Effect of Carbonic Anhydrase on CO2 Separation Performance of Thin Poly(amidoamine) Dendrimer/Poly(ethylene glycol) Hybrid Membranes
Abstract
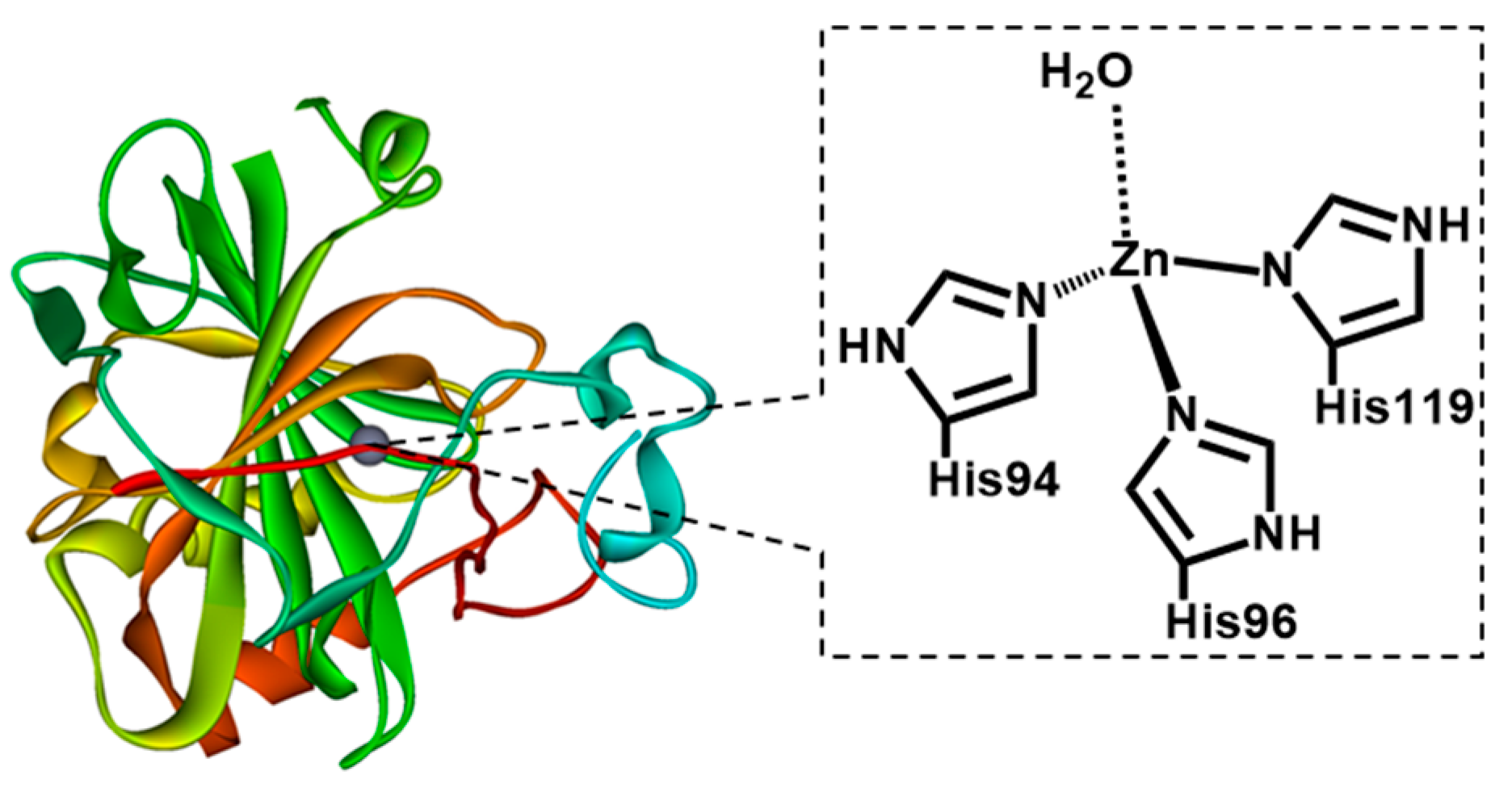
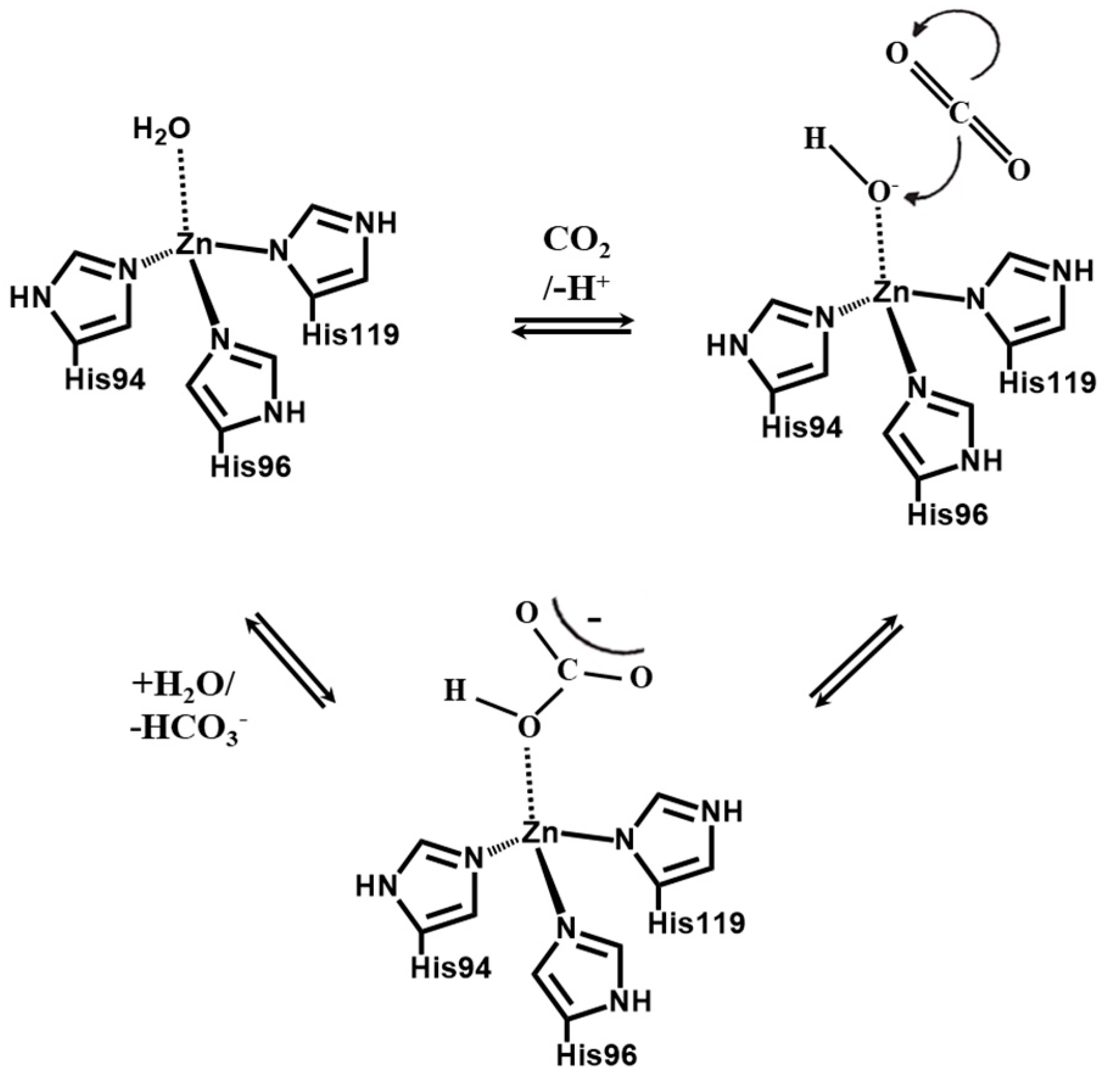
1. Introduction
2. Materials and Methods
2.1. Materials
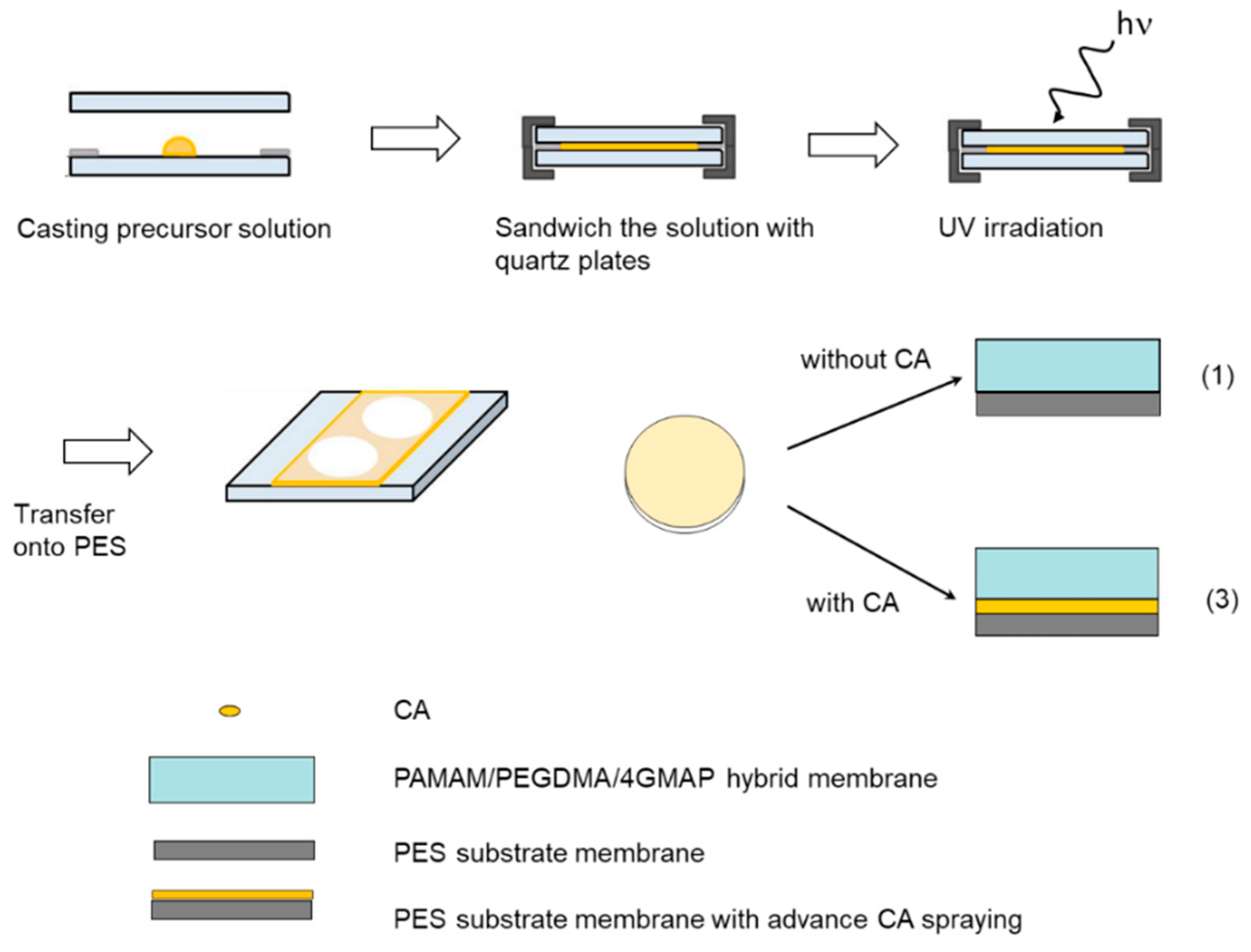
2.2. Membrane Preparation
2.3. Gas Separation Experimental
3. Results and Discussion
3.1. Effect of Membrane Thickness on CO2 Permeance and CO2/H2 Selectivity
3.2. Effect of CA Addition on the CO2 Separation Properties
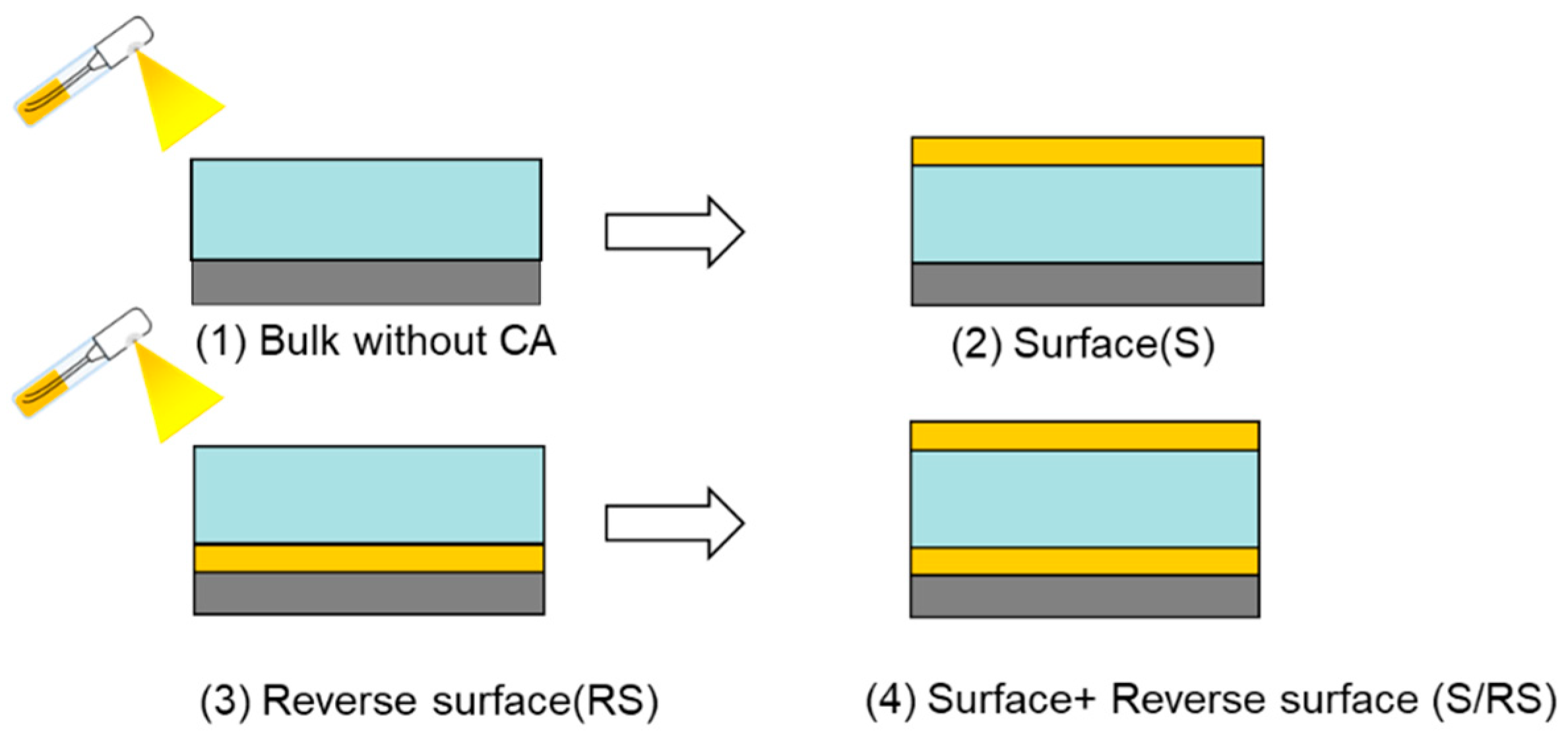
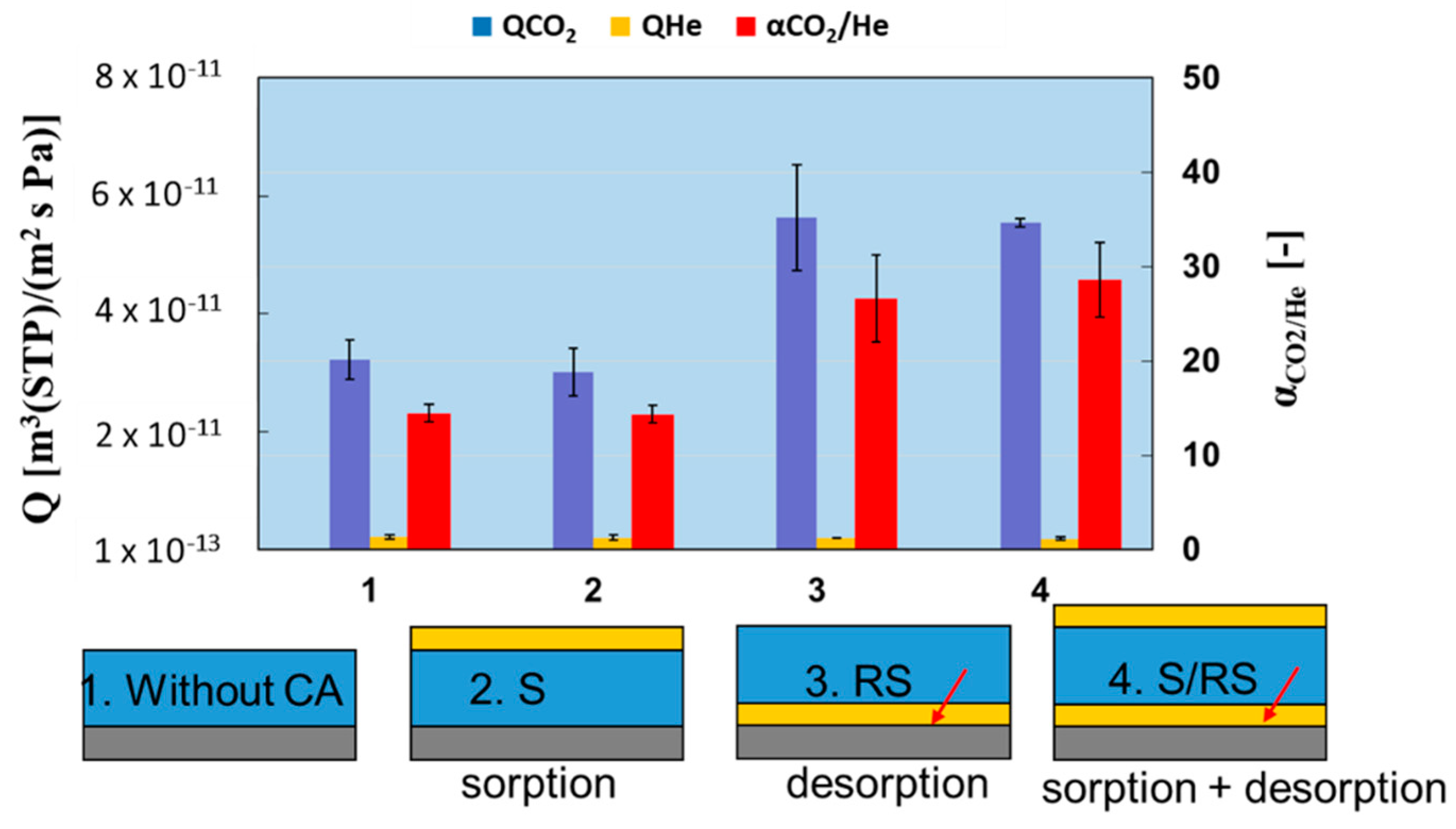
3.3. Effect of Position of CA on CO2 Separation Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Liang, X. Strategy for promoting low-carbon technology transfer to developing countries: The case of CCS. Energy Policy 2011, 39, 3106–3116. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Azzi, M. A critical review of existing strategies for emission control in the monoethanolamine-based carbon capture process and some recommendations for improved strategies. Fuel 2014, 121, 178–188. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.S.; Litwiller, E.; Pinnau, I. Pure- and mixed- gas CO2/CH4 separation properties of PIM-1 and an amidoxime-functionalized PIM1. J. Membr. Sci. 2014, 457, 95–102. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer based membrane materials for CO2 separations. Energy Env. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Caro, J. Organosilica functionalized zeolitic imidazolate framework ZIF-90 membrane for CO2/CH4 separation. Microporous Mesoporous 2014, 192, 18–22. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Kasahara, S.; Kamio, E.; Ishigami, T.; Matsuyama, H. Effect of water in ionic liquids on CO2 permeability in amino acid ionic liquid-based facilitated transport membranes. J. Membr. Sci. 2012, 415–416, 168–175. [Google Scholar] [CrossRef]
- Deng, L.; Kim, T.; Hägg, M. Facilitated transport of CO2 in novel PVAm/PVA blend membrane. J. Membr. Sci. 2009, 340, 154–163. [Google Scholar] [CrossRef]
- Kim, T.; Vralstad, H.; Sandru, M.; Hägg, M. Separation performance of PVAm composite membrane for CO2 capture at various pH levels. J. Membr. Sci. 2013, 428, 218–224. [Google Scholar] [CrossRef]
- Taniguchi, I.; Duan, S.; Kazama, S.; Fujioka, Y. Facile fabrication of a novel high performance CO2 separation membrane: Immobilization of poly(amidoamine) dendrimers in poly(ethyleneglycol) networks. J. Membr. Sci. 2008, 322, 277–280. [Google Scholar] [CrossRef]
- Taniguchi, I.; Urai, H.; Kai, T.; Duan, S.; Kazama, S. A CO2-selective molecular gate of poly(amidoamine) dendrimer immobilized in a poly(ethylene glycol) network. J. Membr. Sci. 2013, 444, 96–100. [Google Scholar] [CrossRef]
- Taniguchi, I.; Kai, T.; Duan, S.; Kazama, S.; Jinnai, H. A compatible crosslinker for enhancement of CO2 capture of poly(amidoamine) dendrimer-containing polymeric membranes. J. Membr. Sci. 2015, 475, 175–183. [Google Scholar] [CrossRef]
- Kovvali, A.S.; Chen, H.; Sirkar, K.K. Dendrimer membranes: A CO2-selective molecular gate. J. Am. Chem. Soc. 2000, 122, 7594–7595. [Google Scholar] [CrossRef]
- Watari, T.; Huang, Q.; Teramoto, M. Effects of Support Membrane Properties on Facilitated Transport of CO2 through Supported Liquid Membrane of Aqueous Amine Solution. J. Chem. Eng. Jpn. 1998, 24, 155–157. [Google Scholar]
- Bao, L.; Trachtenberg, M.C. Facilitated transport of CO2 across a liquid membrane: Comparing enzyme, amine, and alkaline. J. Membr. Sci. 2006, 280, 330–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Chen, H.; Zhang, H. Selective separation of low concentration CO2 using hydrogel immobilized CA enzyme based hollow fiber membrane reactors. Chem. Eng. Sci. 2010, 65, 3199–3207. [Google Scholar] [CrossRef]
- Duan, S.; Kai, T.; Saito, T.; Yamazaki, K.; Ikeda, K. Effect of cross-linking on mechanical and thermal properties of poly(amidoamine) dendrimer/poly(vinyl alcohol) hybrid membranes for CO2 separation. Membranes 2014, 4, 200–209. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Kai, T.; Nakao, S.-i. Effect of Carbonic Anhydrase on CO2 Separation Performance of Thin Poly(amidoamine) Dendrimer/Poly(ethylene glycol) Hybrid Membranes. Membranes 2019, 9, 167. https://doi.org/10.3390/membranes9120167
Duan S, Kai T, Nakao S-i. Effect of Carbonic Anhydrase on CO2 Separation Performance of Thin Poly(amidoamine) Dendrimer/Poly(ethylene glycol) Hybrid Membranes. Membranes. 2019; 9(12):167. https://doi.org/10.3390/membranes9120167
Chicago/Turabian StyleDuan, Shuhong, Teruhiko Kai, and Shin-ichi Nakao. 2019. "Effect of Carbonic Anhydrase on CO2 Separation Performance of Thin Poly(amidoamine) Dendrimer/Poly(ethylene glycol) Hybrid Membranes" Membranes 9, no. 12: 167. https://doi.org/10.3390/membranes9120167
APA StyleDuan, S., Kai, T., & Nakao, S.-i. (2019). Effect of Carbonic Anhydrase on CO2 Separation Performance of Thin Poly(amidoamine) Dendrimer/Poly(ethylene glycol) Hybrid Membranes. Membranes, 9(12), 167. https://doi.org/10.3390/membranes9120167



