Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants
2.2.1. Inclusion Criteria
- Male and female patients aged 18–75 years
- Patients undergoing elective laparoscopic mesh inguinal hernia repair
- Body mass index 18–30 kg/m2
- ASA classification I–III
- Patients provide signed informed consent
2.2.2. Exclusion Criteria
- Patients with bilateral or recurrent inguinal hernia
- Patients with a history of intolerance, hypersensitivity, or abuse of opioids
- Use of opioids in the past month
- Use of monoamine oxidase and selective serotonin reuptake inhibitors
- Patients wearing a cardiac pacemaker
- Patients with clinically significant cardiovascular, pulmonary, renal, hepatic, and neurological disease
- Patients with skin infections, surgical incision, or scar at the point of application of acupuncture
- Patients who participated in other clinical trials or received other acupuncture therapy in the previous four weeks
2.3. Randomization and Concealment
2.4. Sample Size
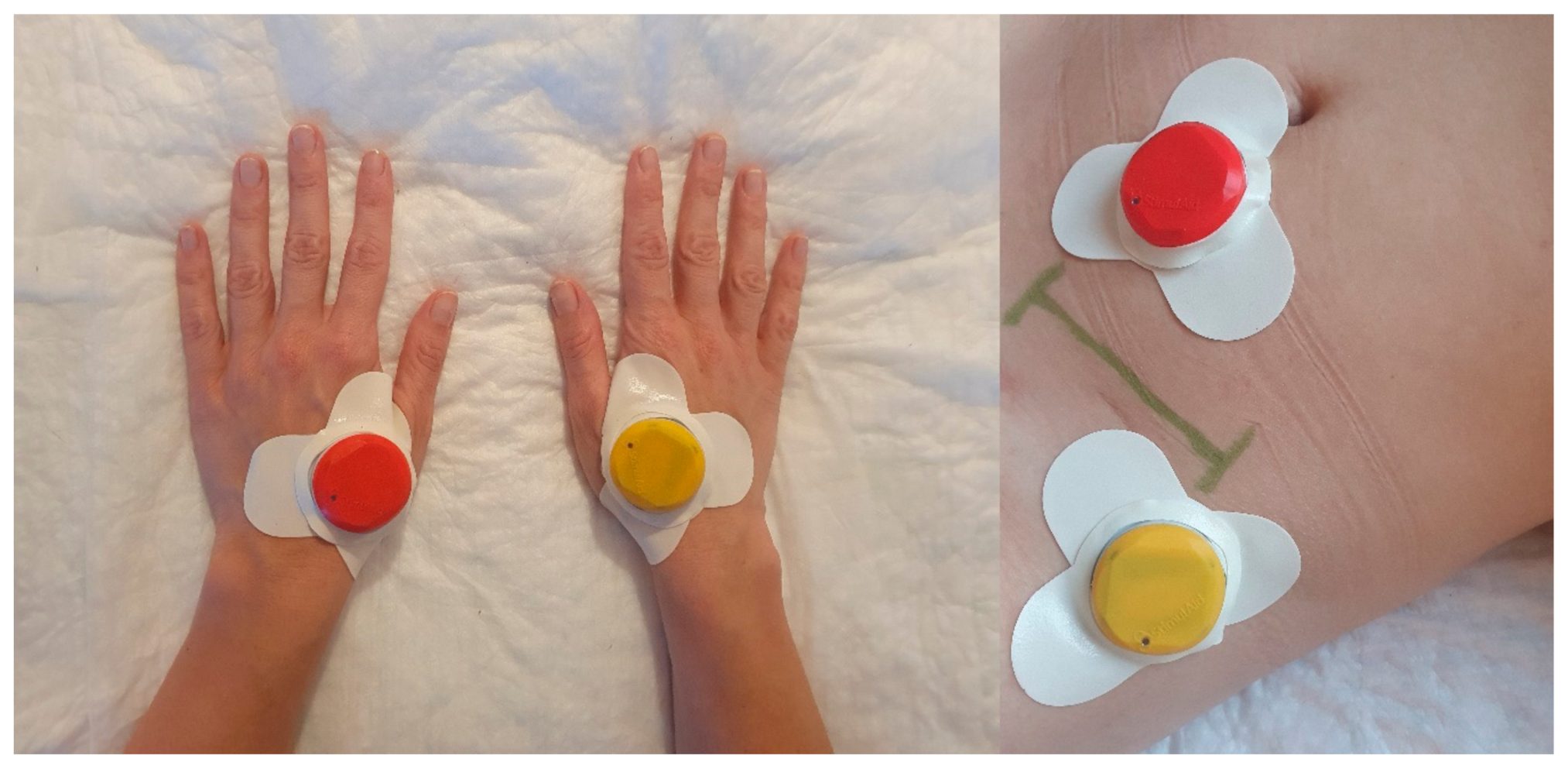
2.5. Interventions
2.5.1. Anesthesia Protocol
2.5.2. Postoperative Analgesia
2.6. Outcomes
2.6.1. Primary Outcome
2.6.2. Secondary Outcomes
2.7. Cortisol Levels Assessment
2.8. Adverse Events
2.9. Statistical Analysis
2.10. Data Availability
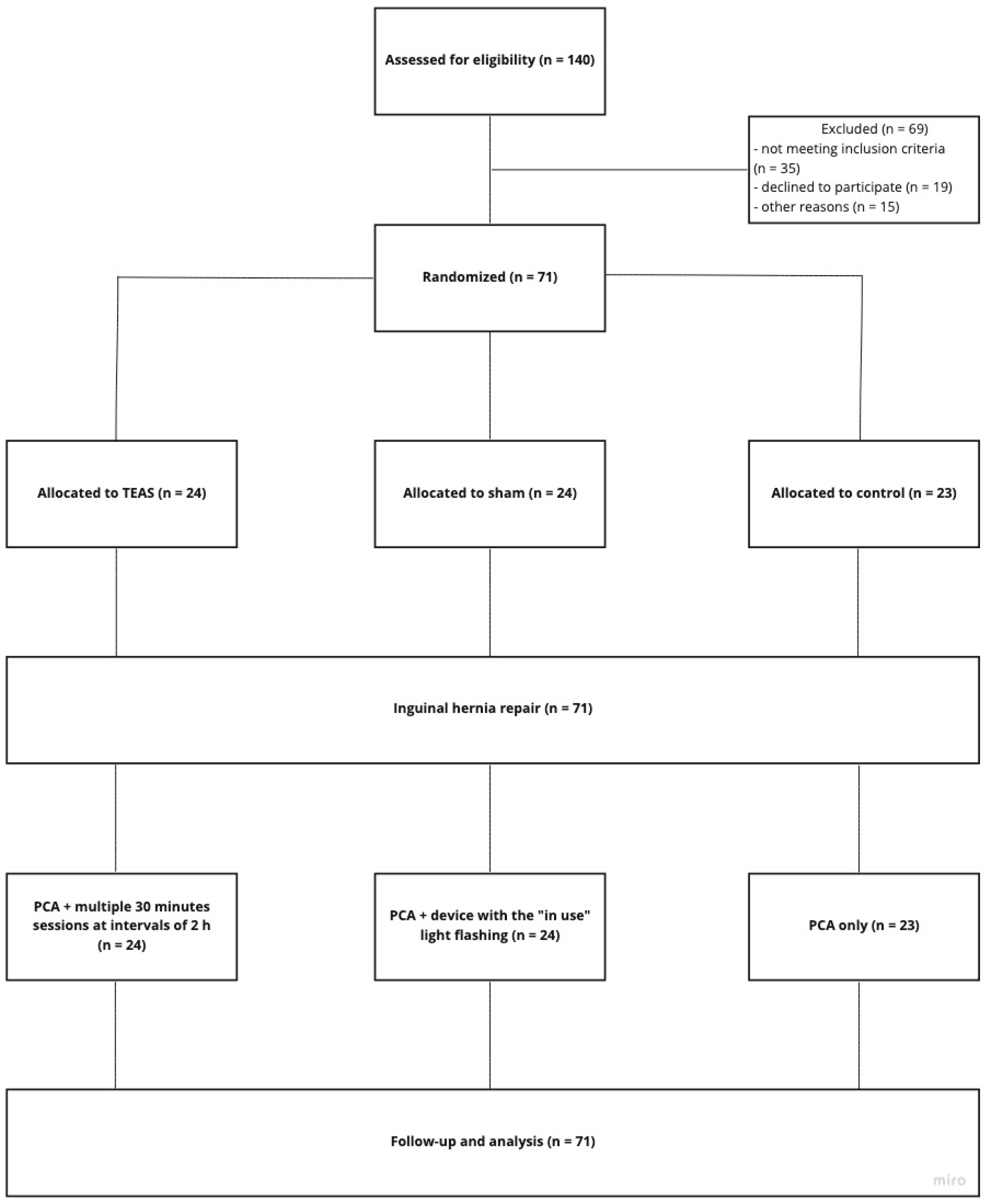
3. Results
3.1. Participants and Baseline Characteristic
3.2. Outcome Comparison between the Groups
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brummett, C.M.; Waljee, J.F.; Goesling, J.; Moser, S.; Lin, P.; Englesbe, M.J.; Bohnert, A.S.B.; Kheterpal, S.; Nallamothu, B.K. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017, 152. [Google Scholar] [CrossRef] [PubMed]
- Oderda, G.M.; Said, Q.; Evans, R.S.; Stoddard, G.J.; Lloyd, J.; Jackson, K.; Rublee, D.; Samore, M.H. Opioid-related adverse drug events in surgical hospitalizations: Impact on costs and length of stay. Ann. Pharmacother. 2007, 41, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Urman, R.D.; Seger, D.L.; Fiskio, J.M.; Neville, B.A.; Harry, E.M.; Weiner, S.G.; Lovelace, B.; Fain, R.; Cirillo, J.; Schnipper, J.L. The Burden of Opioid-Related Adverse Drug Events on Hospitalized Previously Opioid-Free Surgical Patients. J. Patient Saf. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hah, J.M.; Bateman, B.T.; Ratliff, J.; Curtin, C.; Sun, E. Chronic Opioid Use after Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth. Analg. 2017, 125, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S.; Collinsworth, A.W.; Copeland, L.A.; Ogola, G.O.; Qiu, T.; Kouznetsova, M.; Liao, I.C.; Mears, N.; Pham, A.T.; Wan, G.J.; et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018, 153, 757–763. [Google Scholar] [CrossRef]
- Lai, H.C.; Lin, Y.W.; Hsieh, C.L. Acupuncture-Analgesia-Mediated Alleviation of Central Sensitization. Evidence Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Pomeranz, B.; Chiu, D. Naloxone blockade of acupuncture analgesia: Endorphin implicated. Life Sci. 1976, 19, 1757–1762. [Google Scholar] [CrossRef]
- Sjölund, B.; Terenius, L.; Eriksson, M. Increased Cerebrospinal Fluid Levels of Endorphins after Electro-Acupuncture. Acta Physiol. Scand. 1977, 100, 382–384. [Google Scholar] [CrossRef]
- Sun, K.; Xing, T.; Zhang, F.; Liu, Y.; Li, W.; Zhou, Z.; Fang, L.; Yu, L.; Yan, M. Perioperative transcutaneous electrical acupoint stimulation for postoperative pain relief following laparoscopic surgery. Clin. J. Pain 2017, 33, 340–347. [Google Scholar] [CrossRef]
- Lan, F.; Ma, Y.H.; Xue, J.X.; Wang, T.L.; Ma, D.Q. Transcutaneous electrical nerve stimulation on acupoints reduces fentanyl requirement for postoperative pain relief after total hip arthroplasty in elderly patients. Minerva Anestesiol. 2012, 78, 887–895. [Google Scholar]
- Song, B.; Chang, Y.; Li, Y.; Zhu, J. Effects of transcutaneous electrical acupoint stimulation on the postoperative sleep quality and pain of patients after video-assisted thoracoscopic surgery: A prospective, randomized controlled trial. Nat. Sci. Sleep 2020, 12, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, Z.; Wang, H.; Ma, L.; Guo, F.; Zhong, H.; Xiong, L.; Wang, Q. The effect of pre-treatment with transcutaneous electrical acupoint stimulation on the quality of recovery after ambulatory breast surgery: A prospective, randomised controlled trial. Anaesthesia 2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, J.; White, P.F.; Sloninsky, A.; Wender, R.H.; Naruse, R.; Kariger, R. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: Acupoint versus nonacupoint stimulation. Anesth. Analg. 1998, 87, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.H.; Chen, W.S.; Chen, C.H.; Jiang, J.K.; Tang, G.J.; Lui, W.Y.; Lin, J.K. Effect of transcutaneous electrical nerve stimulation for pain relief on patients undergoing hemorrhoidectomy: Prospective, randomized, controlled trial. Dis. Colon Rectum 1999, 42, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Carneiro, N.M.; Vanni Guerra, L.A.; Velarde, G.C.; Teixeira De Souza, P.A.; Damásio Da Silva, L.L.; De Abreu, E.; Souza, R.R.; Nolasco, R.; Olej, B. Effects of electroacupuncture on local anaesthesia for inguinal hernia repair: A randomised placebo-controlled trial. Acupunct. Med. 2010, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Dalamagka, M.; Mavrommatis, C.; Grosomanidis, V.; Karakoulas, K.; Vasilakos, D. Postoperative analgesia after low-frequency electroacupuncture as adjunctive treatment in inguinal hernia surgery with abdominal wall mesh reconstruction. Acupunct. Med. 2015, 33, 360–367. [Google Scholar] [CrossRef] [PubMed]
- DeSantana, J.M.; Santana-Filho, V.J.; Guerra, D.R.; Sluka, K.A.; Gurgel, R.Q.; da Silva, W.M. Hypoalgesic Effect of the Transcutaneous Electrical Nerve Stimulation Following Inguinal Herniorrhaphy: A Randomized, Controlled Trial. J. Pain 2008, 9, 623–629. [Google Scholar] [CrossRef]
- Hamza, M.A.; White, P.F.; Ahmed, H.E.; Ghoname, E.S.A. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 1999, 91, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tang, J.; White, P.F.; Naruse, R.; Sloninsky, A.; Kariger, R.; Gold, J.; Wender, R.H. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth. Analg. 1997, 85, 406–413. [Google Scholar] [CrossRef]
- Lin, J.G.; Lo, M.W.; Wen, Y.R.; Hsieh, C.L.; Tsai, S.K.; Sun, W.Z. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain 2002, 99, 509–514. [Google Scholar] [CrossRef]
- Ng, S.S.M.; Leung, W.W.; Mak, T.W.C.; Hon, S.S.F.; Li, J.C.M.; Wong, C.Y.N.; Tsoi, K.K.F.; Lee, J.F.Y. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology 2013, 144, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.L.; Lee, T.W.; Sihoe, A.D.L.; Wan, I.Y.P.; Ng, C.S.H.; Chan, S.K.C.; Wong, W.W.L.; Liang, Y.M.; Yim, A.P.C. Analgesic Effect of Electroacupuncture in Postthoracotomy Pain: A Prospective Randomized Trial. Ann. Thorac. Surg. 2006, 81, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.K.; Xu, P.C.; Pua, H.L.; Zhang, G.; Lee, T.L. Effects of electroacupuncture on intraoperative and postoperative analgesic requirement. Acupunct. Med. 2002, 20, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.P.; Smietanski, M.; Bonjer, H.J.; Bittner, R.; Miserez, M.; Aufenacker, T.J.; Fitzgibbons, R.J.; Chowbey, P.K.; Tran, H.M.; Sani, R.; et al. International guidelines for groin hernia management. Hernia 2018, 22, 1–165. [Google Scholar] [CrossRef]
- Callesen, T. Inguinal hernia repair: Anaesthesia, pain and convalescence. Dan. Med. Bull. 2003, 50, 203–218. [Google Scholar]
- Wu, M.S.; Chen, K.H.; Chen, I.F.; Huang, S.K.; Tzeng, P.C.; Yeh, M.L.; Lee, F.P.; Lin, J.G.; Chen, C. The efficacy of acupuncture in post-operative pain management: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0150367. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Martyn Lewis, A.; Sim, J.; Mallen, C.D.; Mason, E.E.; Hay, E.M.; Van Der Windt, D.A. Transcutaneous electrical nerve stimulation as adjunct to primary care management for tennis elbow: Pragmatic randomised controlled trial (TATE trial). BMJ 2013, 347. [Google Scholar] [CrossRef]
- Gavronsky, S.; Koeniger-Donohue, R.; Steller, J.; Hawkins, J.W. Postoperative Pain: Acupuncture versus Percutaneous Electrical Nerve Stimulation. Pain Manag. Nurs. 2012, 13, 150–156. [Google Scholar] [CrossRef]
- Han, J.S.; Chen, X.H.; Sun, S.L.; Xu, X.J.; Yuan, Y.; Yan, S.C.; Hao, J.X.; Terenius, L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991, 47, 295–298. [Google Scholar] [CrossRef]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, W.; Cao, Y.; Han, J.S. Effects of synchronous or asynchronous electroacupuncture stimulation with low versus high frequency on spinal opioid release and tail flick nociception. Exp. Neurol. 2005, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Hong, C.; Su-Fong, G.; Chung-Gwo, C.; Ji-Sheng, H. Optimal conditions for eliciting maximal electroacupuncture analgesia with dense-and-disperse mode stimulation. Am. J. Acupunct. 1994, 22, 47–53. [Google Scholar]
- Sun, Y.; Gan, T.J.; Dubose, J.W.; Habib, A.S. Acupuncture and related techniques for postoperative pain: A systematic review of randomized controlled trials. Br. J. Anaesth. 2008, 101, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tennant, F. The Physiologic Effects of Pain on the Endocrine System. Pain Ther. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Zhao, Z.Q. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008, 85, 355–375. [Google Scholar] [CrossRef]
- Chernyak, G.V.; Sessler, D.I. Perioperative acupuncture and related techniques. Anesthesiology 2005, 102, 1031–1049. [Google Scholar] [CrossRef]
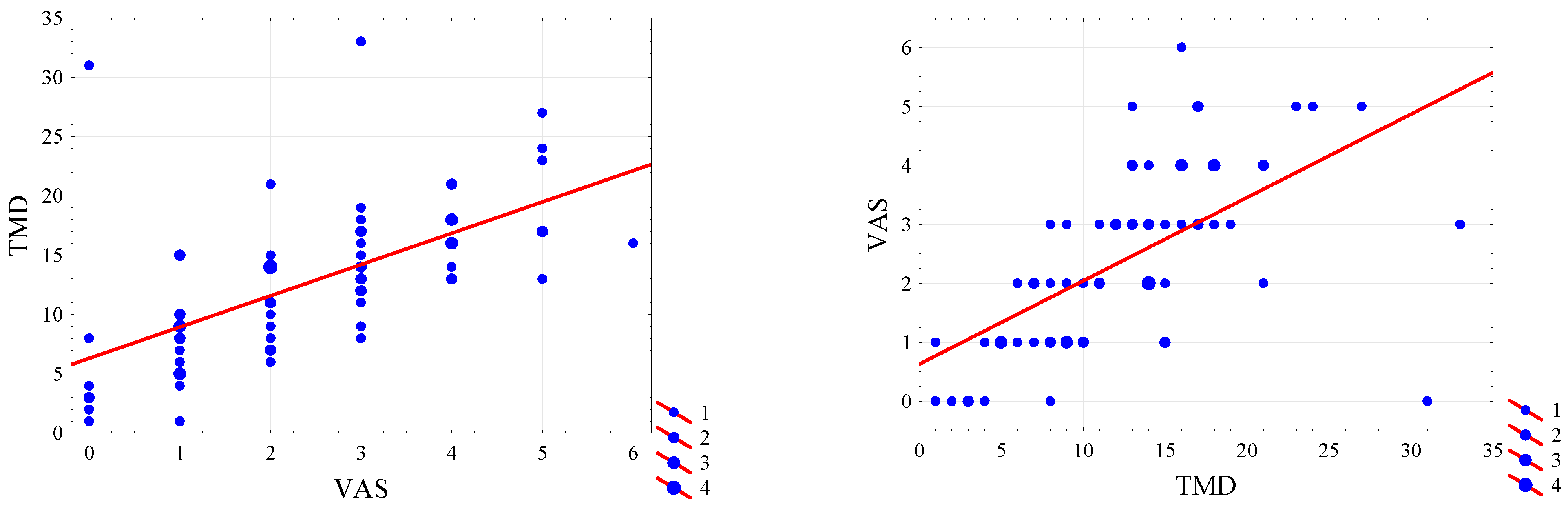
| Characteristic | Group | p-Value | |||||
|---|---|---|---|---|---|---|---|
| TEAS | Sham | Control | |||||
| n = 24 | n = 24 | n = 23 | |||||
| Gender | n | % | n | % | n | % | 0.881 |
| Females | 3 | 12.5% | 4 | 16.7% | 4 | 17.4% | |
| Males | 21 | 87.5% | 20 | 83.3% | 19 | 82.6% | |
| Age (years) | 0.408 | ||||||
| M (SD) | 54.6 (15.8) | 57.8 (15.6) | 60.6 (12.1) | ||||
| Me (IQR) | 55 (43; 68) | 63 (43; 70) | 63 (56; 69) | ||||
| Min–Max | 27–74 | 23–74 | 27–75 | ||||
| Characteristic | Group | p-Value | |||||
|---|---|---|---|---|---|---|---|
| TEAS | Sham | Control | |||||
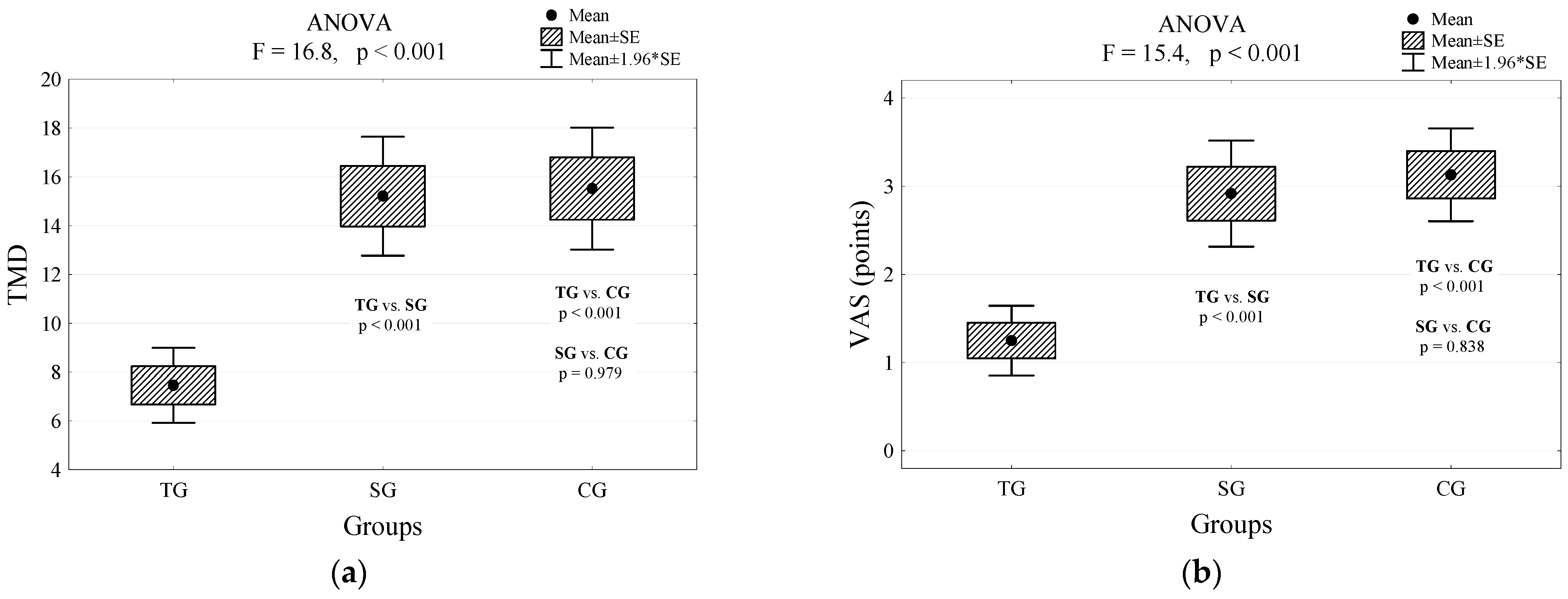
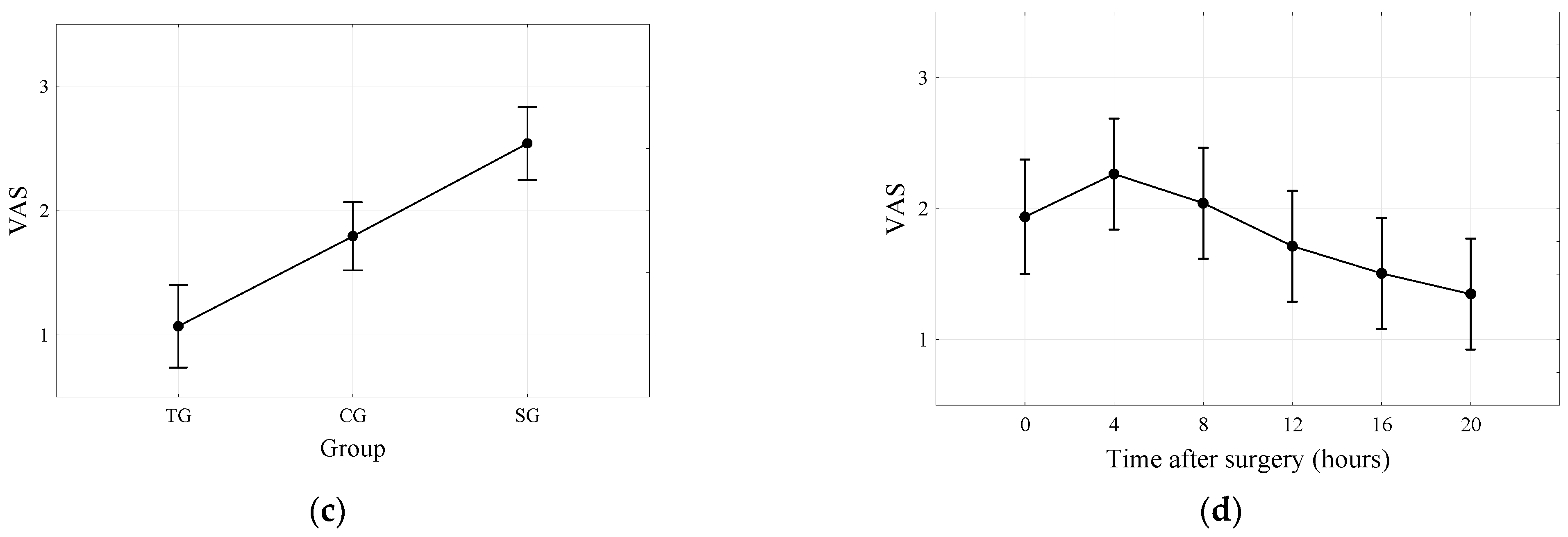
| VAS (points) | <0.001 | ||||||
| M (SD) | 1.3 (1.0) | 2.9 (1.5) | 3.1 (1.3) | ||||
| Me (IQR) | 1 (0.5; 2) | 3 (2; 4) | 3 (2; 4) | ||||
| Min–Max | 0–3 | 0–5 | 1–6 | ||||
| TMD (mg): | <0.001 | ||||||
| M (SD) | 7.5 (3.8) | 15.2 (6,24) | 15.5 (6.1) | ||||
| Me (IQR) | 8 (5; 9) | 15 (13; 18) | 15 (11; 18) | ||||
| Min–Max | 1–18 | 1–31 | 4–33 | ||||
| Nausea | n | % | n | % | n | % | 0.116 |
| No | 24 | 100,0% | 20 | 83.3% | 21 | 91.3% | |
| Yes | 0 | 0,0% | 4 | 16.7% | 2 | 8.7% | |
| Characteristic | Gender | p-Value | |
|---|---|---|---|
| Females | Males | ||
| n = 11 | n = 60 | ||
| VAS (points) | 0.190 | ||
| M (SD) | 3.0 (1.7) | 2.3 (1.5) | |
| Me (IQR) | 3 (2; 5) | 2 (1; 3) | |
| Min–Max | 0–5 | 0–6 | |
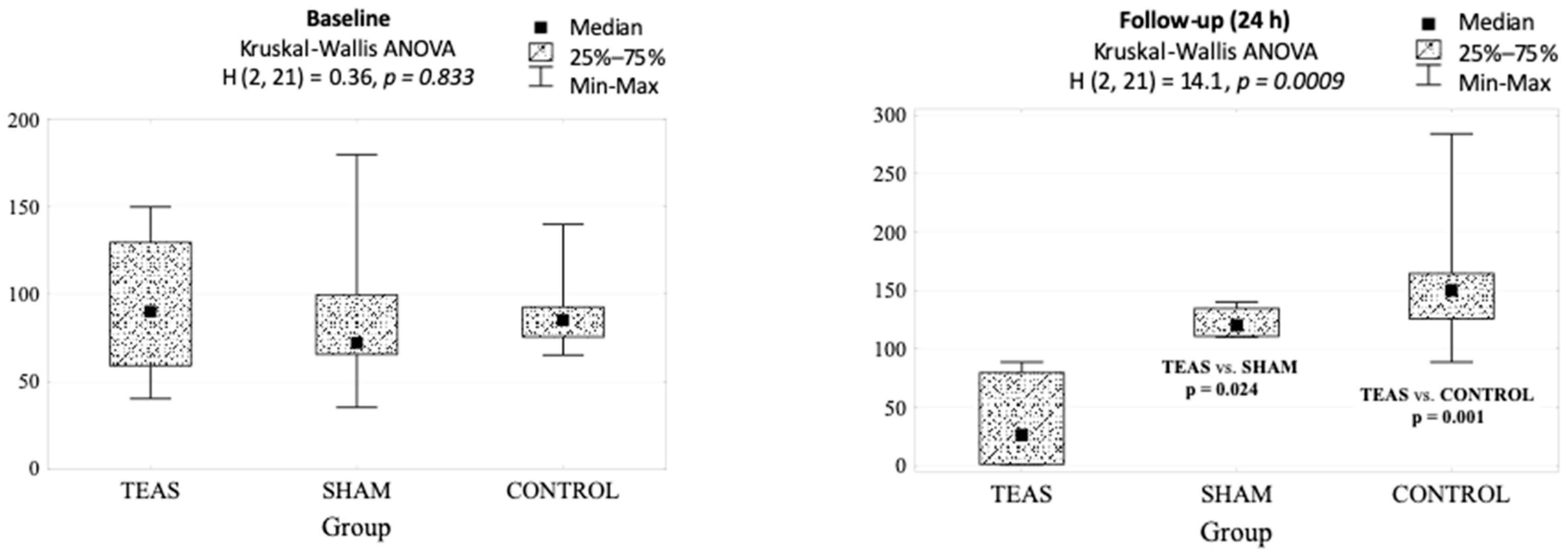
| Group | p-Value | |||
|---|---|---|---|---|
| TEAS | Sham | Control | ||
| Baseline | 90 (40–150) | 72 (35–180) | 85 (65–140) | 0.833 |
| Follow-up (24 h) | 26 (1–89) | 120 (110–140) | 150 (89–284) | 0.001 |
| p-value | 0.063 | 0.128 | 0.018 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmit, M.; Agrawal, S.; Goździk, W.; Kübler, A.; Agrawal, A.; Pruchnicki, P.; Woźniak, M.; Nowak, M.; Bartoszewicz, B.; Rudnicki, J. Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study. J. Clin. Med. 2021, 10, 146. https://doi.org/10.3390/jcm10010146
Szmit M, Agrawal S, Goździk W, Kübler A, Agrawal A, Pruchnicki P, Woźniak M, Nowak M, Bartoszewicz B, Rudnicki J. Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study. Journal of Clinical Medicine. 2021; 10(1):146. https://doi.org/10.3390/jcm10010146
Chicago/Turabian StyleSzmit, Mateusz, Siddarth Agrawal, Waldemar Goździk, Andrzej Kübler, Anil Agrawal, Piotr Pruchnicki, Marta Woźniak, Matylda Nowak, Bartłomiej Bartoszewicz, and Jerzy Rudnicki. 2021. "Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study" Journal of Clinical Medicine 10, no. 1: 146. https://doi.org/10.3390/jcm10010146
APA StyleSzmit, M., Agrawal, S., Goździk, W., Kübler, A., Agrawal, A., Pruchnicki, P., Woźniak, M., Nowak, M., Bartoszewicz, B., & Rudnicki, J. (2021). Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study. Journal of Clinical Medicine, 10(1), 146. https://doi.org/10.3390/jcm10010146





