Characterization of the Initial Fouling Layer on the Membrane Surface in a Membrane Bioreactor: Effects of Permeation Drag
Abstract
:1. Introduction
2. Materials and Methods
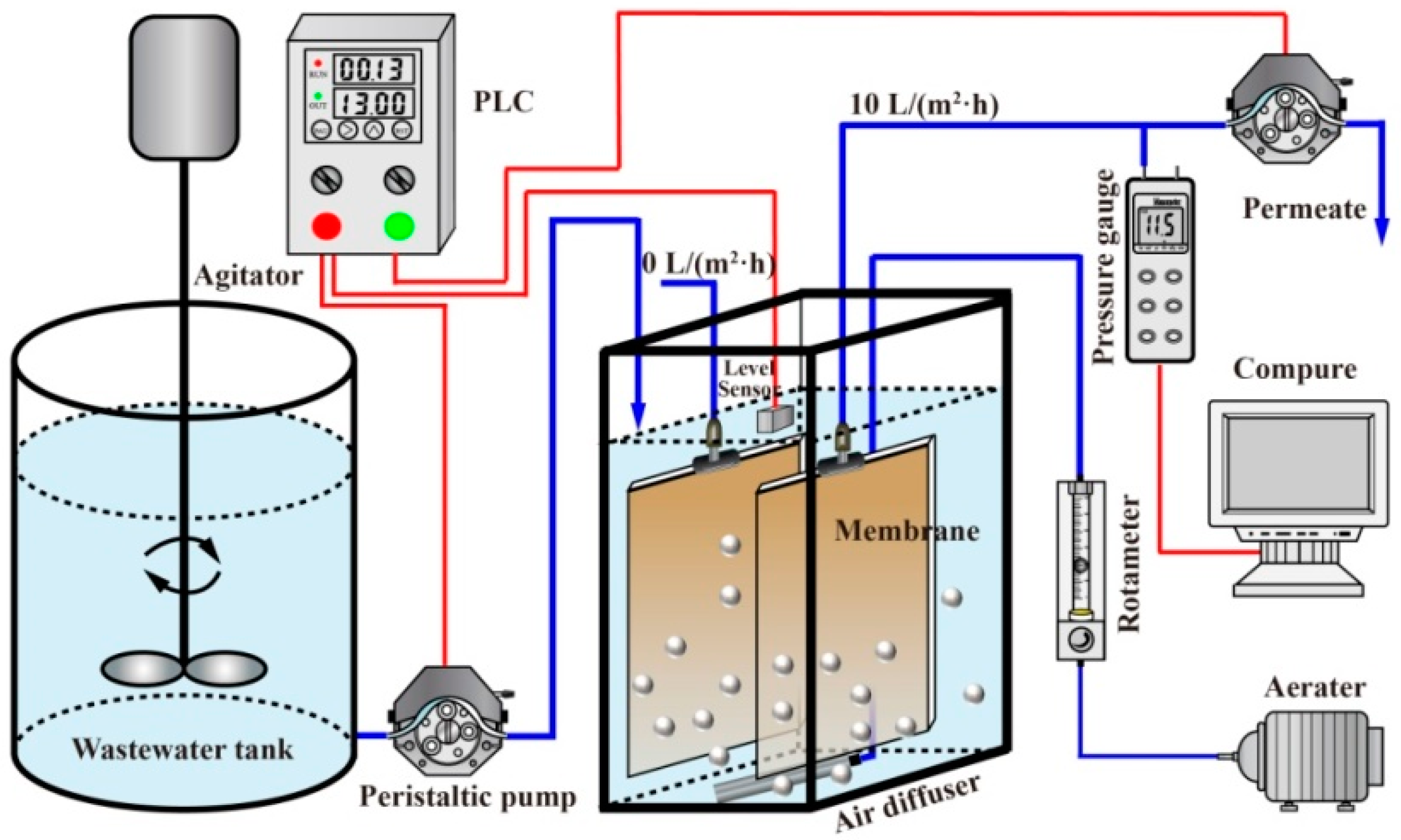
2.1. Experimental Setup and Operation
2.2. Contact Angle and Zeta Potential Analysis
2.3. EPS Extraction and Analysis
2.4. Surface Thermodynamics and XDLVO Approach
2.5. Others
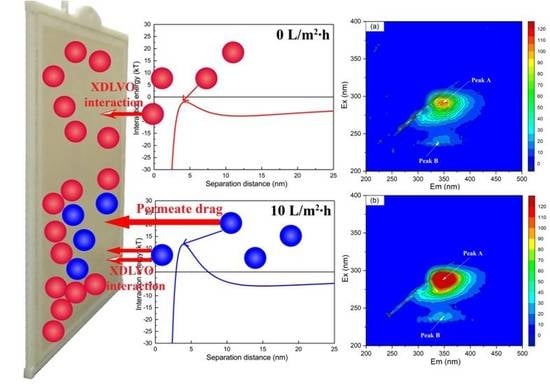
3. Results and Discussion
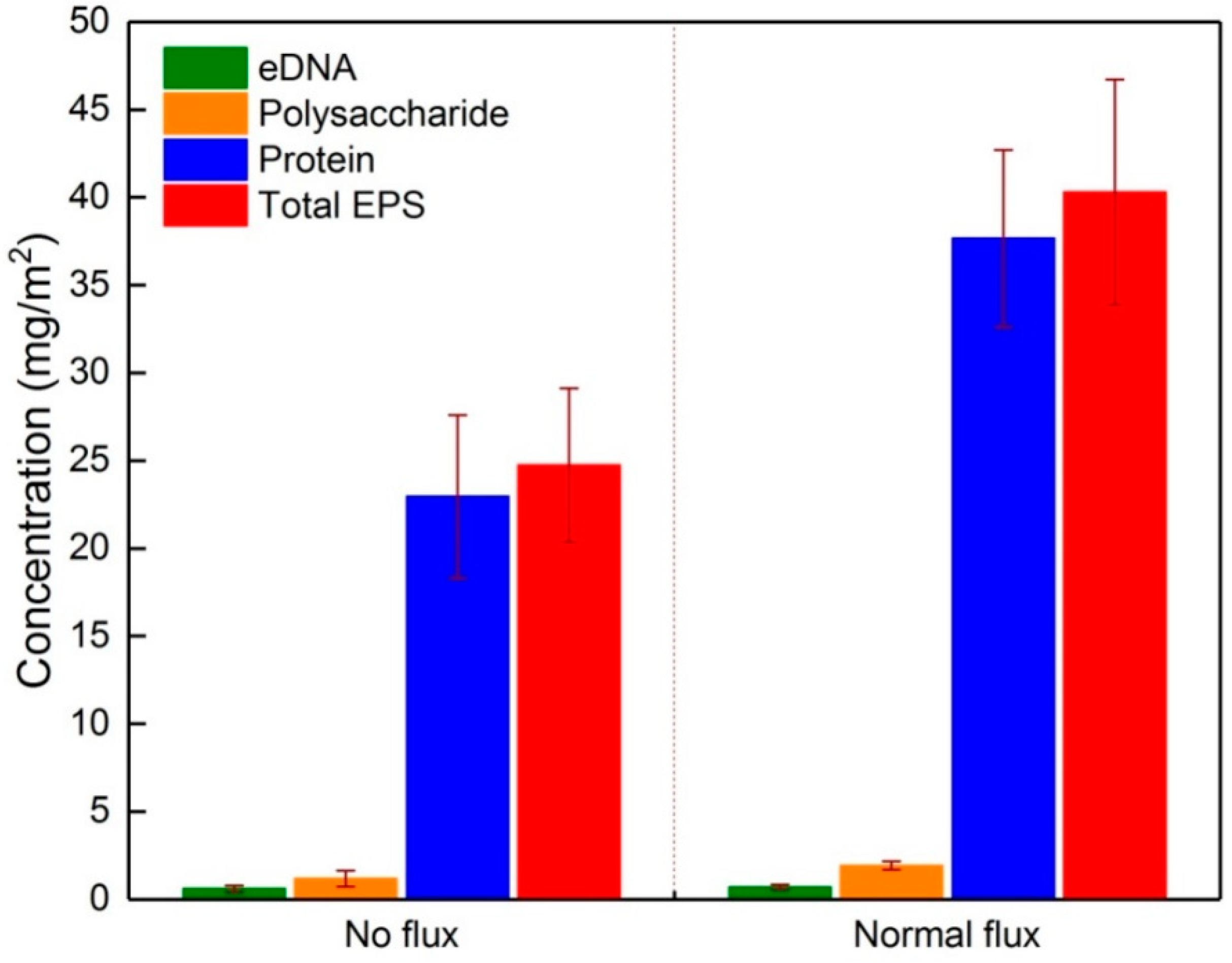
3.1. Composition and Content of EPS Fractions in the Initial Fouling Layer
3.2. Surficial Properties of Membrane and Initial Fouling Layer
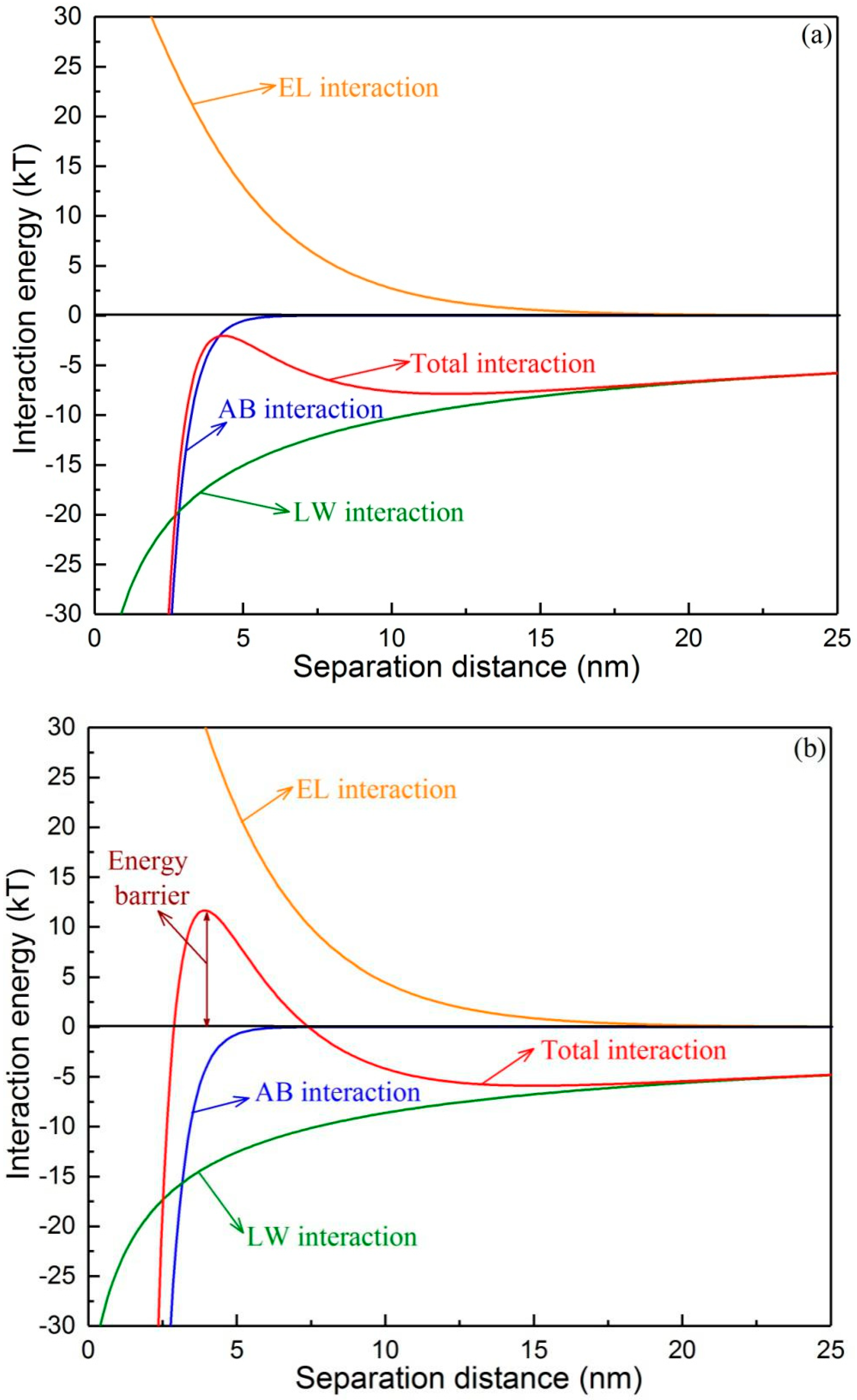
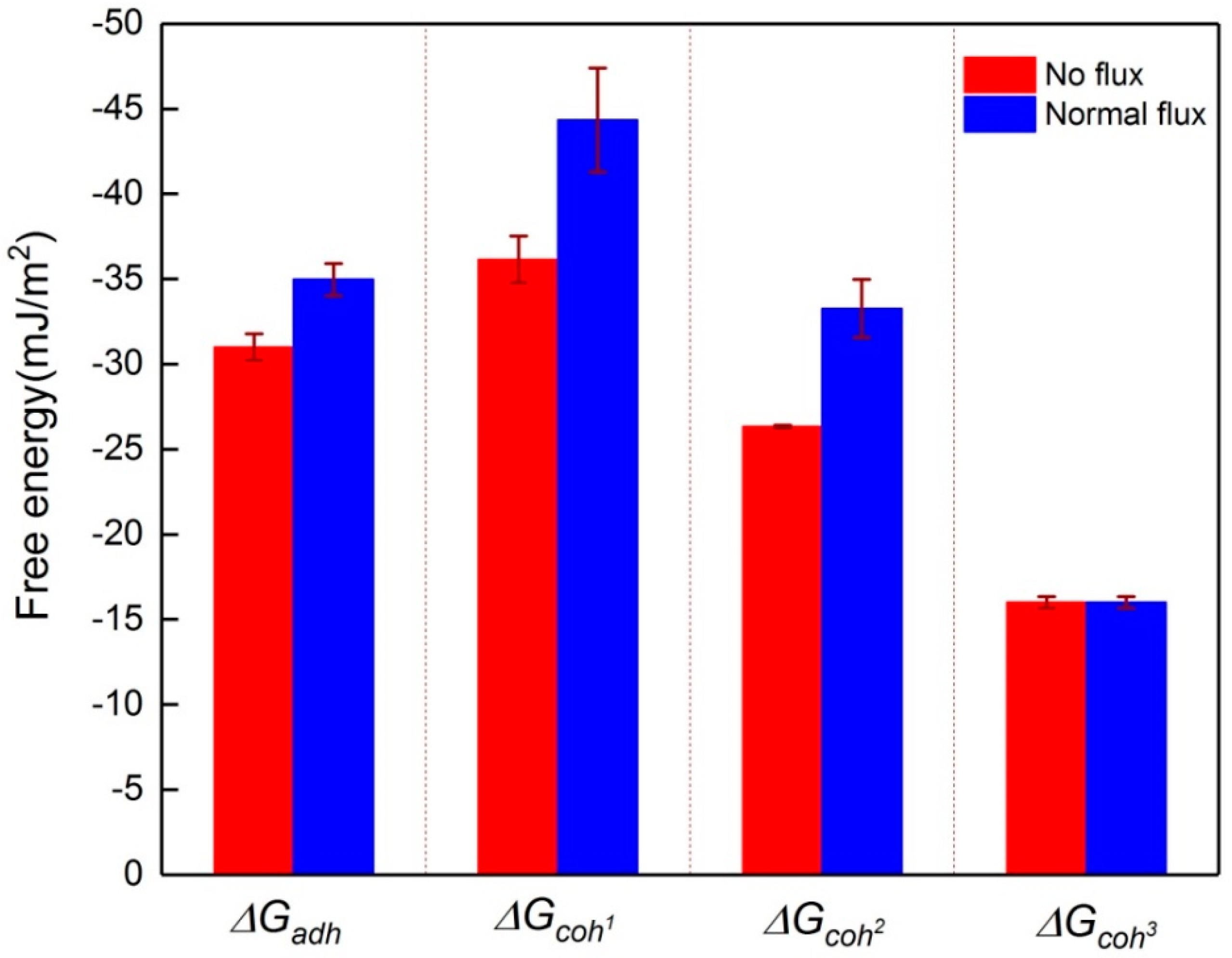
3.3. Changes in the Interaction Energy during Membrane Fouling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| surface tension parameter | |
| interaction energy per unit area | |
| interaction energy | |
| AB | Lewis acid-base |
| EL | electrostatic double layer |
| LW | Lishitz-van der Waals |
| Superscripts | |
| tol | total |
| + | electron acceptor |
| − | electron donor |
| Subscripts | |
| adh | adhesion between foulants and membrane |
| coh | cohesion among foulants |
| s | solid |
| w | water |
References
- Tian, Y.; Su, X. Relation between the stability of activated sludge flocs and membrane fouling in MBR: Under different SRTs. Bioresour. Technol. 2012, 118, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, X.; Wang, b.; Song, L. Calcium ion on membrane fouling reduction and bioflocculation promotion in membrane bioreactor at high salt shock. Bioresour. Technol. 2016, 200, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, M.; Song, L.; Guo, J.; Zhang, L. Fate of NaClO and membrane foulants during in-situ cleaning of membrane bioreactors: Combined effect on thermodynamic properties of sludge. Biochem. Eng. J. 2019, 147, 146–152. [Google Scholar] [CrossRef]
- Yan, L.; Li, R.; Song, Y.; Jia, Y.; Li, Z.; Song, L.; Zhang, H. Characterization of the Fouling Layer on the Membrane Surface in a Membrane Bioreactor: Evolution of the Foulants’ Composition and Aggregation Ability. Membranes 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Matar, G.; Gonzalez-Gil, G.; Maab, H.; Nunes, S.; Le-Clech, P.; Vrouwenvelder, J.; Saikaly, P.E. Temporal changes in extracellular polymeric substances on hydrophobic and hydrophilic membrane surfaces in a submerged membrane bioreactor. Water Res. 2016, 95, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, W.; Wang, Z.; Song, P. The cake layer formation in the early stage of filtration in MBR: Mechanism and Model. J. Membr. Sci. 2018, 559, 75–86. [Google Scholar] [CrossRef]
- Tansel, B.; Tansel, D.Z. Adhesion strength and spreading characteristics of EPS on membrane surfaces during lateral and central growth. Colloids Surf. B 2013, 111, 594–599. [Google Scholar] [CrossRef]
- Gao, D.; Fu, Y.; Ren, N. Tracing biofouling to the structure of the microbial community and its metabolic products: A study of the three-stage MBR process. Water Res. 2013, 47, 6680–6690. [Google Scholar] [CrossRef]
- Sun, M.; Yan, L.; Zhang, L.; Song, L.; Guo, J.; Zhang, H. New insights into the rapid formation of initial membrane fouling after in-situ cleaning in a membrane bioreactor. Process Biochem. 2019, 78, 108–113. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Ding, Y.; Lu, Y. Identification of the change in fouling potential of soluble microbial products (SMP) in membrane bioreactor coupled with worm reactor. Water Res. 2013, 47, 2015–2024. [Google Scholar] [CrossRef]
- Lei, Q.; Li, F.; Shen, L.; Yang, L.; Liao, B.Q.; Lin, H. Tuning anti-adhesion ability of membrane for a membrane bioreactor by thermodynamic analysis. Bioresour. Technol. 2016, 216, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, F.; Weng, X.; Li, R.; Zhou, X.; Lin, H.; Yu, H.; Liao, B.Q. Novel indicators for thermodynamic prediction of interfacial interactions related with adhesive fouling in a membrane bioreactor. J. Colloid Interface Sci. 2017, 487, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Peng, W.; Zhang, M.; Chen, J.; He, Y.; Wang, F.; Weng, X.; Yu, H.; Lin, H. Thermodynamic analysis of membrane fouling in a submerged membrane bioreactor and its implications. Bioresour. Technol. 2013, 146, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Elimelech, M.; Bhattacharjee, S. A novel approach for modeling concentration polarization in crossflow membrane filtration based on the equivalence of osmotic pressure model and filtration theory. J. Membr. Sci. 1998, 145, 223–241. [Google Scholar] [CrossRef]
- Sunny, W.; Greg, G.; Hoek, E.M.V. Direct observation of microbial adhesion to membranes. Environ. Sci. Technol. 2005, 39, 6461–6469. [Google Scholar]
- Judd, S. The Commercial Status of Membrane Bioreactors for Municipal Wastewater. Sep. Sci. Technol. 2010, 45, 850–857. [Google Scholar]
- Hong, H.; Zhang, M.; He, Y.; Chen, J.; Lin, H. Fouling mechanisms of gel layer in a submerged membrane bioreactor. Bioresour. Technol. 2014, 166, 295–302. [Google Scholar] [CrossRef]
- Zhang, H.; Xin, L.; Song, L.; Zhang, L. Effects of Loosely Bound EPS Release and Floc Reconstruction on Sludge Dewaterability. Water, Air, Soil Pollut. 2018, 229, 27. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wu, Z.; Wei, T.; Lu, F.; Tong, J.; Mai, S. Membrane fouling in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Characteristics of membrane foulants and bulk sludge. Process Biochem. 2011, 46, 1538–1544. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Burton, K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J 1956, 62, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oss, C.J.V. Hydrophobicity of biosurfaces-Origin, quantitative determination and interaction energies. Colloids Surf. B 1995, 5, 91–110. [Google Scholar]
- Zhang, M.; Liao, B.Q.; Zhou, X.; He, Y.; Hong, H.; Lin, H.; Chen, J. Effects of hydrophilicity/hydrophobicity of membrane on membrane fouling in a submerged membrane bioreactor. Bioresour. Technol. 2015, 175, 59–67. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; Am. Public Health Assoc.: Washington, DC, USA, 1981. [Google Scholar]
- Zhu, L.; Zhou, J.; Lv, M.; Yu, H.; Zhao, H.; Xu, X. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere 2015, 121, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Dan, H.; Wang, Y.; Zhu, J. The membrane fouling characteristics of MBRs with different aerobic granular sludges at high flux. Bioresour. Technol. 2013, 136, 488–495. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Wei, P.; He, Y.; Lin, H.; Chen, J.; Hong, H.; Wang, A.; Yu, H. Effects of ionic strength on membrane fouling in a membrane bioreactor. Bioresour. Technol. 2014, 156, 35–41. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: measurements and mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef]
- Qu, X.; Xiang, C.; Zhang, M.; Lin, H.; Liao, B.Q. A facile method for simulating randomly rough membrane surface associated with interface behaviors. Appl. Surf. Sci. 2017, 427, 915–921. [Google Scholar] [CrossRef]
- Patsios, S.I.; Goudoulas, T.B.; Kastrinakis, E.G.; Nychas, S.G.; Karabelas, A.J. A novel method for rheological characterization of biofouling layers developing in Membrane Bioreactors (MBR). J. Membr. Sci. 2015, 482, 13–24. [Google Scholar] [CrossRef]
| Parameter | Content |
|---|---|
| COD in influent (mg/L) | 212.18 ± 2.15 |
| NH4+–N in influent (mg/L) | 19.10 ± 2.90 |
| TP in influent (mg/L) | 5.72 ± 0.62 |
| COD removal (%) | 92.84 ± 4.31 |
| NH4+–N removal (%) | 92.04 ± 6.04 |
| TP removal (%) | 19.19 ± 1.09 |
| MLSS (mg/L) | 4.50 ± 0.27 |
| MLVSS (mg/L) | 3.46 ± 0.13 |
| MLVSS/MLSS(%) | 0.77 ± 0.01 |
| Components | EPS Fractions | Content (mg/g SS) |
|---|---|---|
| eDNA | S-EPS | 0.65 ± 0.21 |
| LB-EPS | 0.64 ± 0.28 | |
| TB-EPS | 2.13 ± 0.54 | |
| Protein | S-EPS | 2.27 ± 1.58 |
| LB-EPS | 3.12 ± 0.46 | |
| TB-EPS | 18.07 ± 2.23 | |
| Polysaccharide | S-EPS | 2.24 ± 0.29 |
| LB-EPS | 1.84 ± 0.44 | |
| TB-EPS | 21.36 ± 1.16 |
| Samples | Peak A | Peak B | ||
|---|---|---|---|---|
| Ex/Em (nm) | Intensity (a.u.) | Ex/Em (nm) | Intensity (a.u.) | |
| No flux | 280/345 | 108.80 | 235/340 | 23.86 |
| Normal flux | 280/345 | 195.02 | 235/340 | 32.15 |
| Materials | Contact Angle (°) | Zeta Potential (mV) b | ||
|---|---|---|---|---|
| Water | Glycerol | Diiodomethane | ||
| Virgin membrane | 58.86 ± 2.27 | 53.08 ± 1.17 | 20.69 ± 0.99 | −31.22 ± 0.13 |
| Fouled membrane (no flux) | 72.51 ± 0.29 | 69.45 ± 1.24 | 38.79 ± 3.34 | −9.59 ± 1.68 |
| Fouled membrane (normal flux) | 78.55 ± 4.39 | 70.32 ± 5.54 | 45.43 ± 0.99 | −15.59 ± 4.95 |
| Sludge flocs | 78.32 ± 2.13 | 85.52 ± 1.39 | 57.59 ± 3.45 | −26.23 ± 1.73 |
| Materials | (mJ/m2) | |||||
|---|---|---|---|---|---|---|
| Virgin membrane | 45.86 | 0.13 | 17.45 | 2.98 | 48.84 | −25.22 |
| Fouled membrane (no flux) | 40.21 | 0.02 | 13.26 | −0.95 | 39.26 | −34.77 |
| Fouled membrane (normal flux) | 36.78 | 0.06 | 7.76 | 1.37 | 38.15 | −47.39 |
| Sludge flocs | 29.96 | 0.67 | 19.57 | −7.24 | 22.72 | −15.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Lu, X.; Zhang, L.; Guo, J.; Zhang, H. Characterization of the Initial Fouling Layer on the Membrane Surface in a Membrane Bioreactor: Effects of Permeation Drag. Membranes 2019, 9, 121. https://doi.org/10.3390/membranes9090121
Wang S, Lu X, Zhang L, Guo J, Zhang H. Characterization of the Initial Fouling Layer on the Membrane Surface in a Membrane Bioreactor: Effects of Permeation Drag. Membranes. 2019; 9(9):121. https://doi.org/10.3390/membranes9090121
Chicago/Turabian StyleWang, Shengli, Xin Lu, Lanhe Zhang, Jingbo Guo, and Haifeng Zhang. 2019. "Characterization of the Initial Fouling Layer on the Membrane Surface in a Membrane Bioreactor: Effects of Permeation Drag" Membranes 9, no. 9: 121. https://doi.org/10.3390/membranes9090121