Vanillin as an Antifouling and Hydrophilicity Promoter Agent in Surface Modification of Polyethersulfone Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Characterization
2.2.1. Fourier Transform Infrared Spectroscopy
2.2.2. Contact Angle Measurement
2.2.3. Surface Charge Measurements
2.2.4. Extraction of Vanillin from Membrane and UV Analysis
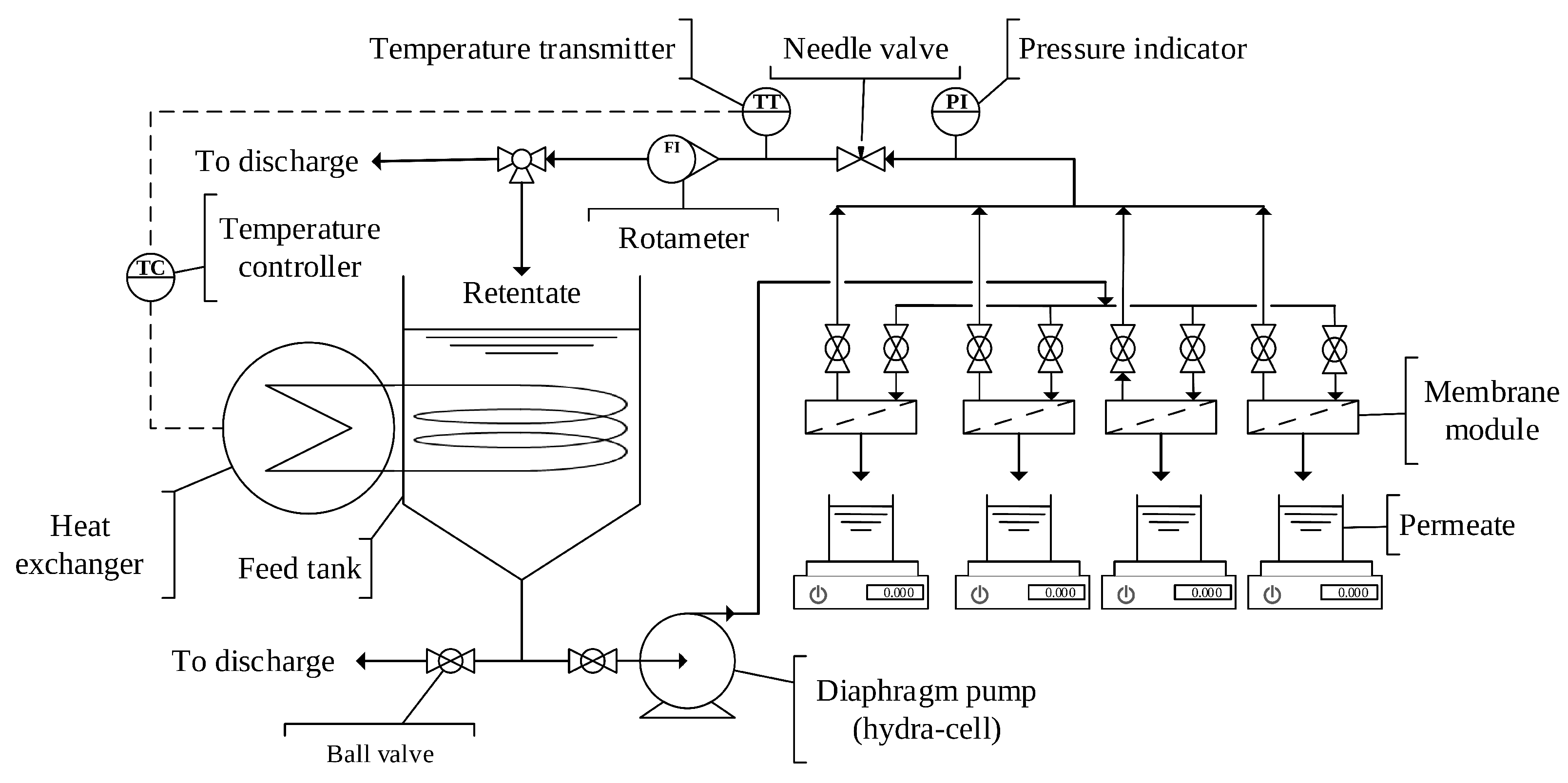
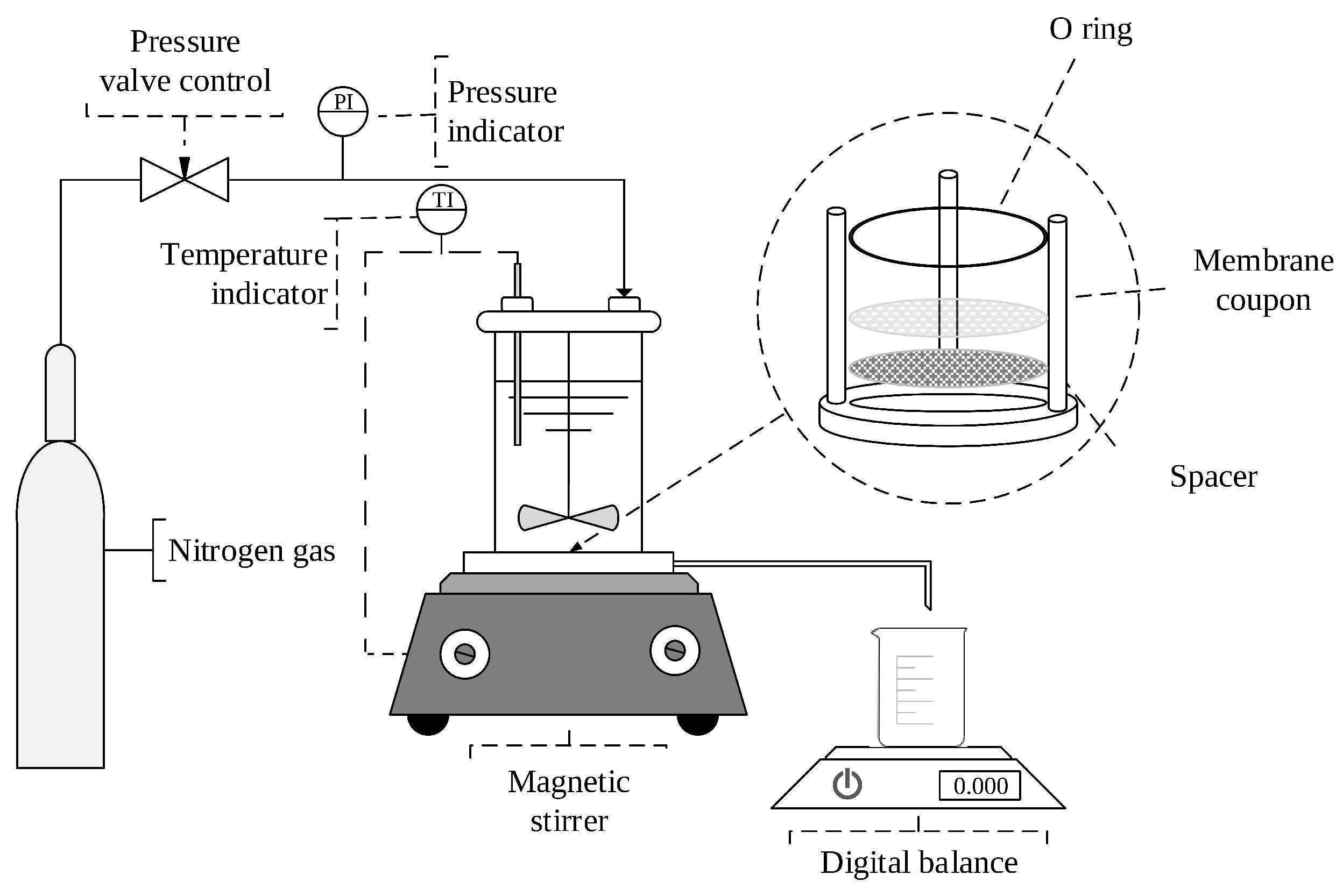
2.3. Experimental Design and Procedure
2.3.1. Preparation of Pressurized Hot Water Extract
2.3.2. Surface Modification of PES Membrane with Vanillin
2.3.3. Fouling Study of the Virgin and Modified Membranes with Wood Extract
3. Results and Discussion
3.1. Characterization of Membranes by the Means of FTIR Spectroscopy
3.1.1. Spectral Analysis of Virgin Membranes
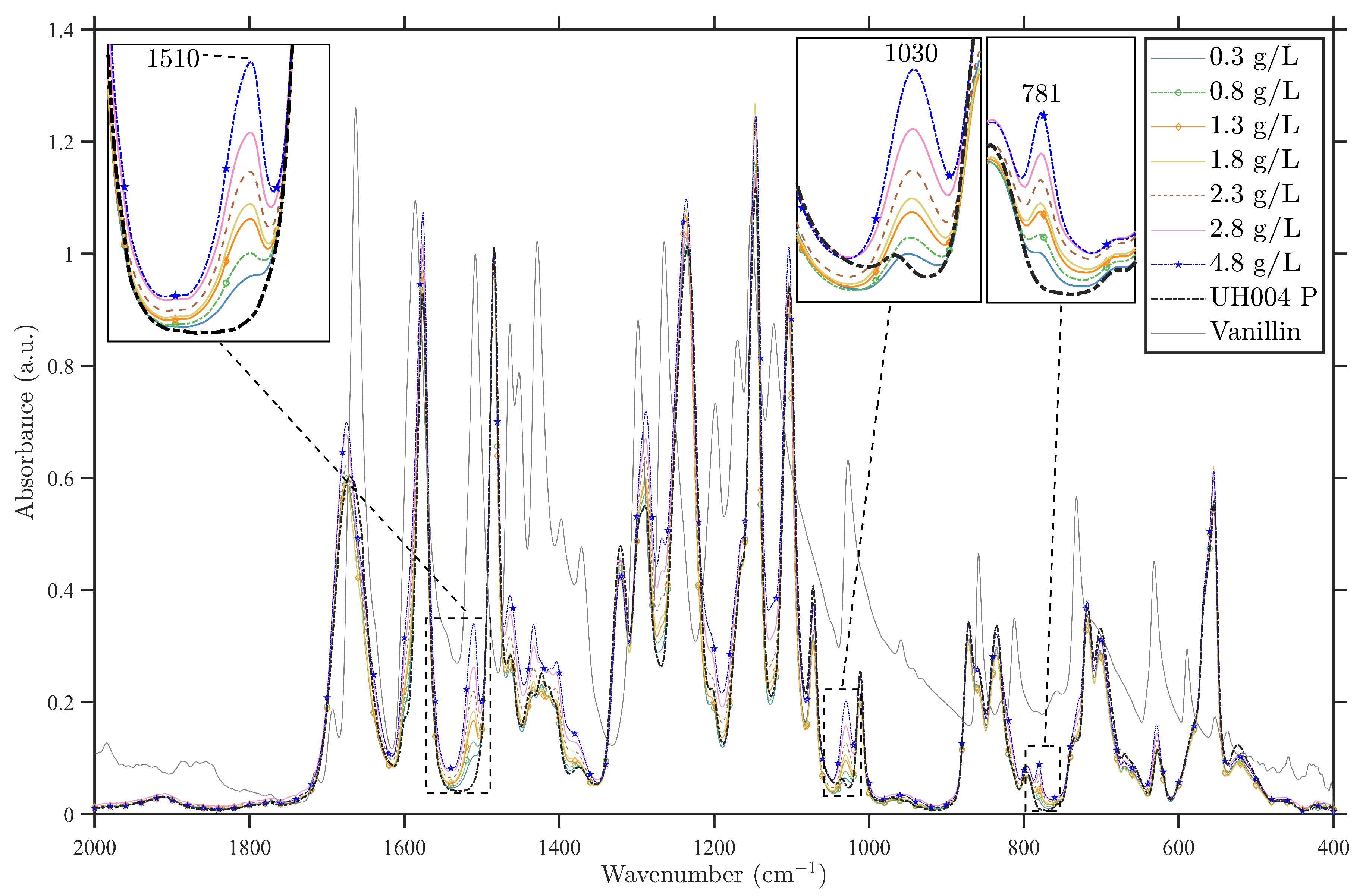
3.1.2. Spectral Analysis of the Commercial Membrane Modified by Vanillin
3.2. The Effect of Modification on the Membrane Performance
3.3. The Effect of Modification on the Contact Angle of the Membrane
3.4. The Quantity of Vanillin Remaining in the Membrane Structure after Experiments
3.5. The Influence of Modification on the Membrane Performance in the Filtration of Wood Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Ding, Z.; Zhong, L.; Wang, X.; Zhang, L. Effect of lignin-cellulose nanofibrils on the hydrophilicity and mechanical properties of polyethersulfone ultrafiltration membranes. High Perform. Polym. 2016, 28, 1192–1200. [Google Scholar] [CrossRef]
- Esmaeili, M.; Anugwom, I.; Mänttäri, M.; Kallioinen, M. Utilization of DES-Lignin as a Bio-Based Hydrophilicity Promoter in the Fabrication of Antioxidant Polyethersulfone Membranes. Membranes 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Natarajan, G.; Cameron, D.C.; Nyström, M. Performance of RC and PES ultrafiltration membranes in filtration of pulp mill process waters. Desalination 2010, 264, 249–255. [Google Scholar] [CrossRef]
- Koivula, E.; Kallioinen, M.; Sainio, T.; Antón, E.; Luque, S.; Mänttäri, M. Enhanced membrane filtration of wood hydrolysates for hemicelluloses recovery by pretreatment with polymeric adsorbents. Bioresour. Technol. 2013, 143, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Wan, L.S.; Liu, Z.M.; Xu, Z.K. Surface engineering of macroporous polypropylene membranes. Soft Matter 2009, 5, 1775–1785. [Google Scholar] [CrossRef]
- Xie, Y.J.; Yu, H.Y.; Wang, S.Y.; Xu, Z.K. Improvement of antifouling characteristics in a bioreactor of polypropylene microporous membrane by the adsorption of Tween 20. J. Environ. Sci. 2007, 19, 1461–1465. [Google Scholar] [CrossRef]
- Reddy, A.; Mohan, D.; Bhattacharya, A.; Shah, V.; Ghosh, P. Surface modification of ultrafiltration membranes by preadsorption of a negatively charged polymer: I. Permeation of water soluble polymers and inorganic salt solutions and fouling resistance properties. J. Membr. Sci. 2003, 214, 211–221. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Li, W.; Xing, W.; Wang, Y. Depositing lignin on membrane surfaces for simultaneously upgraded reverse osmosis performances: An upscalable route. AIChE J. 2017, 63, 2221–2231. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Katebian, L.; Gomez, E.; Skillman, L.; Li, D.; Ho, G.; Jiang, S.C. Inhibiting quorum sensing pathways to mitigate seawater desalination RO membrane biofouling. Desalination 2016, 393, 135–143. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Paul, D.; Kweon, J. Inhibition of quorum sensing mechanism and Aeromonas hydrophila biofilm formation by vanillin. Environ. Eng. Sci. 2009, 26, 1359–1363. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Kappachery, S.; Thekeettle, M.; Song, J.H.; Kweon, J.H. Anti-biofouling property of vanillin on Aeromonas hydrophila initial biofilm on various membrane surfaces. World J. Microbiol. Biotechnol. 2013, 29, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Kappachery, S.; Paul, D.; Yoon, J.; Kweon, J.H. Vanillin, a potential agent to prevent biofouling of reverse osmosis membrane. Biofouling 2010, 26, 667–672. [Google Scholar] [CrossRef]
- Katebian, L.; Hoffmann, M.R.; Jiang, S.C. Incorporation of quorum sensing inhibitors onto reverse osmosis membranes for biofouling prevention in seawater desalination. Environ. Eng. Sci. 2017, 1–9. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM–41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423, 238–246. [Google Scholar] [CrossRef]
- Virtanen, T.; Reinikainen, S.P.; Kögler, M.; Mänttäri, M.; Viitala, T.; Kallioinen, M. Real-time fouling monitoring with Raman spectroscopy. J. Membr. Sci. 2017, 525, 312–319. [Google Scholar] [CrossRef]
- Fengel, S.Y.; Dence, G. Wood: Chemistry, Ultrastructure, Reactions, 1st ed.; Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Lin, D.; Wegener, C.W. Methods in Lignin Chemistry, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Liu, S.; Kim, J.T.; Kim, S. Effect of polymer surface modification on polymer–protein interaction via hydrophilic polymer grafting. J. Food Sci. 2008, 73, E143–E150. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.M.; Gekas, V.; Trägårdh, G. Study of membrane compaction and its influence on ultrafiltration water permeability. J. Membr. Sci. 1995, 100, 155–162. [Google Scholar] [CrossRef]
- Evans, P.; Bird, M. Solute-membrane fouling interactions during the ultrafiltration of black tea liquor. Food Bioprod. Process. 2006, 84, 292–301. [Google Scholar] [CrossRef]
- Antón, E.; Álvarez, J.R.; Palacio, L.; Prádanos, P.; Hernández, A.; Pihlajamäki, A.; Luque, S. Ageing of polyethersulfone ultrafiltration membranes under long-term exposures to alkaline and acidic cleaning solutions. Chem. Eng. Sci. 2015, 134, 178–195. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Vatsha, B.; Ngila, J.C.; Moutloali, R.M. Preparation of antifouling polyvinylpyrrolidone (PVP 40K) modified polyethersulfone (PES) ultrafiltration (UF) membrane for water purification. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 125–131. [Google Scholar] [CrossRef]
- Thuyavan, Y.L.; Anantharaman, N.; Arthanareeswaran, G.; Ismail, A. Modification of polyethersulfone using sericin and polyvinylpyrrolidone for cadmium ion removal by polyelectrolyte-enhanced ultrafiltration. Desalin. Water Treat. 2015, 56, 366–378. [Google Scholar] [CrossRef]
- Balachandran, V.; Parimala, K. Vanillin and isovanillin: Comparative vibrational spectroscopic studies, conformational stability and NLO properties by density functional theory calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 354–368. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Baltaru, M.C.; Tang, Y.P.; Bernstein, N.J.; Gao, P.; Balta, S.; Vlad, M.; Volodin, A.; Sotto, A.; et al. Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment. J. Membr. Sci. 2016, 514, 217–228. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, X.; Wan, L. Surface Engineering of Polymer Membranes, 2nd ed.; Advanced Topics in Science & Technology in China; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Highly hydrophilic thin-film composite forward osmosis membranes functionalized with surface-tailored nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 5044–5053. [Google Scholar] [CrossRef]
- Liu, K.J.; Parsons, J.L. Solvent effects on the preferred conformation of poly(ethylene glycols). Macromolecules 1969, 2, 529–533. [Google Scholar] [CrossRef]
- Nyström, M.; Kaipia, L.; Luque, S. Fouling and retention of nanofiltration membranes. J. Membr. Sci. 1995, 98, 249–262. [Google Scholar] [CrossRef]
- Virtanen, T.; Parkkila, P.; Koivuniemi, A.; Lahti, J.; Viitala, T.; Kallioinen, M.; Mänttäri, M.; Bunker, A. Characterization of membrane–foulant interactions with novel combination of Raman spectroscopy, surface plasmon resonance and molecular dynamics simulation. Sep. Purif. Technol. 2018, 205, 263–272. [Google Scholar] [CrossRef]
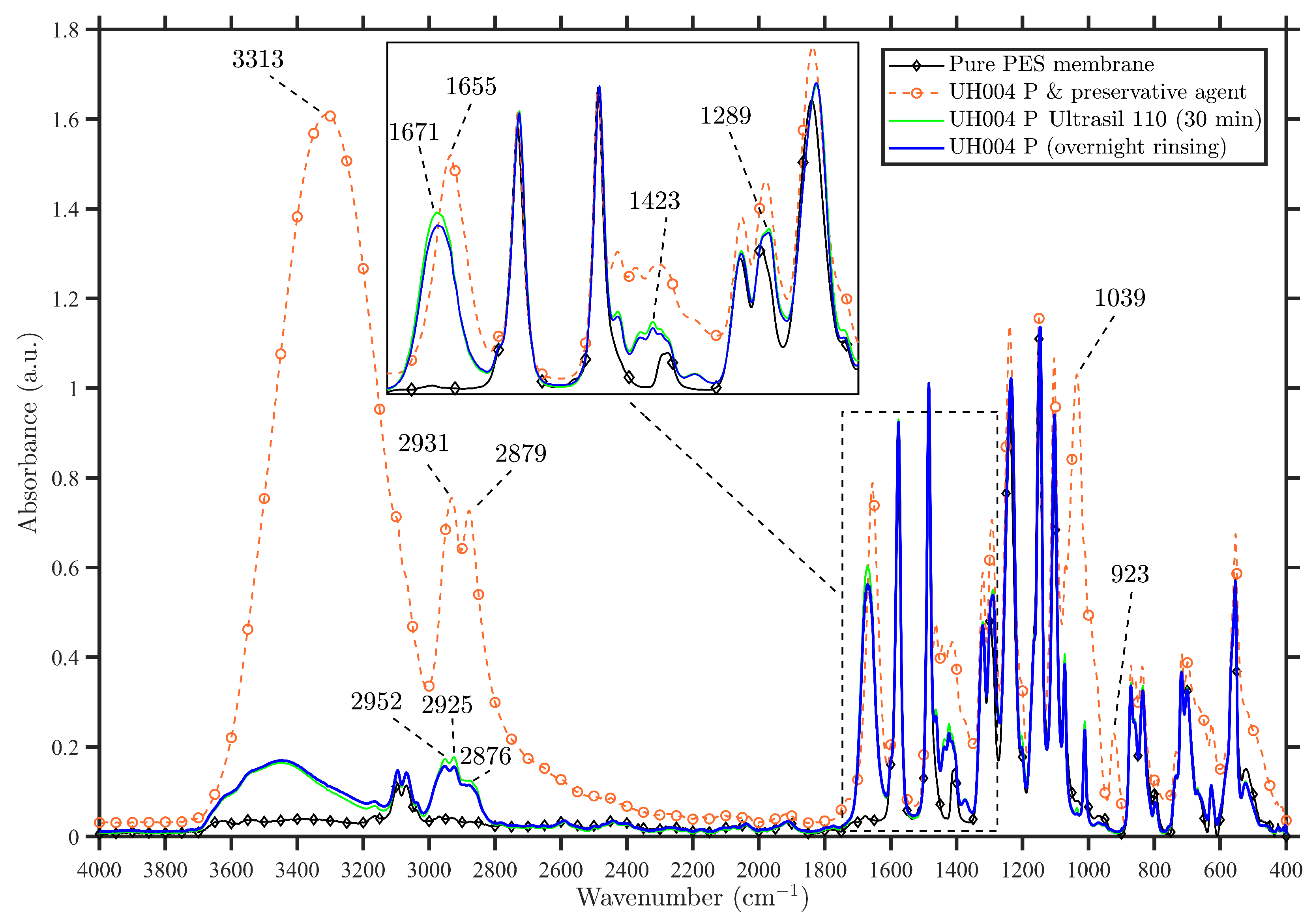
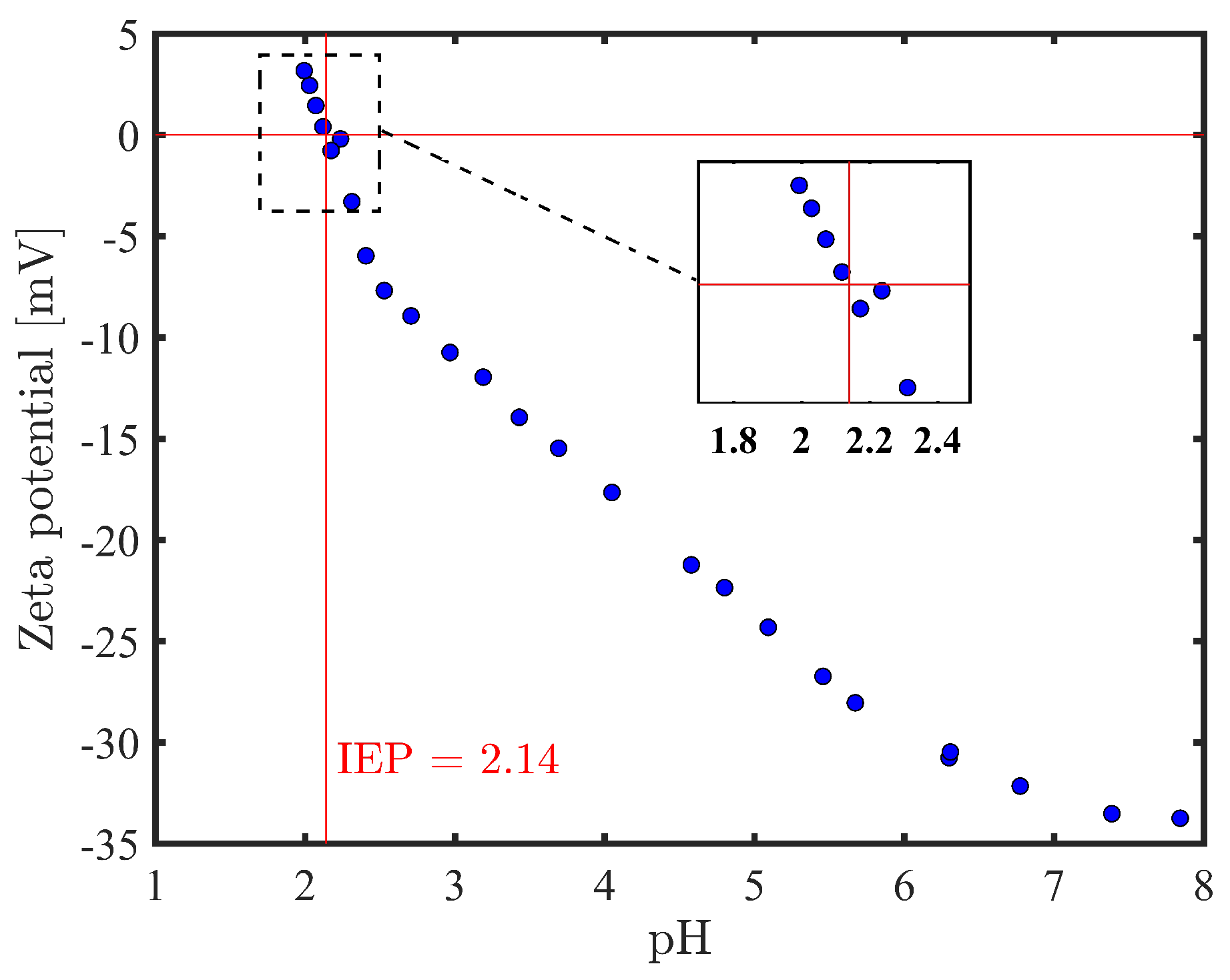
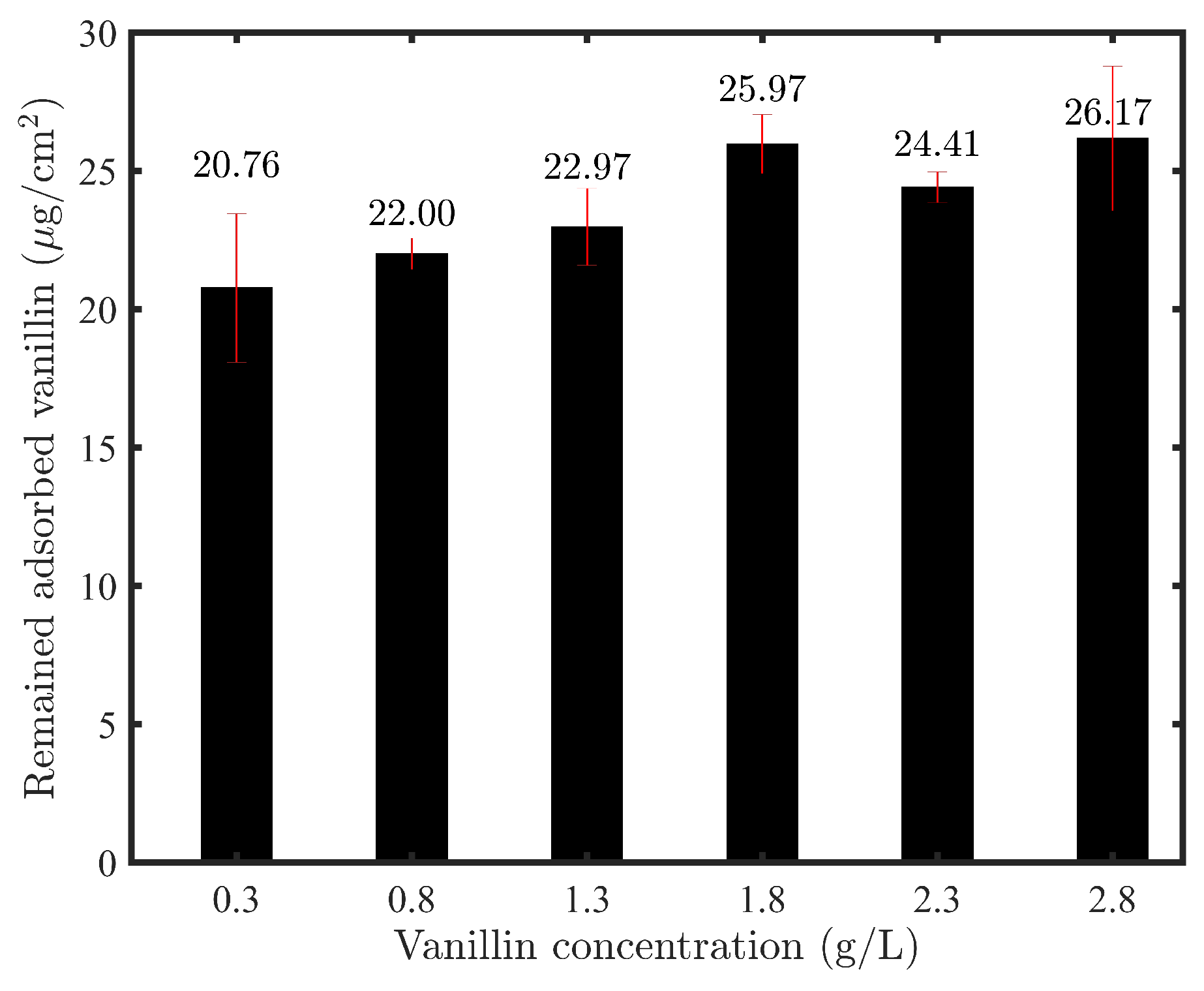
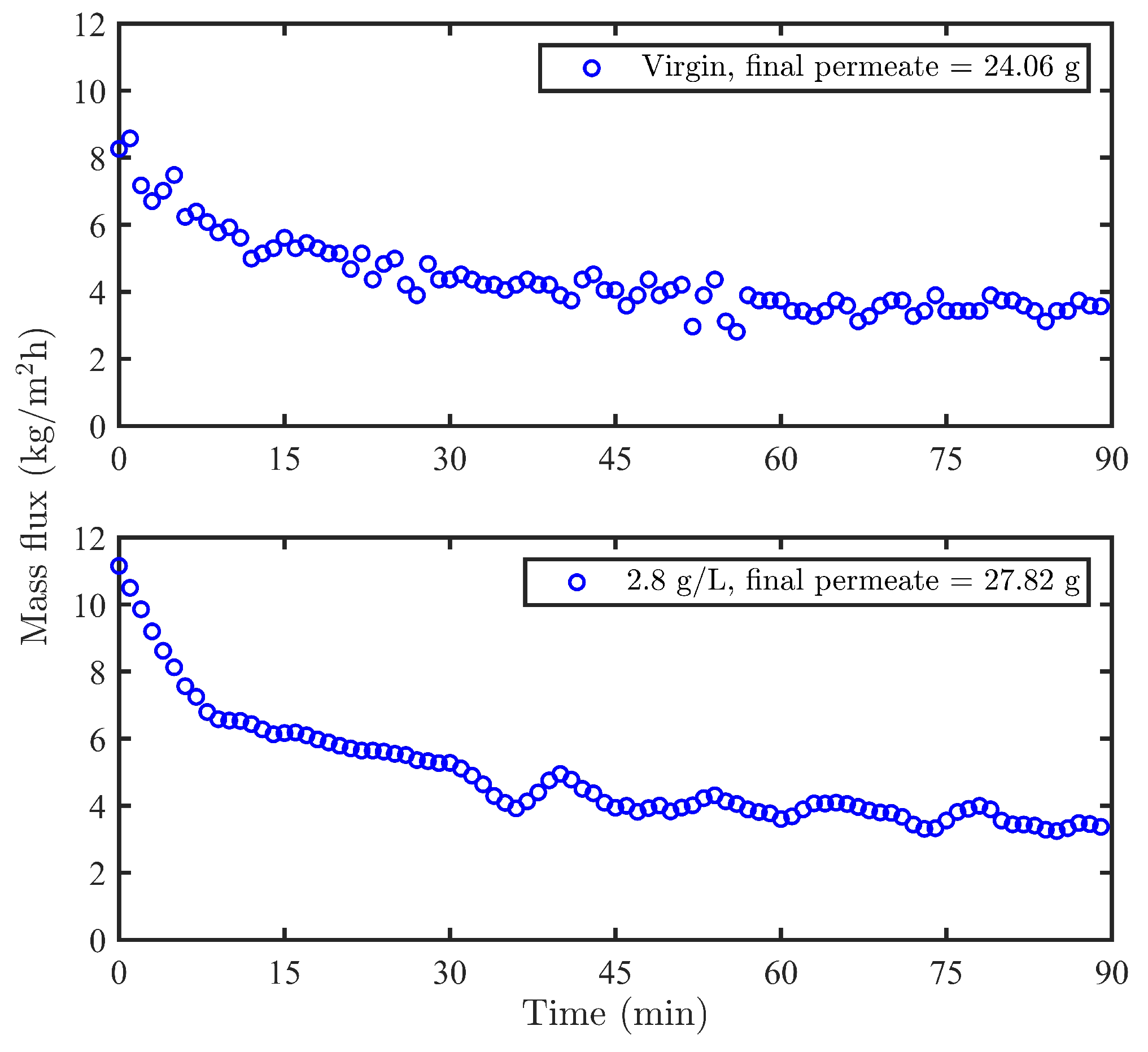
| This Study (cm) | Literature (cm) | Peak Assignments |
|---|---|---|
| 923 | 920 [26], 923 [22] | O–H bending |
| 1039 | 1037 [22] | alcoholic C–O asymmetric stretching vibration |
| 1443–1104 | 1100–450 [26] | C–O stretching from secondary and primary alcohols |
| 1655 | 1650 [26], 1647 [22] | HO bending |
| 1462–1400 | 1400–460 [26] | C–O–H bending |
| 2879 and 2931 | 2880 and 2930 [26] | C–H stretching |
| 3313 | 3313 [22] | O–H stretching |
| FTIR-Peaks (cm) | Peak Assignments |
|---|---|
| 1103 | S=O stretching vibration |
| 1146 | Symmetric SO stretches of sulfone group |
| 1235 | Aromatic ether band |
| 1289 | C–N stretch (PVP) |
| 1321 | Asymmetric SO stretches of sulfone group |
| 1423 | CH bending (PVP) |
| 1486 and 1578 | Aromatic bands (characteristics for PES) |
| 1671 | C=O carbonyl group (PVP) |
| 2850–2856 | s CH symmetric aliphatic stretch (PVP) |
| 2876 | s CH symmetric aliphatic stretch (PVP) |
| 2925 | a CH asymmetric aliphatic stretch (PVP) |
| 2952 | a CH asymmetric aliphatic stretch (PVP) |
| 3096 and 3069 (broad band) | CH–aromatic stretch |
| 3200–3600 | Hydrogen bonded OH band (PVP) |
| Vanillin Concentration (g/L) | Test 1 (%) | Test 2 (%) | Test 3 (%) | Average (%) | Standard Deviation |
|---|---|---|---|---|---|
| 0.0 | −3.70 −5.30 | −4.54 −6.72 | −11.19 – | −6.29 * | 2.96 |
| 0.3 | 0.60 | 0.57 | 0.55 | 0.57 * | 0.03 |
| 0.8 | 2.34 | 3.71 | 2.67 | 2.91 * | 0.72 |
| 1.3 | 27.39 | 21.08 | 21.40 | 23.29 * | 3.55 |
| 1.8 | 22.11 | 14.54 | – | 18.33 * | 5.35 |
| 2.3 | 20.73 | 16.27 | – | 18.50 * | 3.15 |
| 2.8 | 38.83 | 35.90 | – | 37.37 * | 2.07 |
| Samples | Contact Angle (°) |
|---|---|
| UH004 P and preservative agent | 32.08 ± 0.61 |
| UH004 P (precleaned with Ultrasil 110 cleaning agent) | 42.74 ± 1.68 |
| UH004 P reference (water at pH 3.8) * | 41.94 ± 1.86 |
| 0.3 g/L vanillin * | 40.43 ± 0.49 |
| 0.8 g/L vanillin * | 33.40 ± 0.58 |
| 1.3 g/L vanillin * | 29.49 ± 2.05 |
| 1.8 g/L vanillin * | 29.17 ± 1.90 |
| 2.3 g/L vanillin * | 29.88 ± 0.66 |
| 2.8 g/L vanillin * | 39.67 ± 0.74 |
| 4.8 g/L vanillin | 39.14 ± 1.08 |
| Vanillin Concentration (g/L) | PEG Rejection (%) before Adsorption | PEG Rejection (%) after Adsorption | ||||||
|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | Average | Test 1 | Test 2 | Test 3 | Average | |
| 0 | 87 | 85 | 90 | 87 | 86 | 86 | 90 | 87 |
| 0.3 | 92 | 91 | 91 | 91 | 94 | 92 | 92 | 93 |
| 0.8 | 93 | 91 | 88 | 91 | 95 | 93 | 90 | 93 |
| 1.3 | 89 | 87 | 91 | 89 | 90 | 89 | 90 | 90 |
| 1.8 | 85 | 83 | – | 84 | 75 | 85 | – | 80 |
| 2.3 | 82 | 90 | – | 86 | 78 | 90 | – | 84 |
| 2.8 | 88 | 72 | – | 80 | 81 | 68 | – | 75 |
| 0 | 92 | 86 | – | 89 | 86 | 89 | – | 88 |
| Samples | R (%) | R (%) | Final Permeate (g) | RSPF at Last 10 min (kg/mh) |
|---|---|---|---|---|
| Virgin | 76.12 | 42.32 | 24.06 | 3.00 |
| 2.8 g/L | 74.56 ± 1.02 | 34.11 ± 3.38 | 24.92 ± 3.3 | 3.18 ± 0.27 |
| Samples | Pure Water Flux (kg/mh) | PWF (%) | |
|---|---|---|---|
| before Filtration | after Filtration | ||
| Virgin | 62.44 ± 0.12 | 28.92 ± 3.20 | 53.68 ± 5.20 |
| 2.8 g/L | 61.90 ± 0.57 | 35.91 ± 1.33 | 41.99 ± 1.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeili, M.; Virtanen, T.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Vanillin as an Antifouling and Hydrophilicity Promoter Agent in Surface Modification of Polyethersulfone Membrane. Membranes 2019, 9, 56. https://doi.org/10.3390/membranes9040056
Esmaeili M, Virtanen T, Lahti J, Mänttäri M, Kallioinen M. Vanillin as an Antifouling and Hydrophilicity Promoter Agent in Surface Modification of Polyethersulfone Membrane. Membranes. 2019; 9(4):56. https://doi.org/10.3390/membranes9040056
Chicago/Turabian StyleEsmaeili, Mohammadamin, Tiina Virtanen, Jussi Lahti, Mika Mänttäri, and Mari Kallioinen. 2019. "Vanillin as an Antifouling and Hydrophilicity Promoter Agent in Surface Modification of Polyethersulfone Membrane" Membranes 9, no. 4: 56. https://doi.org/10.3390/membranes9040056
APA StyleEsmaeili, M., Virtanen, T., Lahti, J., Mänttäri, M., & Kallioinen, M. (2019). Vanillin as an Antifouling and Hydrophilicity Promoter Agent in Surface Modification of Polyethersulfone Membrane. Membranes, 9(4), 56. https://doi.org/10.3390/membranes9040056






