Thermoelectric Behavior of BaZr0.9Y0.1O3−d Proton Conducting Electrolyte
Abstract
:1. Introduction
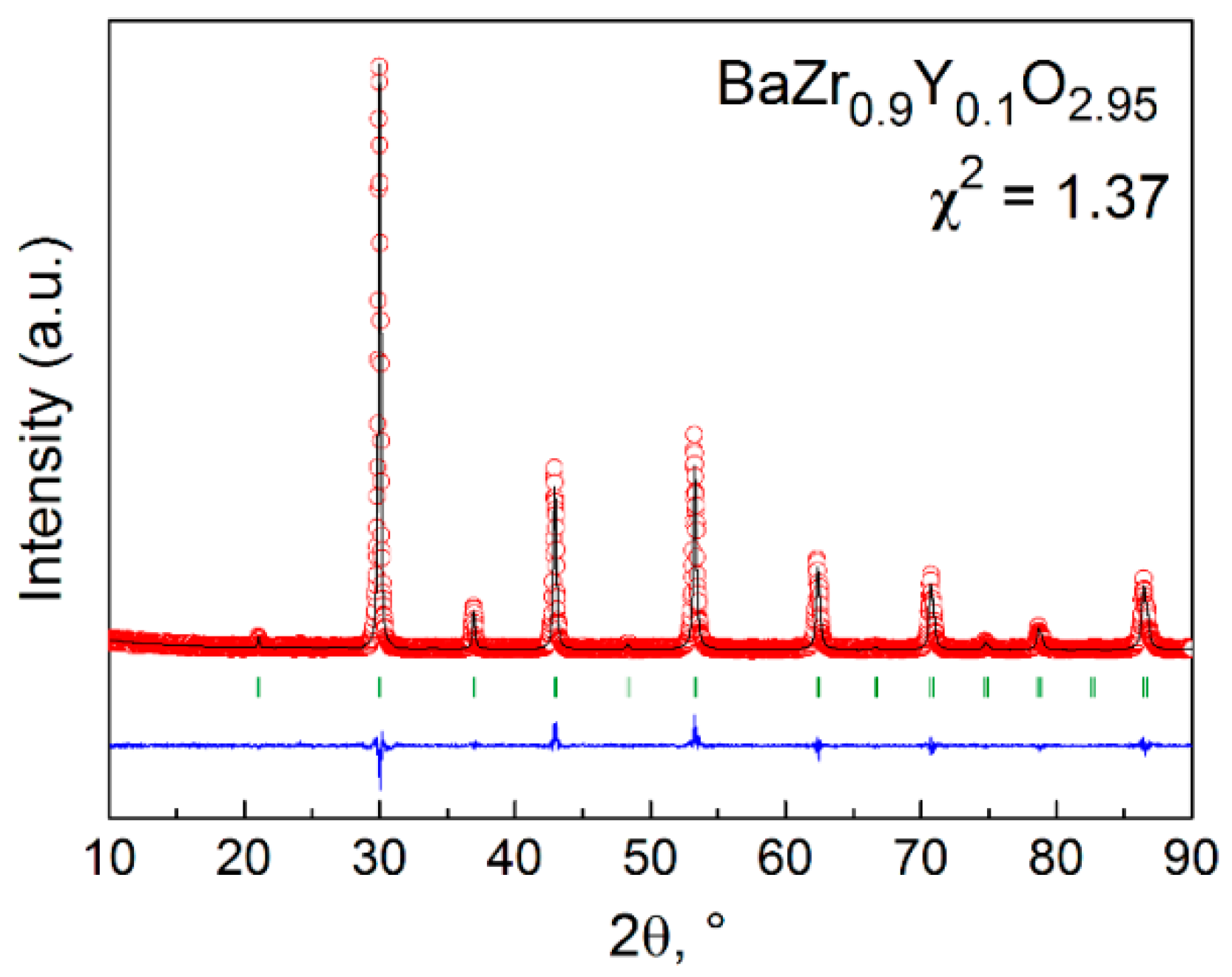
2. Materials and Methods
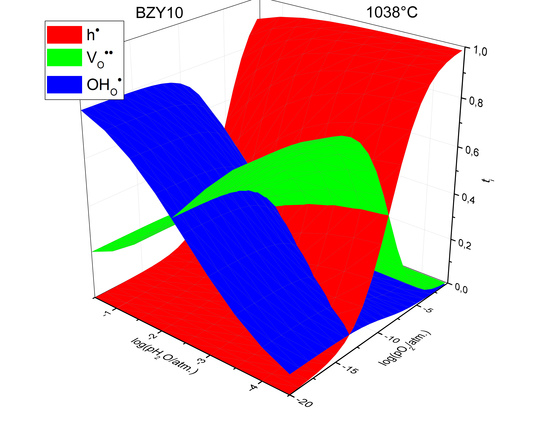
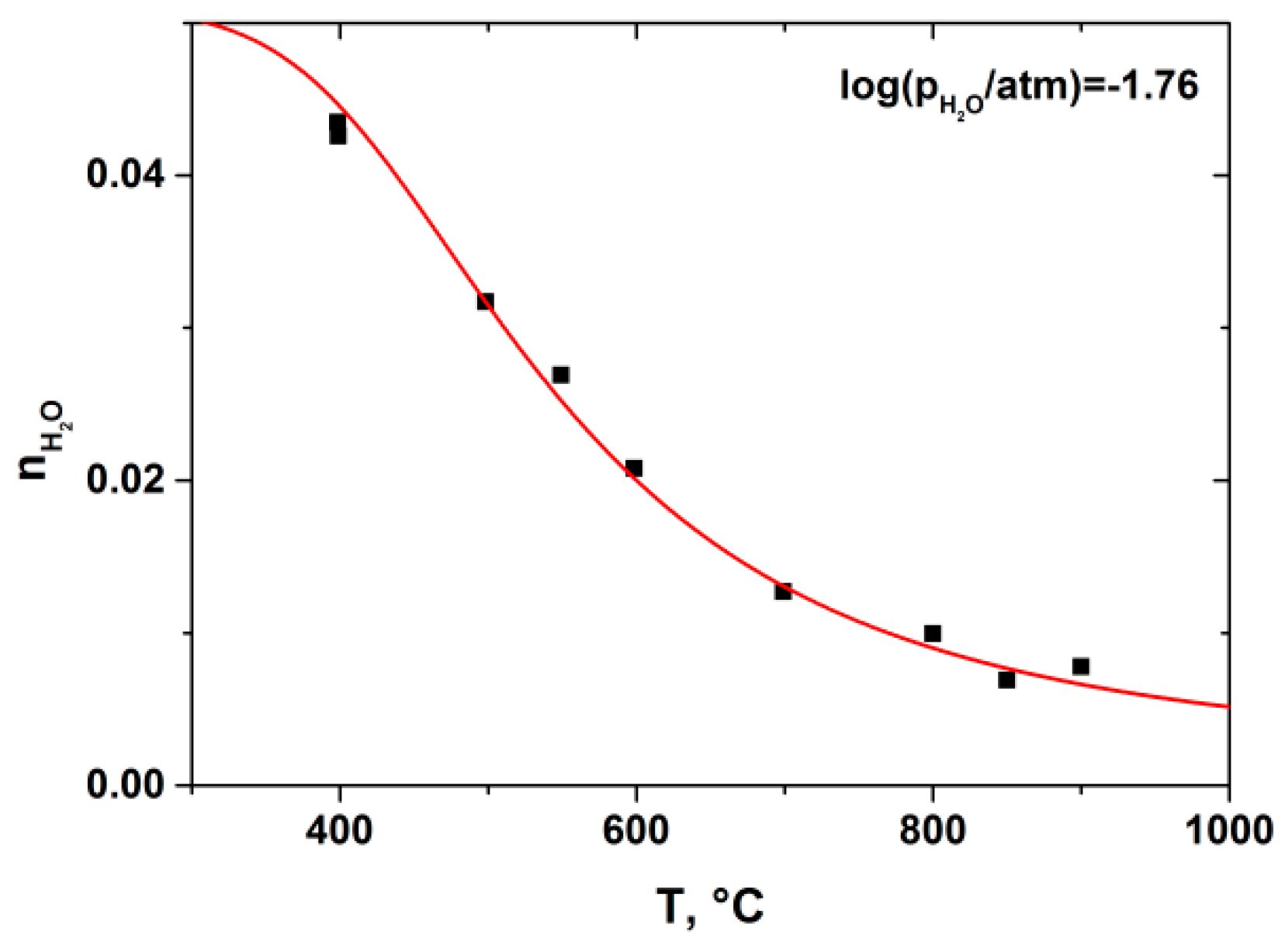
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| R2 | ||
|---|---|---|
| −79 ± 2 | −91 ± 2 | 0.992 |
References
- Sažinas, R.; Einarsrud, M.-A.; Grande, T. Toughening of Y-doped BaZrO3 proton conducting electrolytes by hydration. J. Mater Chem. A 2017, 5, 5846–5857. [Google Scholar] [CrossRef]
- Kreuer, K.D. Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ionics 1999, 125, 285–302. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Medvedev, D.; Brouzgou, A.; Demin, A.; Tsiakaras, P. Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; Boaro, M., Salvatore, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 77–118. [Google Scholar]
- Schober, T.; Bohn, H.G. Water vapor solubility and electrochemical characterization of the high temperature proton conductor BaZr0.9Y0.1O2.95. Solid State Ionics 2000, 127, 351–360. [Google Scholar] [CrossRef]
- Ji, H.; Kim, B.; Yu, J.H.; Choi, S.; Kim, H.; Son, J.; Lee, H.; Lee, J. Three dimensional representations of partial ionic and electronic conductivity based on defect structure analysis of BaZr0.85Y0.15O3-δ. Solid State Ionics 2011, 203, 9–17. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Balakireva, V.B. Synthesis and properties of high-density protonic solid electrolyte BaZr0.9Y0.1O3-α. Russ. J. Electrochem. 2009, 45, 476–482. [Google Scholar] [CrossRef]
- Karlsson, M. Proton dynamics in oxides: Insight into the mechanics of proton conduction from quasielastic neutron scattering. Phys. Chem. Chem. Phys. 2015, 17, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Noferini, D.; Koza, M.M.; Karlsson, M. Localized proton motions in acceptor-doped barium zirconates. J. Phys. Chem. C 2017, 121, 7088–7093. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Blanc, F.; Okuyama, Y.; Buannic, L.; Lucio-Vega, J.C.; Grey, C.P.; Haile, S.M. Proton trapping in yttrium-doped barium zirconate. Nat. Mater 2013, 12, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Iguchi, F.; Nagao, Y.; Sata, N.; Liu, Y.-S.; Glans, P.-A.; Guo, J.; Yugami, H. Surface Electronic Structure of BaZr1-xYxO3-δ by Soft-X-Ray Spectroscopy. Trans. Mater Res. Soc. Japan 2012, 37, 575–578. [Google Scholar] [CrossRef]
- Björketun, M.E.; Sundell, P.G.; Wahnström, G. Structure and thermodynamic stability of hydrogen interstitials in BaZrO3 perovskite oxide from density functional calculations. Faraday Discuss. 2007, 134, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Sundell, P.G.; Björketun, M.E.; Wahnström, G. Density-functional calculations of prefactors and activation energies for H diffusion in BaZrO3. Phys. Rev. B 2007, 76, 094301. [Google Scholar] [CrossRef]
- Raiteri, P.; Gale, J.D.; Bussi, G. Reactive force field simulation of proton diffusion in BaZrO3 using an empirical valence bond approach. J. Phys. Cond. Mater 2011, 23, 334213. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Akola, J.; Kohara, S.; Fujimoto, K.; Idemoto, Y. Proton Distribution and Dynamics in Y- and Zn-Doped BaZrO3. J. Phys. Chem. C 2014, 118, 18846–18852. [Google Scholar] [CrossRef]
- Haro-González, P.; Karlsson, M.; Gaita, S.M.; Knee, C.S.; Bettinelli, M. Eu3+ as a luminescent probe for the local structure of trivalent dopant ions in barium zirconate-based proton conductors. Solid State Ionics 2013, 247, 94–97. [Google Scholar] [CrossRef]
- Noferini, D.; Koza, M.M.; Rahman, S.M.H.; Evenson, Z.; Nilsen, G.J.; Eriksson, S.; Wildes, A.R.; Karlsson, M. Role of the doping level in localized proton motions in acceptor-doped barium zirconate proton conductors. Phys. Chem. Chem. Phys. 2018, 20, 13697–13704. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Yang, C.-K.; Haile, S.M. Unraveling the defect chemistry and proton uptake of yttrium-doped barium zirconate. Scripta Mater 2011, 65, 102–107. [Google Scholar] [CrossRef]
- Wang, W.; Virkar, A.V. Ionic and electron–hole conduction in BaZr0.93Y0.07O3-δ by 4-probe dc measurements. J. Power Sources. 2005, 142, 1–9. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Babilo, P.; Haile, S.M. Defect chemistry of yttrium-doped barium zirconate. Chem. Mater 2008, 20, 6352–6357. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Sereda, V.V.; Zuev, A.Y. Red-Ox energetics and holes trapping in yttrium-substituted barium zirconate BaZr0.9Y0.1O2.95. J. Electrochem. Soc. 2019, 166, F232–F238. [Google Scholar] [CrossRef]
- Babilo, P.; Haile, S.M. Enhanced sintering of yttrium-doped barium zirconate by addition of ZnO. J. Am. Ceram. Soc. 2005, 88, 2362–2368. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Sanders, M.; O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 2010, 20, 6333–6341. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; O’Hayre, R. Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ionics 2013, 253, 201–210. [Google Scholar] [CrossRef]
- Lindman, A.; Erhart, P.; Wahnström, G. Polaronic contributions to oxidation and hole conductivity in acceptor-doped BaZrO3. Phys. Rev. B 2016, 94, 075204. [Google Scholar] [CrossRef]
- Schirmer, O.F. O− bound small polarons in oxide materials. J. Phys. Cond. Mater 2006, 18, R667–R704. [Google Scholar] [CrossRef]
- Wagner, C. The thermoelectric power of cells with ionic compounds involving ionic and electronic conduction. Prog. Solid State Chem. 1972, 7, 1–37. [Google Scholar] [CrossRef]
- Tai, L.-W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 1. The system La0.8Sr0.2Co1-yFeyO3. Solid State Ionics 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Tai, L.-W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 2. The system La1-xSrxCo0.2Fe0.8O3. Solid State Ionics 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Sehlin, S.R.; Anderson, H.U.; Sparlin, D.M. Semiempirical model for the electrical properties of La1-xCaxCoO3. Phys. Rev. B. 1995, 52, 11681–11689. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Kobayashi, K.; Abe, K.; Yamazaki, S.; Iguchi, Y. Electrical conductivity and thermoelectric power measurements of Y2Ti2O7. Solid State Ionics 1998, 113–115, 393–402. [Google Scholar] [CrossRef]
- Koshibae, W.; Tsutsui, K.; Maekawa, S. Thermopower in cobalt oxides. Phys. Rev. B. 2000, 62, 6869–6872. [Google Scholar] [CrossRef]
- Park, S.-H.; Yoo, H.-I. Thermoelectric behavior of a mixed ionic electronic conductor, Ce1-xGdxO2-x/2-d. Phys. Chem. Chem. Phys. 2009, 11, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, E.; Poulsen, F.W. Thermoelectric power of YSZ. Solid State Ionics 1994, 70, 528–532. [Google Scholar] [CrossRef]
- Sereda, V.; Malyshkin, D.; Tsvetkov, D.; Zuev, A. Hydration thermodynamics of proton-conducting perovskite Ba4Ca2Nb2O11. Mater Lett. 2019, 235, 97–100. [Google Scholar] [CrossRef]
- Bohn, H.G.; Schober, T. Electrical conductivity of the high-temperature proton conductor BaZr0.9Y0.1O2.95. J. Am. Ceram. Soc. 2000, 83, 768–772. [Google Scholar] [CrossRef]
- Kjølseth, C.; Wang, L.-Y.; Haugsrud, R.; Norby, T. Determination of the enthalpy of hydration of oxygen vacancies in Y-doped BaZrO3 and BaCeO3 by TG-DSC Solid State Ionics. Solid State Ionics 2010, 181, 1740–1745. [Google Scholar]
- Gurvich, L.V. Thermodynamic Properties of Individual Substances, 4th ed.; Hemisphere Publishing Corp.: New York, NY, USA, 1989. [Google Scholar]
- Tsidilkovski, V.I.; Putilov, L.P. The role of deep acceptor levels in hydration and transport processes in BaZr1-xYxO3-δ and related materials. J. Solid State Electrochem. 2016, 20, 629–643. [Google Scholar] [CrossRef]
- Putilov, L.P.; Tsidilkovski, V.I. The role of deep acceptor centers in the oxidation of acceptor-doped wideband- gap perovskites ABO3. J. Solid State Chem. 2017, 247, 147–155. [Google Scholar] [CrossRef]
- Stokes, S.J.; Islam, M.S. Defect chemistry and proton-dopant association in BaZrO3 and BaPrO3. J. Mater. Chem. 2010, 20, 6258–6264. [Google Scholar] [CrossRef]
- Zhu, H.; Ricote, S.; Coors, W.G.; Kee, R.J. Interpreting equilibrium-conductivity and conductivity-relaxation measurements to establish thermodynamic and transport properties for multiple charged defect conducting ceramics. Faraday Discuss. 2015, 182, 49–74. [Google Scholar] [CrossRef]
- Zhu, H.; Kee, R.J. Membrane polarization in mixed-conducting ceramic fuel cells and electrolyzers. Int. J. Hydrogen Energy 2016, 41, 2931–2943. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Ricote, S.; Duan, C.; O’Hayre, R.P.; Tsvetkov, D.S.; Kee, R.J. Defect incorporation and transport within dense BaZr0.8Y0.2O3-δ (BZY20) proton-conducting membranes. J. Electrochem. Soc. 2018, 165, F581–F588. [Google Scholar] [CrossRef]
- Ma, F.; Raymond, M.V.; Smyth, D.M. Hole-trapping in ATiO3, (A = Ba, Sr, Ca). Ferroelectrics 2006, 331, 43–51. [Google Scholar] [CrossRef]
- Raymond, M.V.; Smyth, D.M. Defect chemistry and transport properties of Pb(Zr1/2Ti1/2)O3. Integr. Ferroelectr. 1994, 4, 145–154. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Balakireva, V.B.; Kleshchev, Y.N.; Brusentsov, V.P. Preparation and Electrical Conductivity of BaZr1-xRxO3–α (R = Sc, Y, Ho, Dy, Gd, In). Inorg. Mater 2001, 37, 535–538. [Google Scholar] [CrossRef]
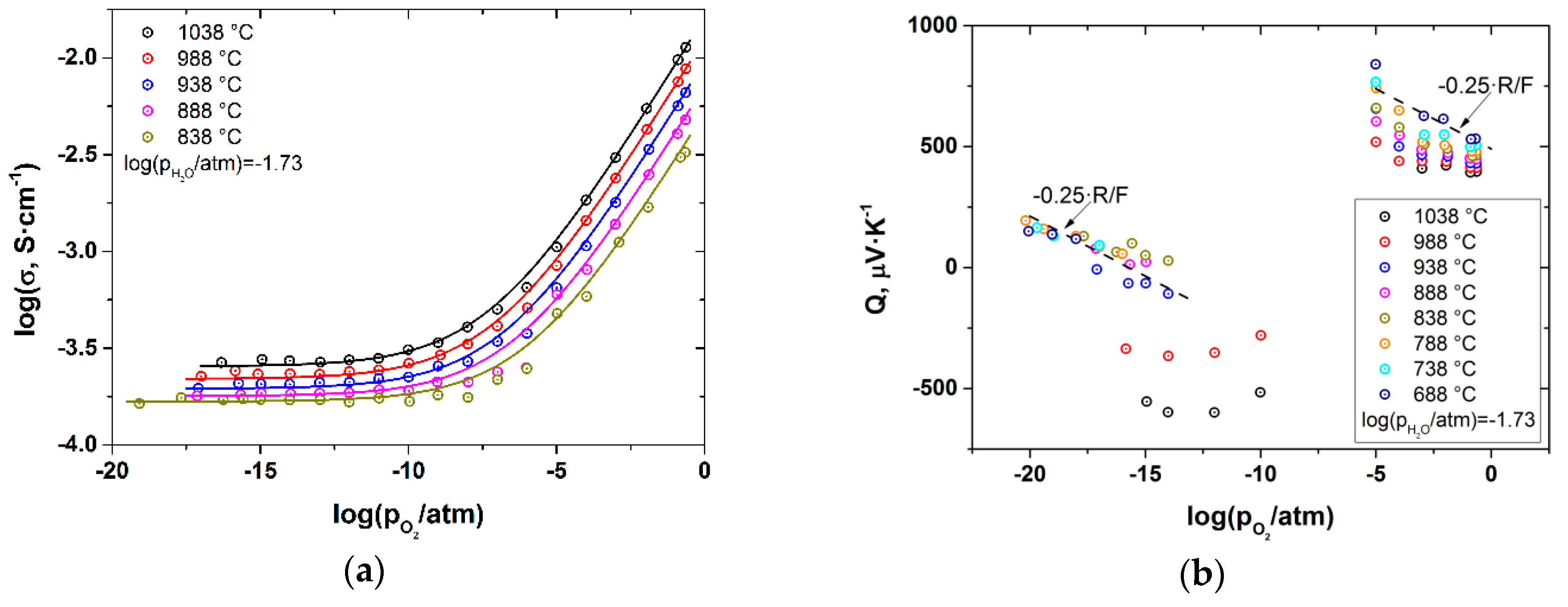
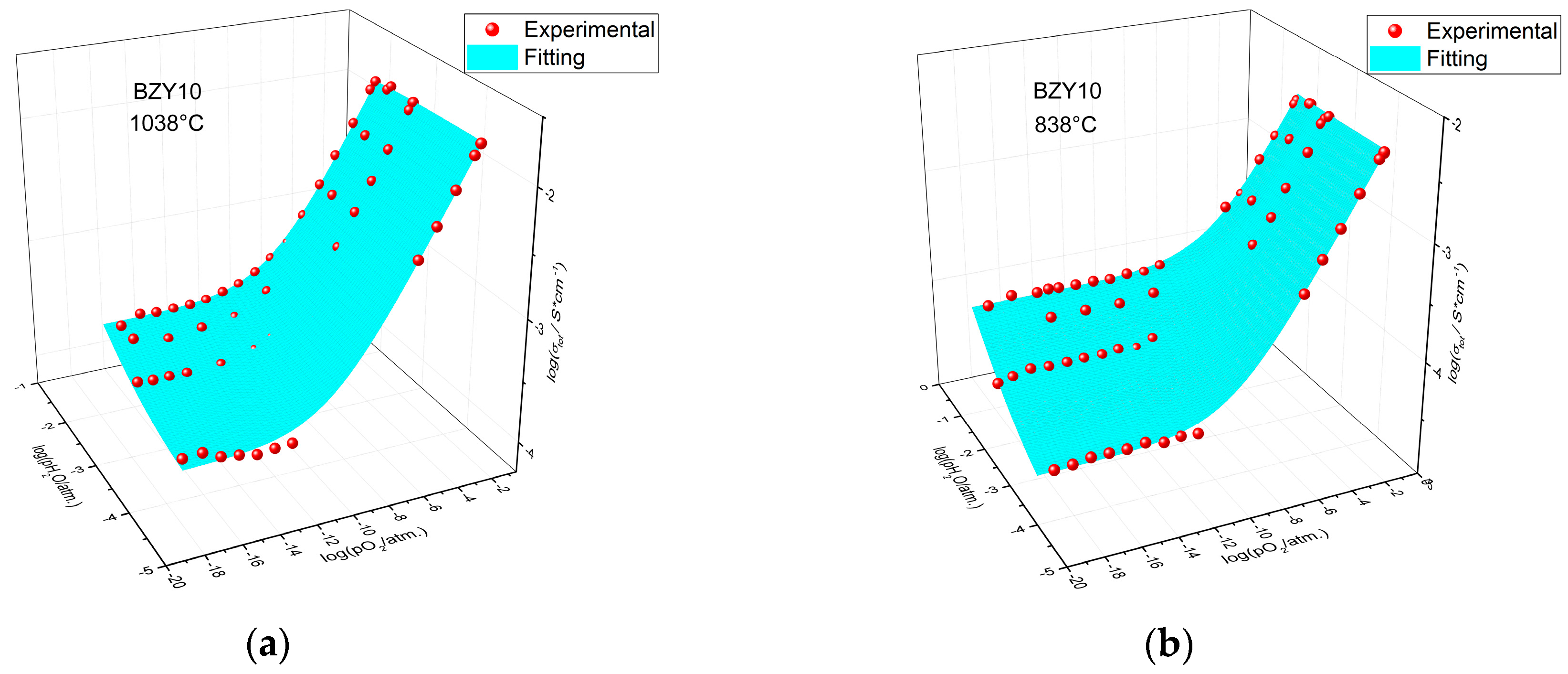
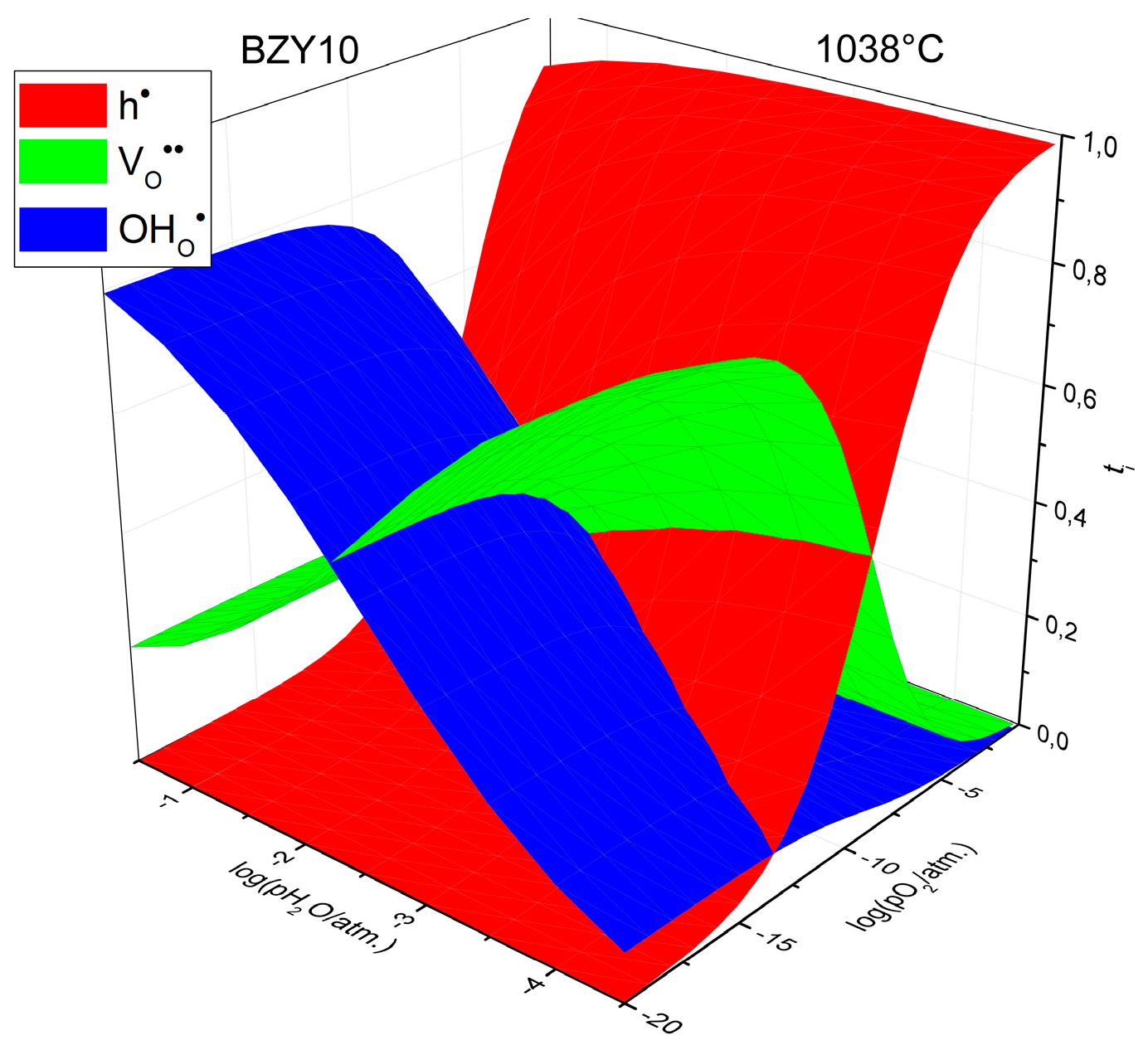
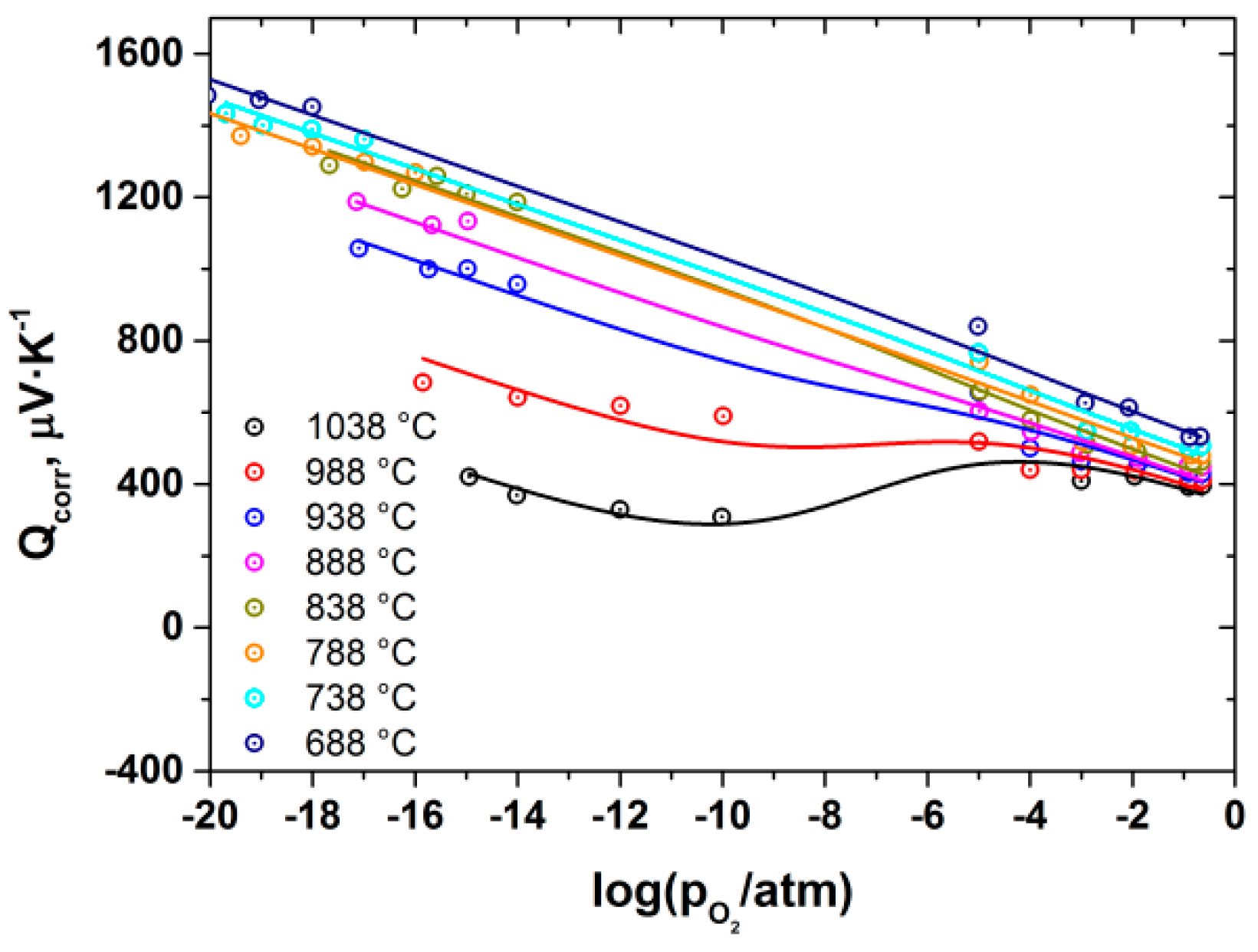
| Charge Carrier | Pre-Exponential Factor 1, S·cm−1 | Activation Energy 1, eV | R2 |
|---|---|---|---|
| Oxide ion | 25.43 ± 13.00 | 1.42 ± 0.01 | 0.997 |
| Hole | 10.42 ± 3.00 | 0.73 ± 0.05 | |
| Proton | (1.47 ± 0.60)·10−3 | 0.02 ± 0.09 |
| Charge Carrier | Heat of Transport, eV * | R2 | ||
|---|---|---|---|---|
| Oxide ion | 0.73 ± 0.03 | 34.6 ± 1.4 (N = 2.95) | 0.74 ± 0.04 (does not depend on N) | 0.990 |
| Hole | 0 ** | 6.5 ± 1.4 (N = 0.1) | ||
| Proton | 0.43 ± 0.10 | −12.3 ± 1.4 (N = 0.01) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetkov, D.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. Thermoelectric Behavior of BaZr0.9Y0.1O3−d Proton Conducting Electrolyte. Membranes 2019, 9, 120. https://doi.org/10.3390/membranes9090120
Tsvetkov D, Ivanov I, Malyshkin D, Sereda V, Zuev A. Thermoelectric Behavior of BaZr0.9Y0.1O3−d Proton Conducting Electrolyte. Membranes. 2019; 9(9):120. https://doi.org/10.3390/membranes9090120
Chicago/Turabian StyleTsvetkov, Dmitry, Ivan Ivanov, Dmitry Malyshkin, Vladimir Sereda, and Andrey Zuev. 2019. "Thermoelectric Behavior of BaZr0.9Y0.1O3−d Proton Conducting Electrolyte" Membranes 9, no. 9: 120. https://doi.org/10.3390/membranes9090120
APA StyleTsvetkov, D., Ivanov, I., Malyshkin, D., Sereda, V., & Zuev, A. (2019). Thermoelectric Behavior of BaZr0.9Y0.1O3−d Proton Conducting Electrolyte. Membranes, 9(9), 120. https://doi.org/10.3390/membranes9090120