalcoholic Fatty Liver Disease. Curr. Opin. Toxicol. 2019, 16, 9–16. [Google Scholar] [CrossRef]
- Kim, B.-M.; Abdelfattah, A.M.; Vasan, R.; Fuchs, B.C.; Choi, M.Y. Hepatic Stellate Cells Secrete Ccl5 to Induce Hepatocyte Steatosis. Sci. Rep. 2018, 8, 7499. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.C.Y.; Tompkins, R.G.; Yarmush, M.L. Long-Term in Vitro Function of Adult Hepatocytes in a Collagen Sandwich Configuration. Biotechnol. Prog. 1991, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, F.; Moghe, P.V.; Toner, M.; Yarmush, M.L. Effect of Extracellular Matrix Topology on Cell Structure, Function, and Physiological Responsiveness: Hepatocytes Cultured in a Sandwich Configuration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1996, 10, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Harris, L.M.; Murphey, B.S.; Sowell, B.L. Use of Three-Dimensional Spheroids of Hepatocyte-Derived Reporter Cells to Study the Effects of Intracellular Fat Accumulation and Subsequent Cytokine Exposure. Biotechnol. Bioeng. 2011, 108, 1171–1180. [Google Scholar] [CrossRef]
- Ota, H.; Miki, N. Microtechnology-Based Three-Dimensional Spheroid Formation. Front. Biosci. Elite Ed. 2013, 5, 37–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Grunsven, L.A. 3D in Vitro Models of Liver Fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 133–146. [Google Scholar] [CrossRef]
- Kozyra, M.; Johansson, I.; Nordling, Å.; Ullah, S.; Lauschke, V.M.; Ingelman-Sundberg, M. Human Hepatic 3D Spheroids as a Model for Steatosis and Insulin Resistance. Sci. Rep. 2018, 8, 14297. [Google Scholar] [CrossRef]
- Pingitore, P.; Sasidharan, K.; Ekstrand, M.; Prill, S.; Lindén, D.; Romeo, S. Human Multilineage 3D Spheroids as a Model of Liver Steatosis and Fibrosis. Int. J. Mol. Sci. 2019, 20, 1629. [Google Scholar] [CrossRef]
- Bell, C.C.; Hendriks, D.F.G.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Puigvert, L.F.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of Primary Human Hepatocyte Spheroids as a Model System for Drug-Induced Liver Injury, Liver Function and Disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef]
- Rogozhnikov, D.; Luo, W.; Elahipanah, S.; O’Brien, P.J.; Yousaf, M.N. Generation of a Scaffold-Free Three-Dimensional Liver Tissue via a Rapid Cell-to-Cell Click Assembly Process. Bioconjug. Chem. 2016, 27, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Baze, A.; Parmentier, C.; Hendriks, D.F.G.; Hurrell, T.; Heyd, B.; Bachellier, P.; Schuster, C.; Ingelman-Sundberg, M.; Richert, L. Three-Dimensional Spheroid Primary Human Hepatocytes in Monoculture and Coculture with Nonparenchymal Cells. Tissue Eng. Part C Methods 2018, 24, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, T.; Kastrinou-Lampou, V.; Fardellas, A.; Hendriks, D.F.G.; Nordling, Å.; Johansson, I.; Baze, A.; Parmentier, C.; Richert, L.; Ingelman-Sundberg, M. Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis. Cells 2020, 9, 964. [Google Scholar] [CrossRef] [PubMed]
- Underhill, G.H.; Khetani, S.R. Advances in Engineered Human Liver Platforms for Drug Metabolism Studies. Drug Metab. Dispos. 2018, 46, 1626–1637. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345. [Google Scholar] [CrossRef]
- Wu, F.; Wu, D.; Ren, Y.; Huang, Y.; Feng, B.; Zhao, N.; Zhang, T.; Chen, X.; Chen, S.; Xu, A. Generation of Hepatobiliary Organoids from Human Induced Pluripotent Stem Cells. J. Hepatol. 2019, 70, 1145–1158. [Google Scholar] [CrossRef]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef]
- Kostrzewski, T.; Cornforth, T.; Snow, S.A.; Ouro-Gnao, L.; Rowe, C.; Large, E.M.; Hughes, D.J. Three-Dimensional Perfused Human in Vitro Model of Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2017, 23, 204–215. [Google Scholar] [CrossRef]
- Ramli, M.N.B.; Lim, Y.S.; Koe, C.T.; Demircioglu, D.; Tng, W.; Gonzales, K.A.U.; Tan, C.P.; Szczerbinska, I.; Liang, H.; Soe, E.L.; et al. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology 2020, 159, 1471–1486.e12. [Google Scholar] [CrossRef]
- De l’Hortet, A.C.; Takeishi, K.; Guzman-Lepe, J.; Morita, K.; Achreja, A.; Popovic, B.; Wang, Y.; Handa, K.; Mittal, A.; Meurs, N.; et al. Generation of Human Fatty Livers Using Custom-Engineered Induced Pluripotent Stem Cells with Modifiable SIRT1 Metabolism. Cell Metab. 2019, 30, 385–401.e9. [Google Scholar] [CrossRef]
- Ding, R.-B.; Bao, J.; Deng, C.-X. Emerging Roles of SIRT1 in Fatty Liver Diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Banaeiyan, A.A.; Theobald, J.; Paukštyte, J.; Wölfl, S.; Adiels, C.B.; Goksör, M. Design and Fabrication of a Scalable Liver-Lobule-on-a-Chip Microphysiological Platform. Biofabrication 2017, 9, 015014. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Sebastian, S.; Maharjan, S.; Lesha, A.; Carpenter, A.-M.; Liu, X.; Xie, X.; Livermore, C.; Zhang, Y.S.; Zarrinpar, A. Liver-on-a-Chip Models of Fatty Liver Disease. Hepatology 2020, 71, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Rennert, K.; Steinborn, S.; Gröger, M.; Ungerböck, B.; Jank, A.-M.; Ehgartner, J.; Nietzsche, S.; Dinger, J.; Kiehntopf, M.; Funke, H.; et al. A Microfluidically Perfused Three Dimensional Human Liver Model. Biomaterials 2015, 71, 119–131. [Google Scholar] [CrossRef]
- Gori, M.; Simonelli, M.C.; Giannitelli, S.M.; Businaro, L.; Trombetta, M.; Rainer, A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. PLoS ONE 2016, 11, e0159729. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sung, J.H. Gut–Liver on a Chip toward an in Vitro Model of Hepatic Steatosis. Biotechnol. Bioeng. 2018, 115, 2817–2827. [Google Scholar] [CrossRef]
- Green, C.J.; Pramfalk, C.; Morten, K.J.; Hodson, L. From Whole Body to Cellular Models of Hepatic Triglyceride Metabolism: Man Has Got to Know His Limitations. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1–E20. [Google Scholar] [CrossRef]
- Van de Bovenkamp, M.; Groothuis, G.M.M.; Meijer, D.K.F.; Olinga, P. Liver Fibrosis in Vitro: Cell Culture Models and Precision-Cut Liver Slices. Toxicol. Vitr. 2007, 21, 545–557. [Google Scholar] [CrossRef]
- Palma, E.; Doornebal, E.J.; Chokshi, S. Precision-Cut Liver Slices: A Versatile Tool to Advance Liver Research. Hepatol. Int. 2019, 13, 51–57. [Google Scholar] [CrossRef]
- Kartasheva, D.; Gaston, J.; Scatton, O.; Vaillant, J.-C.; Morozov, V.A.; Stanislas, P.; Lagaye, S. Establishment of an Ex Vivo Model of Human Fibrotic Liver Slices Culture: Characterization of Intrahepatic Immune Cells and TH17 Cytokines. J. Hepatol. 2018, 68, S405–S406. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, J.; Tacke, F. How Effective Are Nonalcoholic Fatty Liver Disease Models for Drug Discovery? Expert Opin. Drug Discov. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thiele, G.M.; Duryee, M.J.; Thiele, G.E.; Tuma, D.J.; Klassen, L.W. Review: Precision Cut Liver Slices for the Evaluation of Fatty Liver and Fibrosis. Curr. Mol. Pharmacol. 2017, 10, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Barrera, F.; George, J. The Role of Diet and Nutritional Intervention for the Management of Patients with NAFLD. Clin. Liver Dis. 2014, 18, 91–112. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liao, J.K. A Mouse Model of Diet-Induced Obesity and Insulin Resistance. Methods Mol. Biol. Clifton NJ 2012, 821, 421. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-Fat Diet-Induced Obesity in Animal Models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You Are What You Eat, or Are You? The Challenges of Translating High-Fat-Fed Rodents to Human Obesity and Diabetes. Nutr. Diabetes 2014, 4, e135. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The High-Fat Diet–Fed Mouse: A Model for Studying Mechanisms and Treatment of Impaired Glucose Tolerance and Type 2 Diabetes. Diabetes 2004, 53, S215–S219. [Google Scholar] [CrossRef]
- Aydos, L.R.; Amaral, L.A.D.; de Souza, R.S.; Jacobowski, A.C.; dos Santos, E.F.; Macedo, M.L.R. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 2019, 11, 3067. [Google Scholar] [CrossRef]
- Nevzorova, Y.A.; Boyer-Diaz, Z.; Cubero, F.J.; Gracia-Sancho, J. Animal Models for Liver Disease—A Practical Approach for Translational Research. J. Hepatol. 2020, 73, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Asgharpour, A.; Cazanave, S.C.; Pacana, T.; Seneshaw, M.; Vincent, R.; Banini, B.A.; Kumar, D.P.; Daita, K.; Min, H.-K.; Mirshahi, F.; et al. A Diet-Induced Animal Model of Non-Alcoholic Fatty Liver Disease and Hepatocellular Cancer. J. Hepatol. 2016, 65, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse Strain-Dependent Variation in Obesity and Glucose Homeostasis in Response to High-Fat Feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.F.; Vercesi, A.E.; Castilho, R.F. A Spontaneous Mutation in the Nicotinamide Nucleotide Transhydrogenase Gene of C57BL/6J Mice Results in Mitochondrial Redox Abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Malhi, H.; Gores, G.J. Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig. Dis. Sci. 2016, 61, 1325–1336. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Kohli, R.; Kirby, M.; Xanthakos, S.A.; Softic, S.; Feldstein, A.E.; Saxena, V.; Tang, P.H.; Miles, L.; Miles, M.V.; Balistreri, W.F.; et al. High-Fructose, Medium Chain Trans Fat Diet Induces Liver Fibrosis and Elevates Plasma Coenzyme Q9 in a Novel Murine Model of Obesity and Nonalcoholic Steatohepatitis. Hepatology 2010, 52, 934–944. [Google Scholar] [CrossRef]
- Charlton, M.; Krishnan, A.; Viker, K.; Sanderson, S.; Cazanave, S.; McConico, A.; Masuoko, H.; Gores, G. Fast Food Diet Mouse: Novel Small Animal Model of NASH with Ballooning, Progressive Fibrosis, and High Physiological Fidelity to the Human Condition. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G825–G834. [Google Scholar] [CrossRef]
- Henkel, J.; Coleman, C.D.; Schraplau, A.; Jöhrens, K.; Weber, D.; Castro, J.P.; Hugo, M.; Schulz, T.J.; Krämer, S.; Schürmann, A.; et al. Induction of Steatohepatitis (NASH) with Insulin Resistance in Wild-Type B6 Mice by a Western-Type Diet Containing Soybean Oil and Cholesterol. Mol. Med. 2017, 23, 70–82. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Ebata, Y.; Sakabe, T.; Hama, S.; Kogure, K.; Shiota, G. High-Fat, High-Fructose Diet Induces Hepatic Iron Overload via a Hepcidin-Independent Mechanism Prior to the Onset of Liver Steatosis and Insulin Resistance in Mice. Metabolism 2013, 62, 62–69. [Google Scholar] [CrossRef]
- Itagaki, H.; Shimizu, K.; Morikawa, S.; Ogawa, K.; Ezaki, T. Morphological and Functional Characterization of Non-Alcoholic Fatty Liver Disease Induced by a Methionine-Choline-Deficient Diet in C57BL/6 Mice. Int. J. Clin. Exp. Pathol. 2013, 6, 2683–2696. [Google Scholar] [PubMed]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine Biosynthesis and Lipoprotein Metabolism. Biochim. Biophys. Acta 2012, 1821, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, G.F.; Wiggins, D.; Brown, A.-M.; Hebbachi, A.-M. Synthesis and Function of Hepatic Very-Low-Density Lipoprotein. Biochem. Soc. Trans. 2004, 32, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; Neuman, M. Methionine Deficiency and Hepatic Injury in a Dietary Steatohepatitis Model. Dig. Dis. Sci. 2008, 53, 767–776. [Google Scholar] [CrossRef]
- Gautheron, J.; Vucur, M.; Reisinger, F.; Cardenas, D.V.; Roderburg, C.; Koppe, C.; Kreggenwinkel, K.; Schneider, A.T.; Bartneck, M.; Neumann, U.P.; et al. A Positive Feedback Loop between RIP3 and JNK Controls Non-Alcoholic Steatohepatitis. EMBO Mol. Med. 2014, 6, 1062–1074. [Google Scholar] [CrossRef]
- Caballero, F.; Fernández, A.; Matías, N.; Martínez, L.; Fucho, R.; Elena, M.; Caballeria, J.; Morales, A.; Fernández-Checa, J.C.; García-Ruiz, C. Specific Contribution of Methionine and Choline in Nutritional Nonalcoholic Steatohepatitis: Impact on Mitochondrial S-Adenosyl-L-Methionine and Glutathione. J. Biol. Chem. 2010, 285, 18528–18536. [Google Scholar] [CrossRef]
- Greene, M.W.; Burrington, C.M.; Lynch, D.T.; Davenport, S.K.; Johnson, A.K.; Horsman, M.J.; Chowdhry, S.; Zhang, J.; Sparks, J.D.; Tirrell, P.C. Lipid Metabolism, Oxidative Stress and Cell Death Are Regulated by PKC Delta in a Dietary Model of Nonalcoholic Steatohepatitis. PLoS ONE 2014, 9, e85848. [Google Scholar] [CrossRef]
- Peña, A.D.; Leclercq, I.; Field, J.; George, J.; Jones, B.; Farrell, G. NF-ΚB Activation, Rather Than TNF, Mediates Hepatic Inflammation in a Murine Dietary Model of Steatohepatitis. Gastroenterology 2005, 129, 1663–1674. [Google Scholar] [CrossRef]
- Rinella, M.E.; Green, R.M. The Methionine-Choline Deficient Dietary Model of Steatohepatitis Does Not Exhibit Insulin Resistance. J. Hepatol. 2004, 40, 47–51. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M.; Williams, J.; Bell-Anderson, K.S.; Farrell, G.C. MCD-Induced Steatohepatitis Is Associated with Hepatic Adiponectin Resistance and Adipogenic Transformation of Hepatocytes. J. Hepatol. 2008, 49, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kakizaki, S.; Takizawa, D.; Ichikawa, T.; Sato, K.; Takagi, H.; Mori, M. Interstrain Differences in Susceptibility to Non-Alcoholic Steatohepatitis. J. Gastroenterol. Hepatol. 2008, 23, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tryndyak, V.; de Conti, A.; Kobets, T.; Kutanzi, K.; Koturbash, I.; Han, T.; Fuscoe, J.C.; Latendresse, J.R.; Melnyk, S.; Shymonyak, S.; et al. Interstrain Differences in the Severity of Liver Injury Induced by a Choline- and Folate-Deficient Diet in Mice Are Associated with Dysregulation of Genes Involved in Lipid Metabolism. FASEB J. 2012, 26, 4592–4602. [Google Scholar] [CrossRef]
- Passman, A.M.; Strauss, R.P.; McSpadden, S.B.; Finch-Edmondson, M.L.; Woo, K.H.; Diepeveen, L.A.; London, R.; Callus, B.A.; Yeoh, G.C. A Modified Choline-Deficient, Ethionine-Supplemented Diet Reduces Morbidity and Retains a Liver Progenitor Cell Response in Mice. Dis. Model. Mech. 2015, 8, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Pérez-Martínez, L.; Rubio-Mediavilla, S.; Oteo, J.A.; Martínez, A.; Blanco, J.R. Maraviroc, a CCR5 Antagonist, Prevents Development of Hepatocellular Carcinoma in a Mouse Model. PLoS ONE 2013, 8, e53992. [Google Scholar] [CrossRef]
- Gogoi-Tiwari, J.; Köhn-Gaone, J.; Giles, C.; Schmidt-Arras, D.; Gratte, F.D.; Elsegood, C.L.; McCaughan, G.W.; Ramm, G.A.; Olynyk, J.K.; Tirnitz-Parker, J.E.E. The Murine Choline-Deficient, Ethionine-Supplemented (CDE) Diet Model of Chronic Liver Injury. J. Vis. Exp. 2017, e56138. [Google Scholar] [CrossRef]
- Nakae, D.; Mizumoto, Y.; Andoh, N.; Tamura, K.; Horiguchi, K.; Endoh, T.; Kobayashi, E.; Tsujiuchi, T.; Denda, A.; Lombardi, B.; et al. Comparative Changes in the Liver of Female Fischer-344 Rats after Short-Term Feeding of a Semipurified or a Semisynthetic L-Amino Acid-Defined Choline-Deficient Diet. Toxicol. Pathol. 1995, 23, 583–590. [Google Scholar] [CrossRef]
- Hansen, H.H.; Feigh, M.; Veidal, S.S.; Rigbolt, K.T.; Vrang, N.; Fosgerau, K. Mouse Models of Nonalcoholic Steatohepatitis in Preclinical Drug Development. Drug Discov. Today 2017, 22, 1707–1718. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hada, N.; Sakamaki, Y.; Uno, A.; Shiga, T.; Tanaka, C.; Ito, T.; Katsume, A.; Sudoh, M. An Improved Mouse Model That Rapidly Develops Fibrosis in Non-Alcoholic Steatohepatitis. Int. J. Exp. Pathol. 2013, 94, 93–103. [Google Scholar] [CrossRef]
- Denda, A.; Kitayama, W.; Kishida, H.; Murata, N.; Tamura, K.; Kusuoka, O.; Tsutsumi, M.; Nishikawa, F.; Kita, E.; Nakae, D.; et al. Expression of Inducible Nitric Oxide (NO) Synthase but Not Prevention by Its Gene Ablation of Hepatocarcinogenesis with Fibrosis Caused by a Choline-Deficient, l-Amino Acid-Defined Diet in Rats and Mice. Nitric Oxide 2007, 16, 164–176. [Google Scholar] [CrossRef]
- Hebbard, L.; George, J. Animal Models of Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kodama, Y.; Inokuchi, S.; Schnabl, B.; Aoyama, T.; Ohnishi, H.; Olefsky, J.M.; Brenner, D.A.; Seki, E. Toll-like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1beta in Mice. Gastroenterology 2010, 139, 323–334.e7. [Google Scholar] [CrossRef] [PubMed]
- Rakieten, N.; Rakieten, M.L.; Nadkarni, M.V. Studies on the Diabetogenic Action of Streptozotocin (NSC-37917). Cancer Chemother. Rep. 1963, 29, 91–98. [Google Scholar]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A Murine Model for Non-Alcoholic Steatohepatitis Showing Evidence of Association between Diabetes and Hepatocellular Carcinoma. Med. Mol. Morphol. 2013, 46, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Uebanso, T.; Maekawa, K.; Ishikawa, M.; Taguchi, R.; Nammo, T.; Nishimaki-Mogami, T.; Udagawa, H.; Fujii, M.; Shibazaki, Y.; et al. Characterization of Hepatic Lipid Profiles in a Mouse Model with Nonalcoholic Steatohepatitis and Subsequent Fibrosis. Sci. Rep. 2015, 5, 12466. [Google Scholar] [CrossRef]
- Mann, J.P.; Semple, R.K.; Armstrong, M.J. How Useful Are Monogenic Rodent Models for the Study of Human Non-Alcoholic Fatty Liver Disease? Front. Endocrinol. 2016, 7, 145. [Google Scholar] [CrossRef]
- Frühbeck, G.; Jebb, S.A.; Prentice, A.M. Leptin: Physiology and Pathophysiology. Clin. Physiol. 1998, 18, 399–419. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The Role of Leptin and Ghrelin in the Regulation of Food Intake and Body Weight in Humans: A Review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Wang, B.; Charukeshi Chandrasekera, P.; Pippin, J.J. Leptin- and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- Uygun, A.; Kadayifci, A.; Yesilova, Z.; Erdil, A.; Yaman, H.; Saka, M.; Deveci, M.S.; Bagci, S.; Gulsen, M.; Karaeren, N.; et al. Serum Leptin Levels in Patients with Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2000, 95, 3584–3589. [Google Scholar] [CrossRef]
- Huang, X.-D.; Fan, Y.; Zhang, H.; Wang, P.; Yuan, J.P.; Li, M.-J.; Zhan, X.-Y. Serum Leptin and Soluble Leptin Receptor in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2008, 14, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Roiban, A.L.; Both, E.; Huţanu, A. Serum Leptin and Leptin Resistance Correlations with NAFLD in Patients with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3050. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in Nonalcoholic Fatty Liver Disease: A Narrative Review. Metabolism 2015, 64, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.; Schattenberg, J.M.; Leclercq, I.; Yeh, M.M.; Goldin, R.; Teoh, N.; Schuppan, D. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2241–2257. [Google Scholar] [CrossRef]
- Saxena, N.K.; Ikeda, K.; Rockey, D.C.; Friedman, S.L.; Anania, F.A. Leptin in Hepatic Fibrosis: Evidence for Increased Collagen Production in Stellate Cells and Lean Littermates of Ob/Ob Mice. Hepatology 2002, 35, 762–771. [Google Scholar] [CrossRef]
- Trak-Smayra, V.; Paradis, V.; Massart, J.; Nasser, S.; Jebara, V.; Fromenty, B. Pathology of the Liver in Obese and Diabetic Ob/Ob and Db/Db Mice Fed a Standard or High-Calorie Diet. Int. J. Exp. Pathol. 2011, 92, 413–421. [Google Scholar] [CrossRef]
- Sahai, A.; Malladi, P.; Pan, X.; Paul, R.; Melin-Aldana, H.; Green, R.M.; Whitington, P.F. Obese and Diabetic Db/Db Mice Develop Marked Liver Fibrosis in a Model of Nonalcoholic Steatohepatitis: Role of Short-Form Leptin Receptors and Osteopontin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1035–G1043. [Google Scholar] [CrossRef]
- Hoggard, N.; Hunter, L.; Duncan, J.S.; Rayner, D.V. Regulation of Adipose Tissue Leptin Secretion by Alpha-Melanocyte-Stimulating Hormone and Agouti-Related Protein: Further Evidence of an Interaction between Leptin and the Melanocortin Signalling System. J. Mol. Endocrinol. 2004, 32, 145–153. [Google Scholar] [CrossRef]
- Tao, Y.-X. The Melanocortin-4 Receptor: Physiology, Pharmacology, and Pathophysiology. Endocr. Rev. 2010, 31, 506–543. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.H.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. N. Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Lindgren, C.M.; Li, S.; Wheeler, E.; Zhao, J.H.; Prokopenko, I.; Inouye, M.; Freathy, R.M.; Attwood, A.P.; Beckmann, J.S.; et al. Common Variants near MC4R Are Associated with Fat Mass, Weight and Risk of Obesity. Nat. Genet. 2008, 40, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Marsh, D.J.; Hollopeter, G.; Huszar, D.; Laufer, R.; Yagaloff, K.A.; Fisher, S.L.; Burn, P.; Palmiter, R.D. Response of Melanocortin–4 Receptor–Deficient Mice to Anorectic and Orexigenic Peptides. Nat. Genet. 1999, 21, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Suganami, T.; Nakagawa, N.; Tanaka, M.; Yamamoto, Y.; Kamei, Y.; Terai, S.; Sakaida, I.; Ogawa, Y. Melanocortin 4 Receptor–Deficient Mice as a Novel Mouse Model of Nonalcoholic Steatohepatitis. Am. J. Pathol. 2011, 179, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased Levels of Nuclear SREBP-1c Associated with Fatty Livers in Two Mouse Models of Diabetes Mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-Regulated Lipid Metabolism: Convergent Physiology—Divergent Pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, Regulated by the Insulin and AMPK Signaling Pathways, Plays a Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Lee, E.-J.; Shin, K.-O.; Kim, M.H.; Pewzner-Jung, Y.; Lee, Y.-M.; Park, J.-W.; Futerman, A.H.; Park, W.-J. Hepatic Triglyceride Accumulation via Endoplasmic Reticulum Stress-Induced SREBP-1 Activation Is Regulated by Ceramide Synthases. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Knebel, B.; Haas, J.; Hartwig, S.; Jacob, S.; Köllmer, C.; Nitzgen, U.; Muller-Wieland, D.; Kotzka, J. Liver-Specific Expression of Transcriptionally Active SREBP-1c Is Associated with Fatty Liver and Increased Visceral Fat Mass. PLoS ONE 2012, 7, e31812. [Google Scholar] [CrossRef]
- Jelenik, T.; Kaul, K.; Séquaris, G.; Flögel, U.; Phielix, E.; Kotzka, J.; Knebel, B.; Fahlbusch, P.; Hörbelt, T.; Lehr, S.; et al. Mechanisms of Insulin Resistance in Primary and Secondary Nonalcoholic Fatty Liver. Diabetes 2017, 66, 2241–2253. [Google Scholar] [CrossRef]
- Sanches, S.C.L.; Ramalho, L.N.Z.; Augusto, M.J.; da Silva, D.M.; Ramalho, F.S. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. BioMed Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Miyashita, Y.; Sasaki, M.; Aruga, Y.; Nakamura, Y.; Ishii, Y.; Sasahara, M.; Kanasaki, K.; Kitada, M.; Koya, D.; et al. Eplerenone Ameliorates the Phenotypes of Metabolic Syndrome with NASH in Liver-Specific SREBP-1c Tg Mice Fed High-Fat and High-Fructose Diet. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1415–E1425. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Warda, A.-S.; Verbeek, J.; Cassiman, D.; Spincemaille, P. An Overview of Mouse Models of Nonalcoholic Steatohepatitis: From Past to Present. Curr. Protoc. Mouse Biol. 2016, 6, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Droz, B.A.; Sneed, B.L.; Jackson, C.V.; Zimmerman, K.M.; Michael, M.D.; Emmerson, P.J.; Coskun, T.; Peterson, R.G. Correlation of Disease Severity with Body Weight and High Fat Diet in the FATZO/Pco Mouse. PLoS ONE 2017, 12, e0179808. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.G.; Jackson, C.V.; Zimmerman, K.M.; Alsina-Fernandez, J.; Michael, M.D.; Emmerson, P.J.; Coskun, T. Glucose Dysregulation and Response to Common Anti-Diabetic Agents in the FATZO/Pco Mouse. PLoS ONE 2017, 12, e0179856. [Google Scholar] [CrossRef]
- Sun, G.; Jackson, C.V.; Zimmerman, K.; Zhang, L.-K.; Finnearty, C.M.; Sandusky, G.E.; Zhang, G.; Peterson, R.G.; Wang, Y.-X.J. The FATZO Mouse, a next Generation Model of Type 2 Diabetes, Develops NAFLD and NASH When Fed a Western Diet Supplemented with Fructose. BMC Gastroenterol. 2019, 19, 41. [Google Scholar] [CrossRef]
- Maciejewska, D.; Łukomska, A.; Dec, K.; Skonieczna-Żydecka, K.; Gutowska, I.; Skórka-Majewicz, M.; Styburski, D.; Misiakiewicz-Has, K.; Pilutin, A.; Palma, J.; et al. Diet-Induced Rat Model of Gradual Development of Non-Alcoholic Fatty Liver Disease (NAFLD) with Lipopolysaccharides (LPS) Secretion. Diagnostics 2019, 9, 205. [Google Scholar] [CrossRef]
- Van der Graaff, D.; Kwanten, W.J.; Couturier, F.J.; Govaerts, J.S.; Verlinden, W.; Brosius, I.; D’Hondt, M.; Driessen, A.; De Winter, B.Y.; De Man, J.G.; et al. Severe Steatosis Induces Portal Hypertension by Systemic Arterial Hyporeactivity and Hepatic Vasoconstrictor Hyperreactivity in Rats. Lab. Investig. 2018, 98, 1263–1275. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Tussellino, M.; Carotenuto, R.; Liverini, G.; Iossa, S. Fructose Supplementation Worsens the Deleterious Effects of Short-Term High-Fat Feeding on Hepatic Steatosis and Lipid Metabolism in Adult Rats. Exp. Physiol. 2014, 99, 1203–1213. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical Models of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 230–237. [Google Scholar] [CrossRef]
- Lieber, C.S.; Leo, M.A.; Mak, K.M.; Xu, Y.; Cao, Q.; Ren, C.; Ponomarenko, A.; DeCarli, L.M. Model of Nonalcoholic Steatohepatitis. Am. J. Clin. Nutr. 2004, 79, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Stöppeler, S.; Palmes, D.; Fehr, M.; Hölzen, J.P.; Zibert, A.; Siaj, R.; Schmidt, H.H.-J.; Spiegel, H.-U.; Bahde, R. Gender and Strain-Specific Differences in the Development of Steatosis in Rats. Lab. Anim. 2013, 47, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Murakami, T.; Iida, M.; Kuwajima, M.; Shima, K. Leptin Receptor of Zucker Fatty Rat Performs Reduced Signal Transduction. Diabetes 1997, 46, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Kucera, O.; Cervinkova, Z. Experimental Models of Non-Alcoholic Fatty Liver Disease in Rats. World J. Gastroenterol. 2014, 20, 8364–8376. [Google Scholar] [CrossRef] [PubMed]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A High-Fat Diet Leads to the Progression of Non-Alcoholic Fatty Liver Disease in Obese Rats. FASEB J. 2005, 19, 136–138. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Galsgaard, E.D.; Christoffersen, B.Ø.; Cirera, S.; Holst, D.; Fredholm, M.; Latta, M. NASH-Inducing Diets in Göttingen Minipigs. J. Clin. Exp. Hepatol. 2020, 10, 211–221. [Google Scholar] [CrossRef]
- Lee, L.; Alloosh, M.; Saxena, R.; Alstine, W.V.; Watkins, B.A.; Klaunig, J.E.; Sturek, M.; Chalasani, N. Nutritional Model of Steatohepatitis and Metabolic Syndrome in the Ossabaw Miniature Swine. Hepatology 2009, 50, 56–67. [Google Scholar] [CrossRef]
- Litten-Brown, J.C.; Corson, A.M.; Clarke, L. Porcine Models for the Metabolic Syndrome, Digestive and Bone Disorders: A General Overview. Animal 2010, 4, 899–920. [Google Scholar] [CrossRef]
- Forster, R.; Bode, G.; Ellegaard, L.; van der Laan, J.W. The RETHINK Project: Minipigs as Models for the Toxicity Testing of New Medicines and Chemicals: An Impact Assessment. J. Pharmacol. Toxicol. Methods 2010, 62, 158–159. [Google Scholar] [CrossRef]
- Schumacher-Petersen, C.; Christoffersen, B.Ø.; Kirk, R.K.; Ludvigsen, T.P.; Zois, N.E.; Pedersen, H.D.; Vyberg, M.; Olsen, L.H. Experimental Non-Alcoholic Steatohepatitis in Göttingen Minipigs: Consequences of High Fat-Fructose-Cholesterol Diet and Diabetes. J. Transl. Med. 2019, 17, 110. [Google Scholar] [CrossRef]
- Bergen, W.G.; Mersmann, H.J. Comparative Aspects of Lipid Metabolism: Impact on Contemporary Research and Use of Animal Models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.C.; Liang, Z.; Sun, F.; Yang, Z.; Tang, C.; Chen, Z.; Yubo, S.; Yao, Z.; Wu, M.; Chen, Y.; et al. Nonalcoholic Fatty Liver Disease (NAFLD) in Obese Rhesus Monkeys Provides the First Animal Model That Accurately Reflects the Human Condition. FASEB J. 2017, 31, 895–896. [Google Scholar] [CrossRef]
- Ratziu, V.; Friedman, S.L. Why Do So Many NASH Trials Fail? Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).