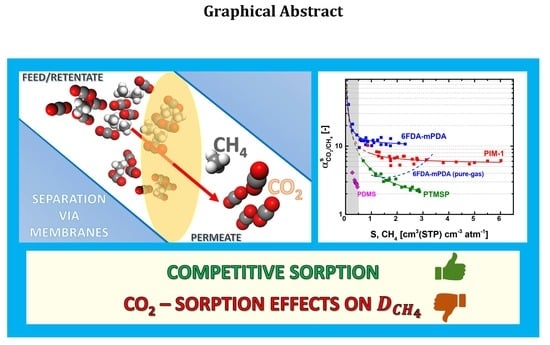
Experimental Mixed-Gas Permeability, Sorption and Diffusion of CO2-CH4 Mixtures in 6FDA-mPDA Polyimide Membrane: Unveiling the Effect of Competitive Sorption on Permeability Selectivity
Abstract
1. Introduction
2. Materials and Methods
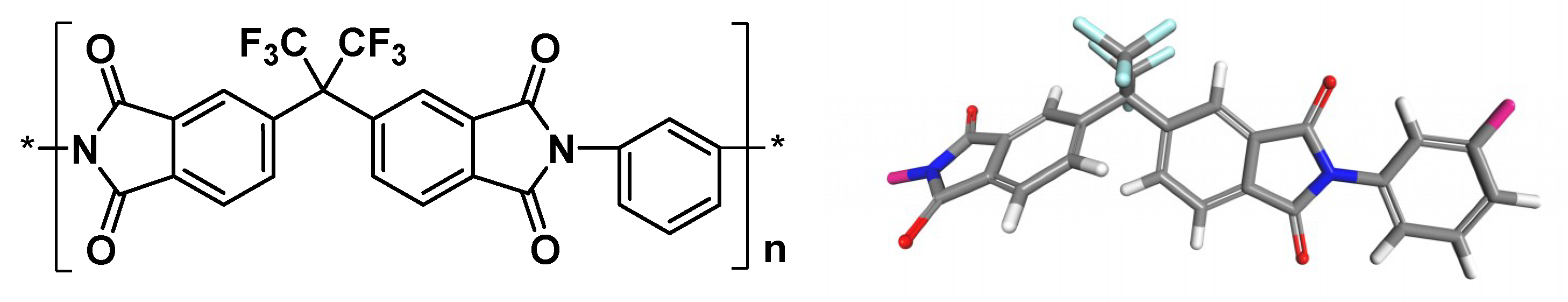
2.1. Materials
2.2. Methods
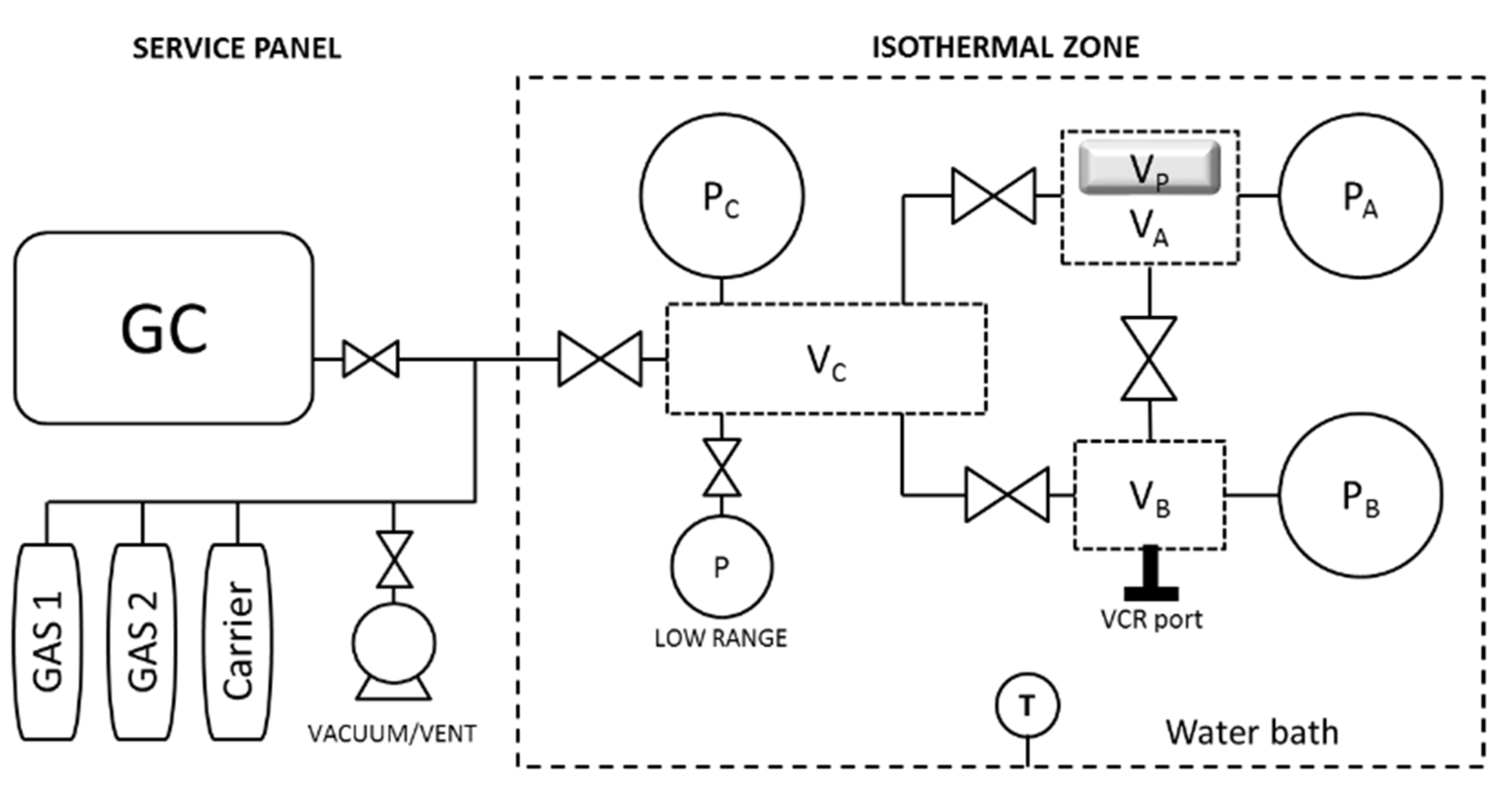
2.2.1. Barometric System
2.2.2. Barometric Pure-Gas Sorption
2.2.3. Gravimetric Gas Sorption
2.2.4. Barometric Mixed-Gas Sorption and Data Analysis
2.2.5. Pure- and Mixed-Gas Permeation and Diffusion Coefficients
3. Results and Discussion
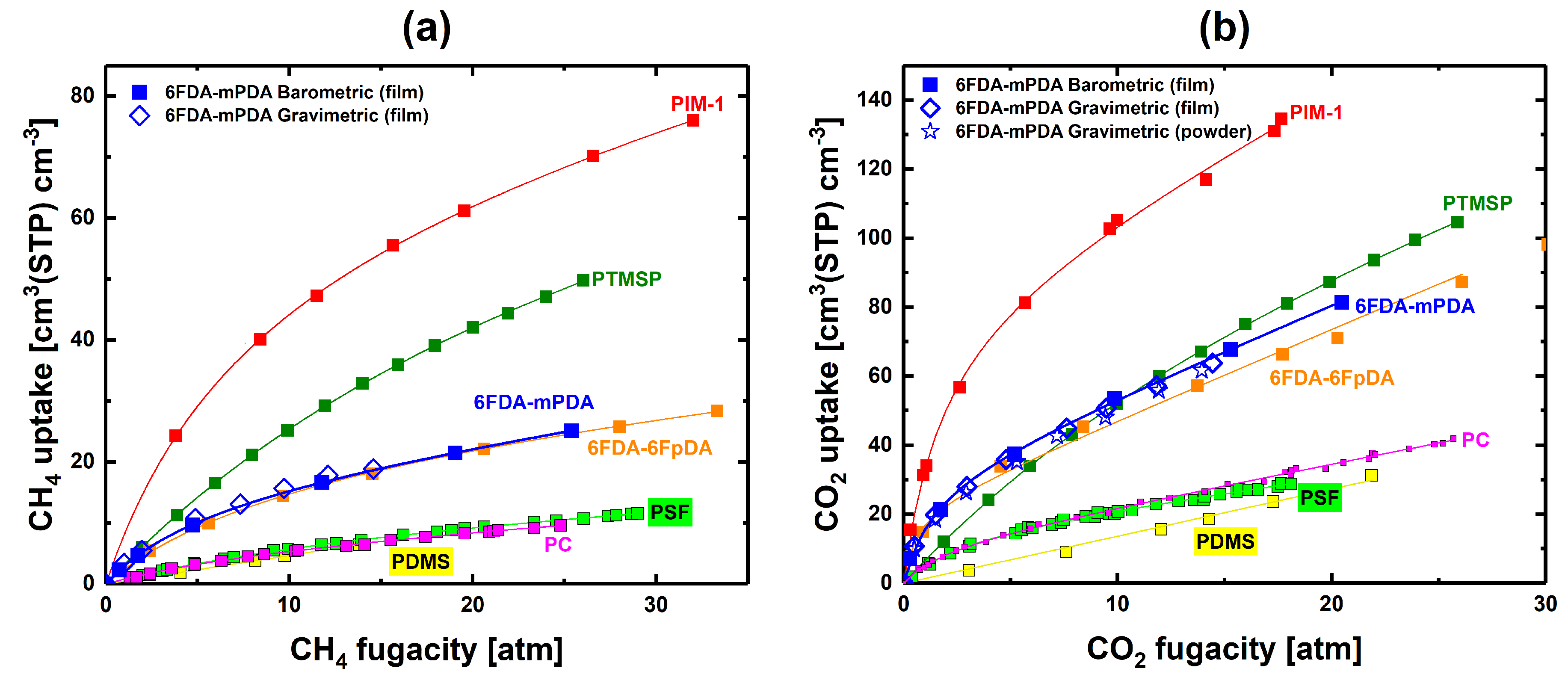
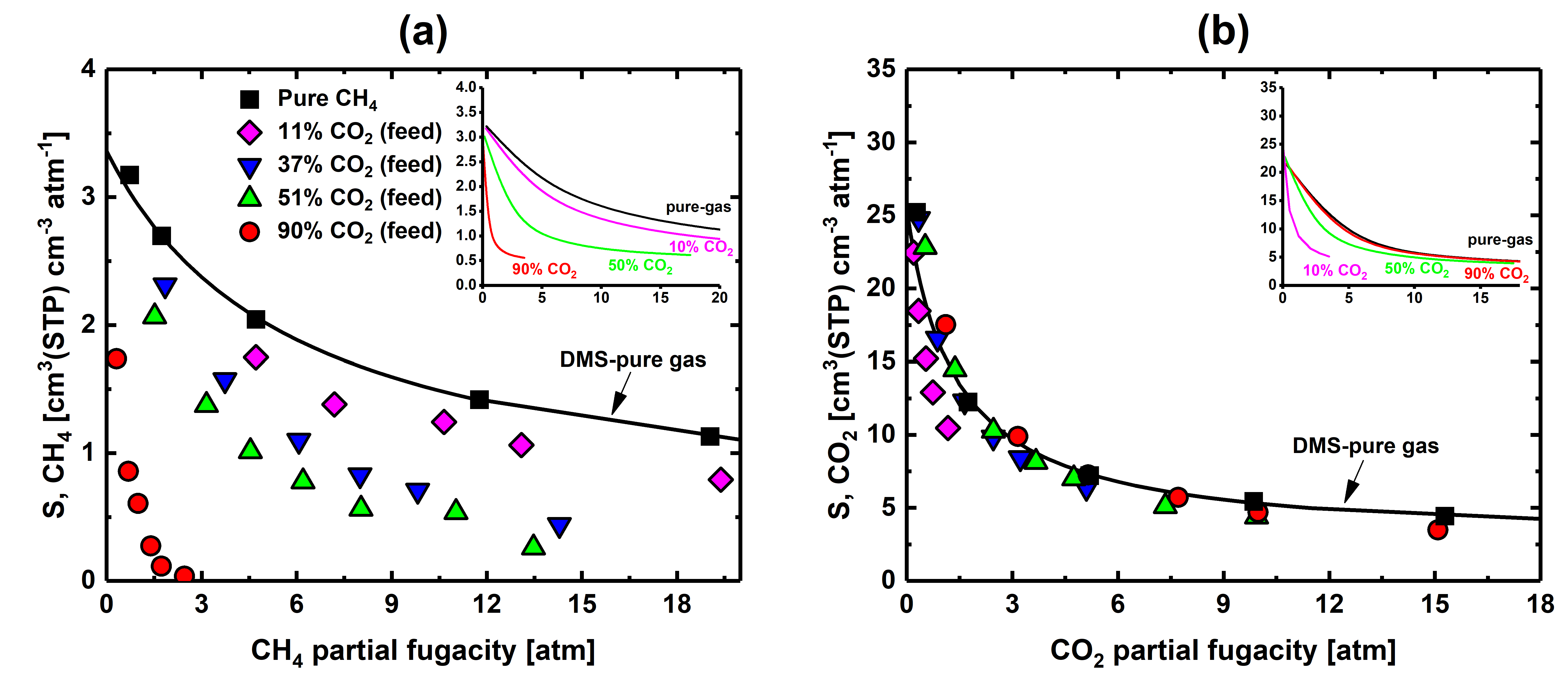
3.1. Experimental Pure- and Mixed-Gas Sorption Data
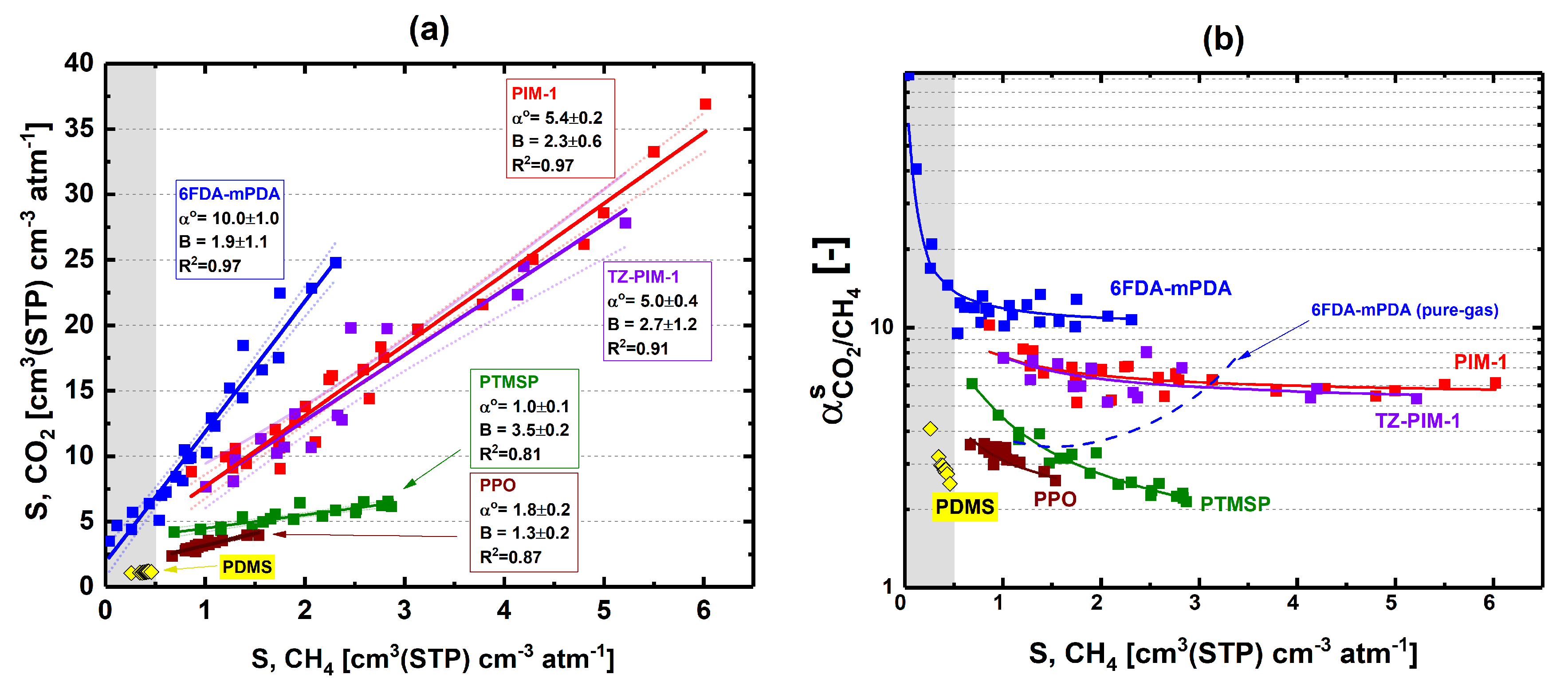
3.2. Solubility Selectivity Analysis
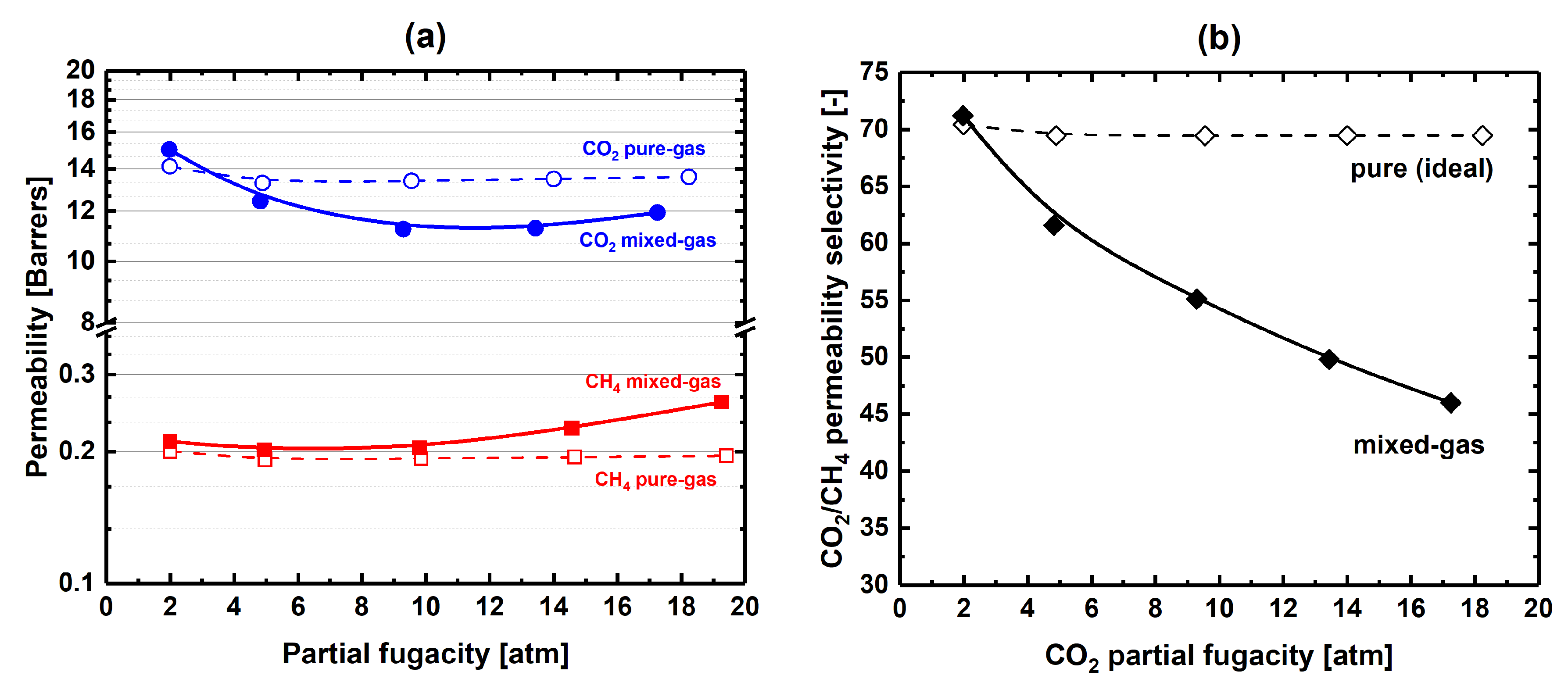
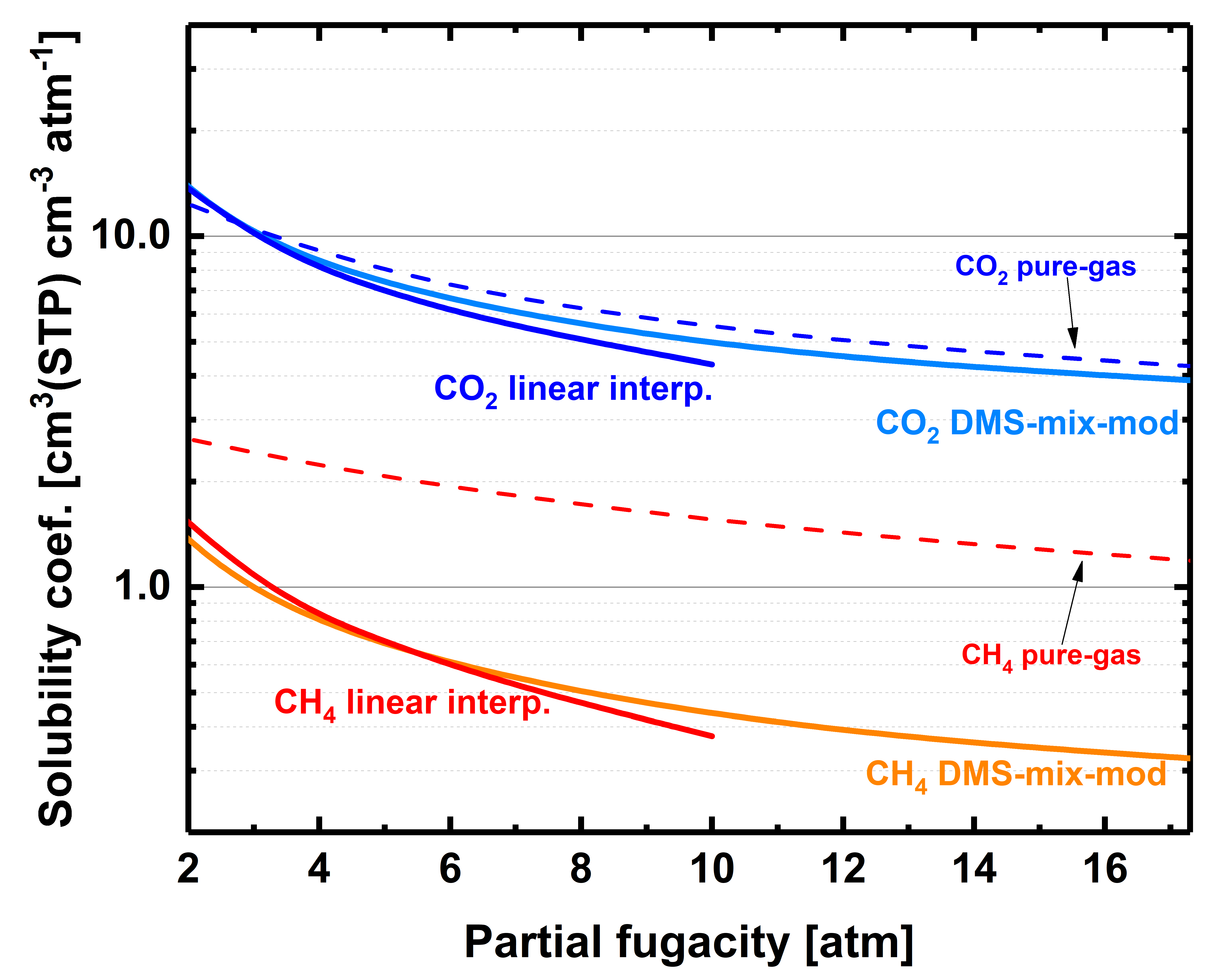
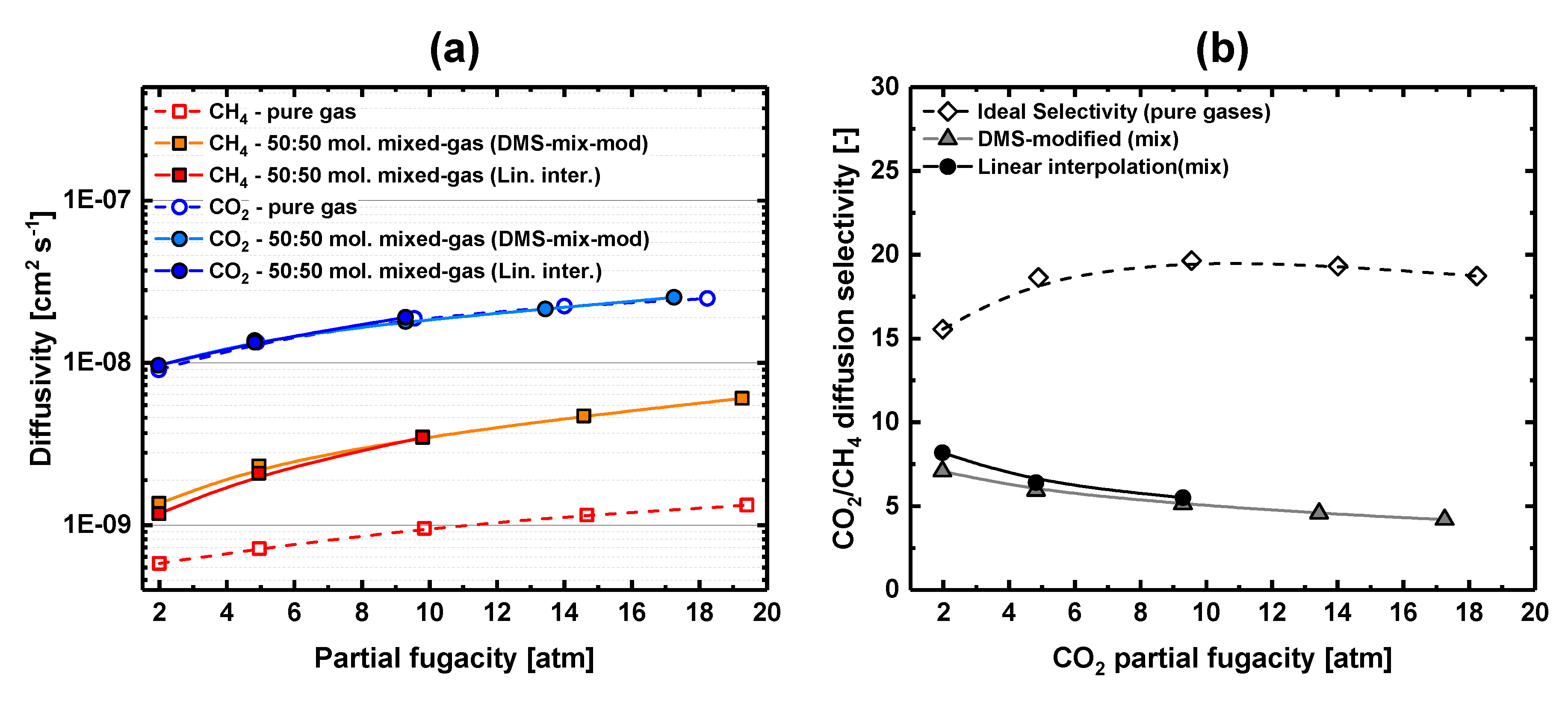
3.3. Equimolar CO2-CH4 Mixed-Gas Diffusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High pressure pure- and mixed-gas separation of CO2/CH4 by thermally-rearranged and carbon molecular sieve membranes derived from a polyimide of intrinsic microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.S.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas CO2/CH4 separation properties of PIM-1 and an amidoxime-functionalized PIM-1. J. Membr. Sci. 2014, 457, 95–102. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.R.; Kelley, S.S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- O’Brien, K.C.; Koros, W.J.; Barbari, T.A.; Sanders, E.S. A new technique for the measurement of multicomponent gas transport through polymeric films. J. Membr. Sci. 1986, 29, 229–238. [Google Scholar] [CrossRef]
- Fraga, S.C.; Monteleone, M.; Lanč, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnáček, K.; Friess, K.; Carta, M.; McKeown, N.B.; et al. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Monteleone, M.; Esposito, E.; Fuoco, A.; Lanč, M.; Pilnáček, K.; Friess, K.; Bezzu, C.G.; Carta, M.; McKeown, N.B.; Jansen, J.C. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Pressure dependence of transport parameters. Membranes 2018, 8, 73. [Google Scholar] [CrossRef]
- Genduso, G.; Litwiller, E.; Ma, X.; Zampini, S.; Pinnau, I. Mixed-gas sorption in polymers via a new barometric test system: Sorption and diffusion of CO2-CH4 mixtures in polydimethylsiloxane (PDMS). Manuscript submitted for publication.
- Koros, W.J.; Paul, D.R. Design considerations for measurement of gas sorption in polymers by pressure decay. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 1903–1907. [Google Scholar] [CrossRef]
- Ribeiro, C.P.; Freeman, B.D. Carbon dioxide/ethane mixed-gas sorption and dilation in a cross-linked poly(ethylene oxide) copolymer. Polymer 2010, 51, 1156–1168. [Google Scholar] [CrossRef]
- Raharjo, R.D.; Freeman, B.D.; Sanders, E.S. Pure and mixed gas CH4 and n-C4H10 sorption and dilation in poly(1-trimethylsilyl-1-propyne). Polymer 2007, 48, 6097–6114. [Google Scholar] [CrossRef]
- Vopička, O.; De Angelis, M.G.; Sarti, G.C. Mixed gas sorption in glassy polymeric membranes: I. CO2/CH4 and n-C4/CH4 mixtures sorption in poly(1-trimethylsilyl-1-propyne) (PTMSP). J. Membr. Sci. 2014, 449, 97–108. [Google Scholar] [CrossRef]
- Sanders, E.S.; Koros, W.J.; Hopfenberg, H.B.; Stannett, V.T. Mixed gas sorption in glassy polymers: Equipment design considerations and preliminary results. J. Membr. Sci. 1983, 13, 161–174. [Google Scholar] [CrossRef]
- Sanders, E.S.; Koros, W.J.; Hopfenberg, H.B.; Stannett, V.T. Pure and mixed gas sorption of carbon dioxide and ethylene in poly(methyl methacrylate). J. Membr. Sci. 1984, 18, 53–74. [Google Scholar] [CrossRef]
- Story, B.J.; Koros, W.J. Sorption of CO2/CH4 mixtures in poly(phenylene oxide) and a carboxylated derivative. J. Appl. Polym. Sci. 1991, 42, 2613–2626. [Google Scholar] [CrossRef]
- Raharjo, R.D.; Freeman, B.D.; Sanders, E.S. Pure and mixed gas CH4 and n-C4H10 sorption and dilation in poly(dimethylsiloxane). J. Membr. Sci. 2007, 292, 45–61. [Google Scholar] [CrossRef]
- Vopička, O.; De Angelis, M.G.; Du, N.; Li, N.; Guiver, M.D.; Sarti, G.C. Mixed gas sorption in glassy polymeric membranes: II. CO2/CH4 mixtures in a polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2014, 459, 264–276. [Google Scholar] [CrossRef]
- Gemeda, A.E.; De Angelis, M.G.; Du, N.; Li, N.; Guiver, M.D.; Sarti, G.C. Mixed gas sorption in glassy polymeric membranes. III. CO2/CH4 mixtures in a polymer of intrinsic microporosity (PIM-1): Effect of temperature. J. Membr. Sci. 2017, 524, 746–757. [Google Scholar] [CrossRef]
- Kamaruddin, H.D.; Koros, W.J. Some observations about the application of Fick's first law for membrane separation of multicomponent mixtures. J. Membr. Sci. 1997, 135, 147–159. [Google Scholar] [CrossRef]
- Gemeda, A.E. Solubility, Diffusivity and Permeability of Gases in Glassy Polymers. Ph.D. Thesis, Università di Bologna, Bologna, Italy, 2015. [Google Scholar]
- Ribeiro, C.P.; Freeman, B.D.; Paul, D.R. Pure- and mixed-gas carbon dioxide/ethane permeability and diffusivity in a cross-linked poly(ethylene oxide) copolymer. J. Membr. Sci. 2011, 377, 110–123. [Google Scholar] [CrossRef]
- Raharjo, R.D.; Freeman, B.D.; Paul, D.R.; Sanders, E.S. Pure and mixed gas CH4 and n-C4H10 permeability and diffusivity in poly(1-trimethylsilyl-1-propyne). Polymer 2007, 48, 7329–7344. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Nijmeijer, K.; Ribeiro, C.P.; Freeman, B.D.; Wessling, M. On the effects of plasticization in CO2/light gas separation using polymeric solubility selective membranes. J. Membr. Sci. 2011, 367, 33–44. [Google Scholar] [CrossRef]
- Raharjo, R.D.; Freeman, B.D.; Paul, D.R.; Sarti, G.C.; Sanders, E.S. Pure and mixed gas CH4 and n-C4H10 permeability and diffusivity in poly(dimethylsiloxane). J. Membr. Sci. 2007, 306, 75–92. [Google Scholar] [CrossRef]
- Tanis, I.; Brown, D.; Neyertz, S.J.; Heck, R.; Mercier, R. A comparison of homopolymer and block copolymer structure in 6FDA-based polyimides. Phys. Chem. Chem. Phys. 2014, 16, 23044–23055. [Google Scholar] [CrossRef]
- Alaslai, N.; Ghanem, B.; Alghunaimi, F.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas permeation properties of highly selective and plasticization resistant hydroxyl-diamine-based 6FDA polyimides for CO2/CH4 separation. J. Membr. Sci. 2016, 505, 100–107. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, K.; Li, F.S.; Zhang, K.; Koros, W.J. Gas separation performance of carbon molecular sieve membranes based on 6FDA-mPDA/DABA (3:2) polyimide. ChemSusChem 2014, 7, 1186–1194. [Google Scholar] [CrossRef]
- Gleason, K.L.; Smith, Z.P.; Liu, Q.; Paul, D.R.; Freeman, B.D. Pure- and mixed-gas permeation of CO2 and CH4 in thermally rearranged polymers based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2015, 475, 204–214. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Physical aging, plasticization and their effects on gas permeation in “rigid” polymers of intrinsic microporosity. Macromolecules 2015, 48, 6553–6561. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Al-Saeedi, M.; Litwiller, E.; Pinnau, I. Role of intrachain rigidity in the plasticization of intrinsically microporous triptycene-based polyimide membranes in mixed-gas CO2/CH4 separations. Macromolecules 2014, 47, 7453–7462. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.J.; Koros, W. Improvement of co2/ch4 separation characteristics of polyimides by chemical crosslinking. J. Membr. Sci. 1999, 155, 145–154. [Google Scholar] [CrossRef]
- Koros, W.J. Model for sorption of mixed gases in glassy polymers. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 981–992. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Hernández, A.; de la Campa, J.G.; de Abajo, J.; Lozano, A.E.; Lee, Y.M. Thermally rearranged polybenzoxazoles and poly(benzoxazole-co-imide)s from ortho-hydroxyamine monomers for high performance gas separation membranes. J. Membr. Sci. 2015, 493, 329–339. [Google Scholar] [CrossRef]
- Cheng, S.-X.; Chung, T.-S.; Wang, R.; Vora, R.H. Gas-sorption properties of 6FDA–durene/1,4-phenylenediamine (pPDA) and 6FDA–durene/1,3-phenylenediamine (mPDA) copolyimides. J. Appl. Polym. Sci. 2003, 90, 2187–2193. [Google Scholar] [CrossRef]
- Heck, R.; Qahtani, M.S.; Yahaya, G.O.; Tanis, I.; Brown, D.; Bahamdan, A.A.; Ameen, A.W.; Vaidya, M.M.; Ballaguet, J.P.R.; Alhajry, R.H.; et al. Block copolyimide membranes for pure- and mixed-gas separation. Sep. Purif. Technol. 2017, 173, 183–192. [Google Scholar] [CrossRef]
- Joly, C.; Le Cerf, D.; Chappey, C.; Langevin, D.; Muller, G. Residual solvent effect on the permeation properties of fluorinated polyimide films. Sep. Purif. Technol. 1999, 16, 47–54. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef]
- Soave, G. 20 years of Redlich-Kwong equation of state. Fluid Phase Equilib. 1993, 82, 345–359. [Google Scholar] [CrossRef]
- Koros, W.J.; Chan, A.H.; Paul, D.R. Sorption and transport of various gases in polycarbonate. J. Membr. Sci. 1977, 2, 165–190. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Shahidi, K.; Mohammadi, T. Effect of operating parameters on pure and mixed gas permeation properties of a synthesized composite PDMS/PA membrane. J. Membr. Sci. 2009, 342, 327–340. [Google Scholar] [CrossRef]
- Coleman, M.R. Isomers of Fluorine-Containing Polyimides for Gas Separation Membranes. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 1992. [Google Scholar]
- Erb, A.J.; Paul, D.R. Gas sorption and transport in polysulfone. J. Membr. Sci. 1981, 8, 11–22. [Google Scholar] [CrossRef]
- Koros, W.J.; Paul, D.R.; Rocha, A.A. Carbon dioxide sorption and transport in polycarbonate. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 687–702. [Google Scholar] [CrossRef]
- Lou, Y.; Hao, P.; Lipscomb, G. NELF predictions of a solubility–solubility selectivity upper bound. J. Membr. Sci. 2014, 455, 247–253. [Google Scholar] [CrossRef]
- Walker, D.R.B. Synthesis and Characterization of Polypyrrolones for Gas Separation Membranes. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 1993. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genduso, G.; Ghanem, B.S.; Pinnau, I. Experimental Mixed-Gas Permeability, Sorption and Diffusion of CO2-CH4 Mixtures in 6FDA-mPDA Polyimide Membrane: Unveiling the Effect of Competitive Sorption on Permeability Selectivity. Membranes 2019, 9, 10. https://doi.org/10.3390/membranes9010010
Genduso G, Ghanem BS, Pinnau I. Experimental Mixed-Gas Permeability, Sorption and Diffusion of CO2-CH4 Mixtures in 6FDA-mPDA Polyimide Membrane: Unveiling the Effect of Competitive Sorption on Permeability Selectivity. Membranes. 2019; 9(1):10. https://doi.org/10.3390/membranes9010010
Chicago/Turabian StyleGenduso, Giuseppe, Bader S. Ghanem, and Ingo Pinnau. 2019. "Experimental Mixed-Gas Permeability, Sorption and Diffusion of CO2-CH4 Mixtures in 6FDA-mPDA Polyimide Membrane: Unveiling the Effect of Competitive Sorption on Permeability Selectivity" Membranes 9, no. 1: 10. https://doi.org/10.3390/membranes9010010
APA StyleGenduso, G., Ghanem, B. S., & Pinnau, I. (2019). Experimental Mixed-Gas Permeability, Sorption and Diffusion of CO2-CH4 Mixtures in 6FDA-mPDA Polyimide Membrane: Unveiling the Effect of Competitive Sorption on Permeability Selectivity. Membranes, 9(1), 10. https://doi.org/10.3390/membranes9010010