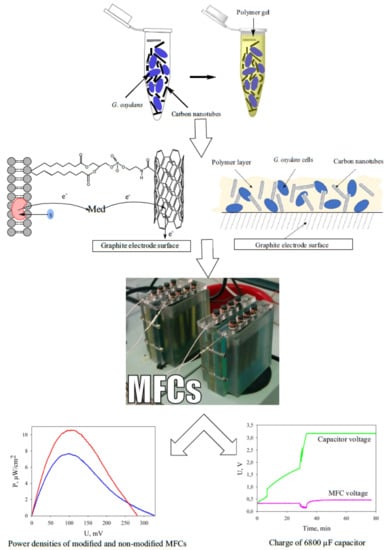
Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Formation of the Working Electrode
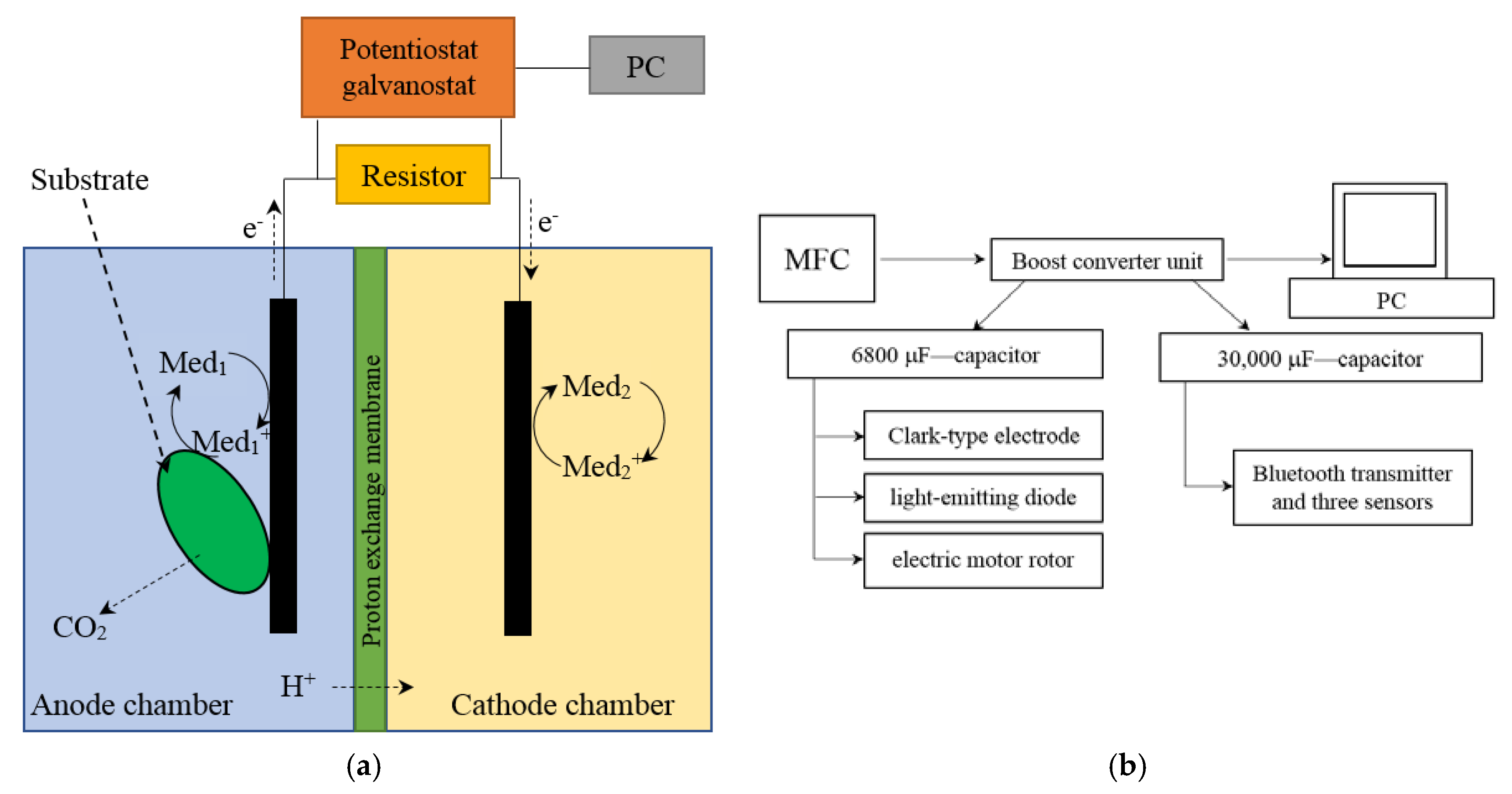
2.3. MFC Setup and Operation
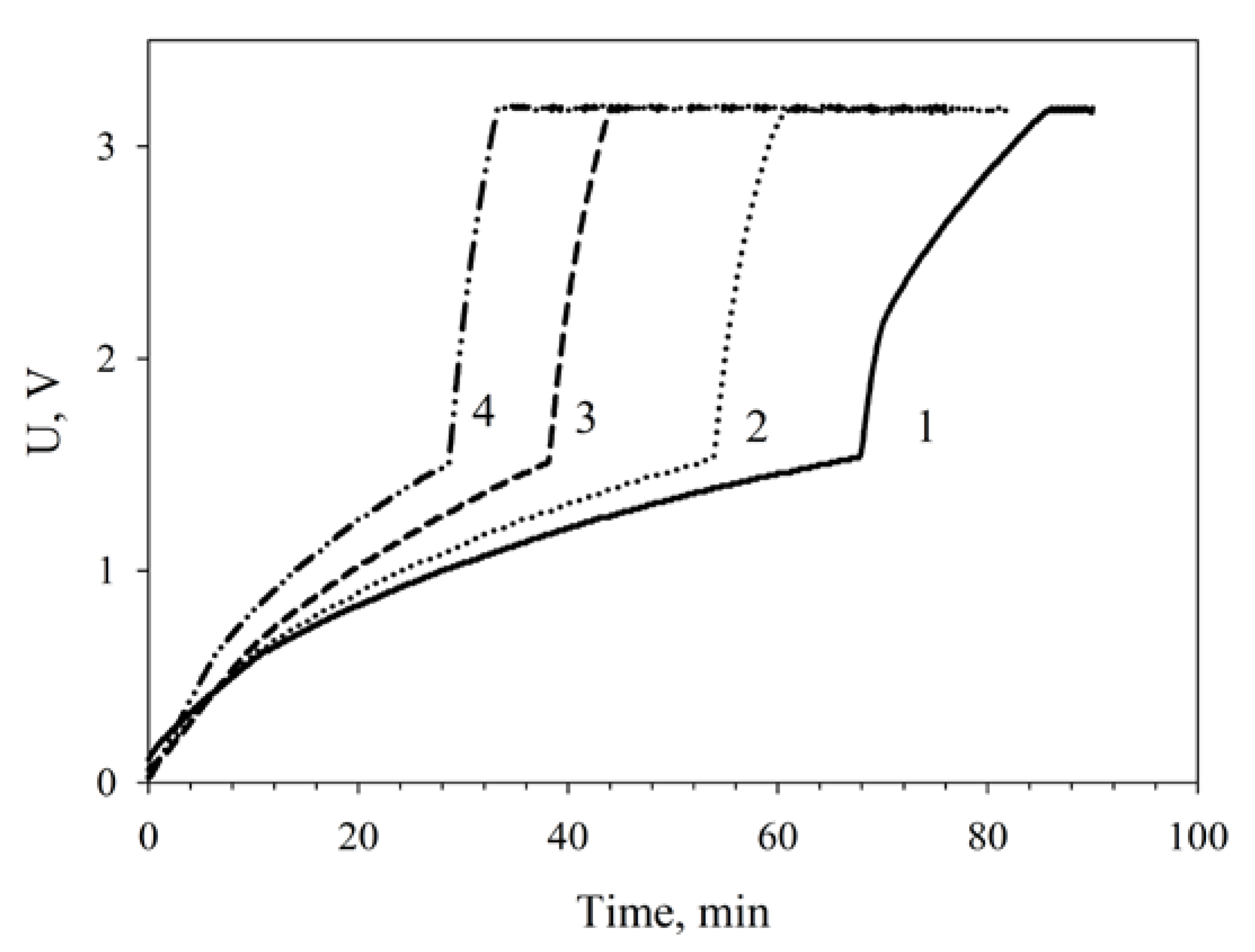
3. Results and Discussion
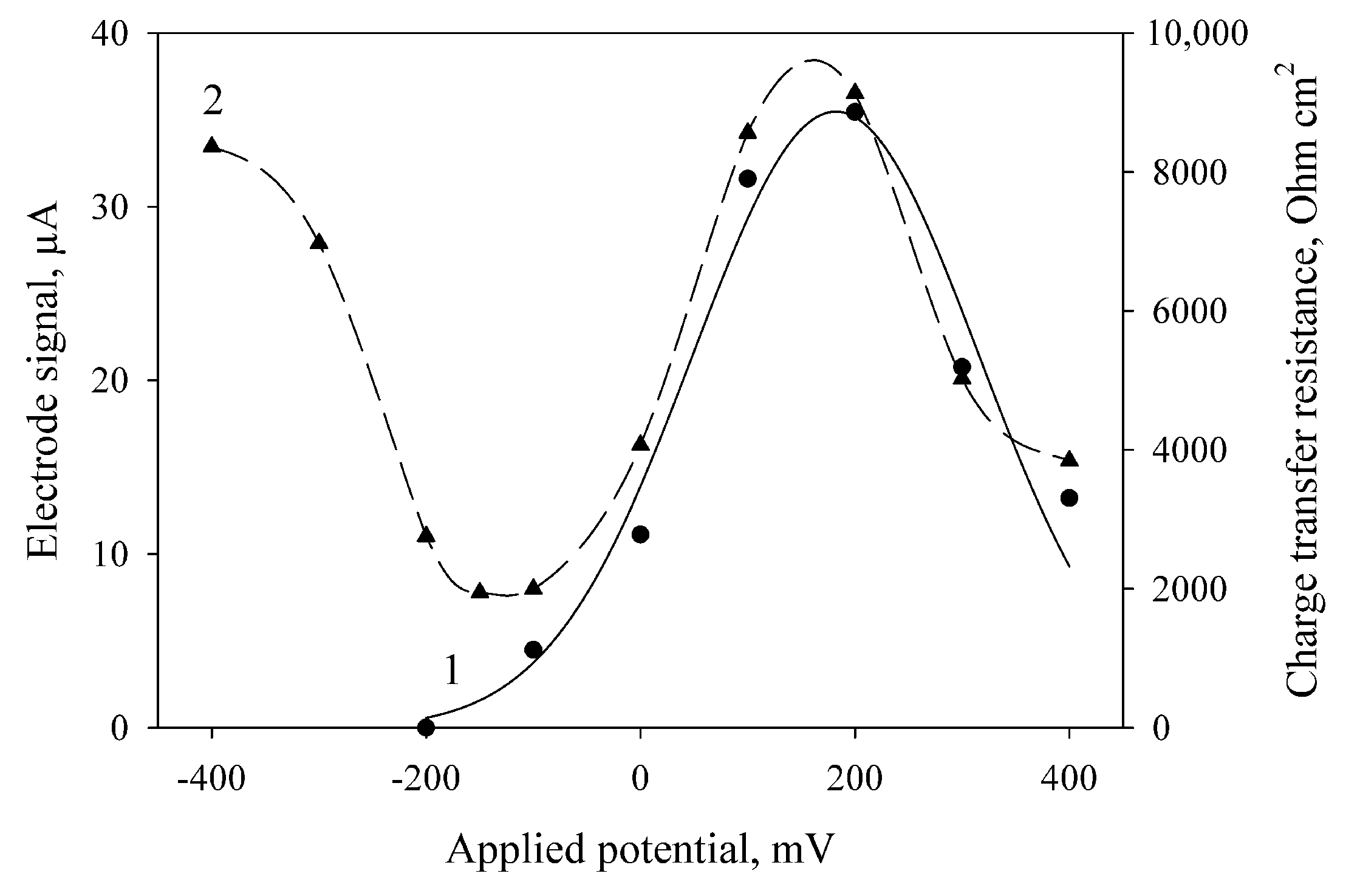
3.1. Applied Potential Selection
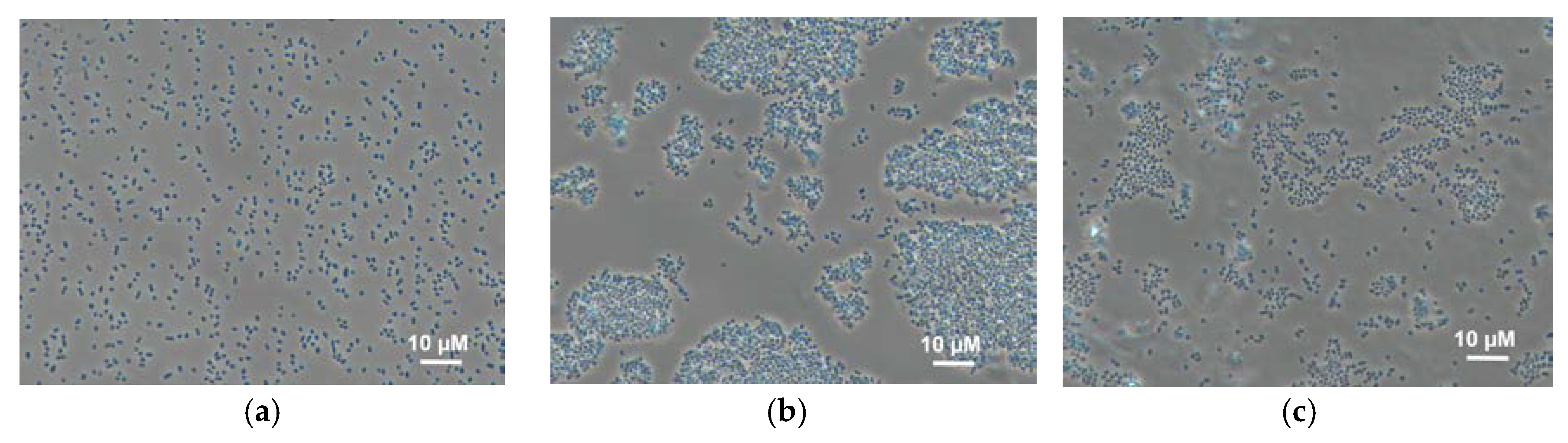
3.2. Behaviour of Bacterial Cells in Polymer Gels
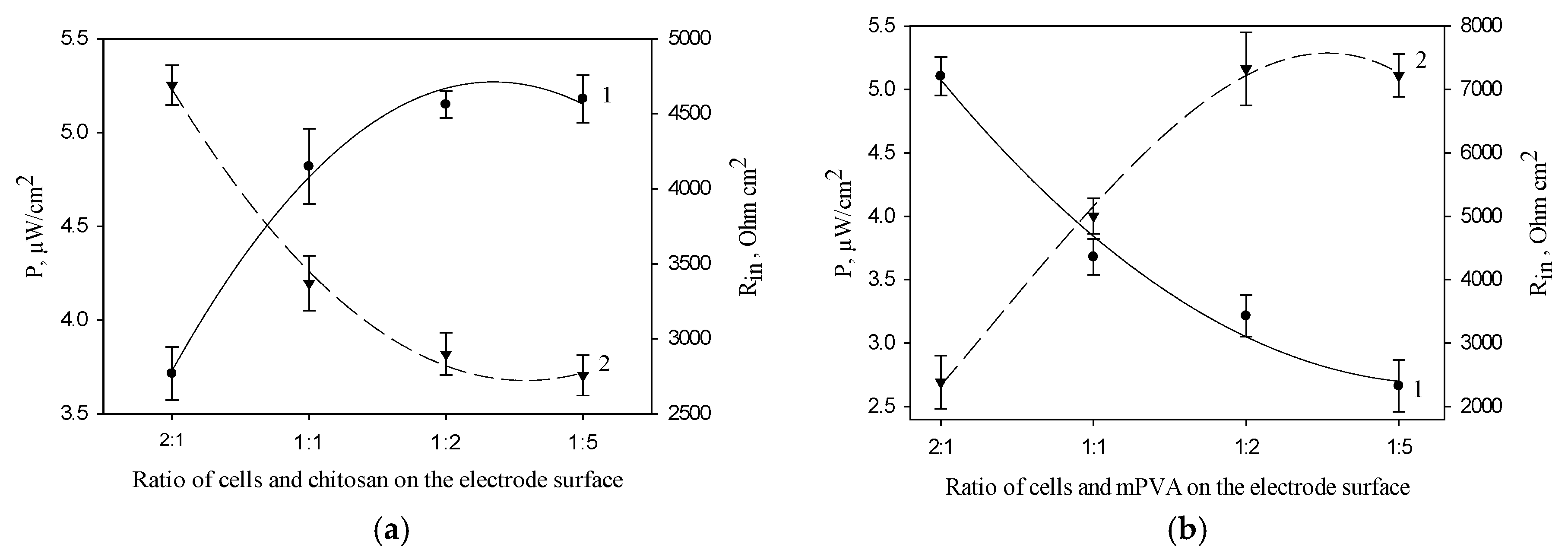
3.3. Effect of the Concentration of Polymer and Bacterial Cells on the Electrochemical Characteristics of MFC Bioanode
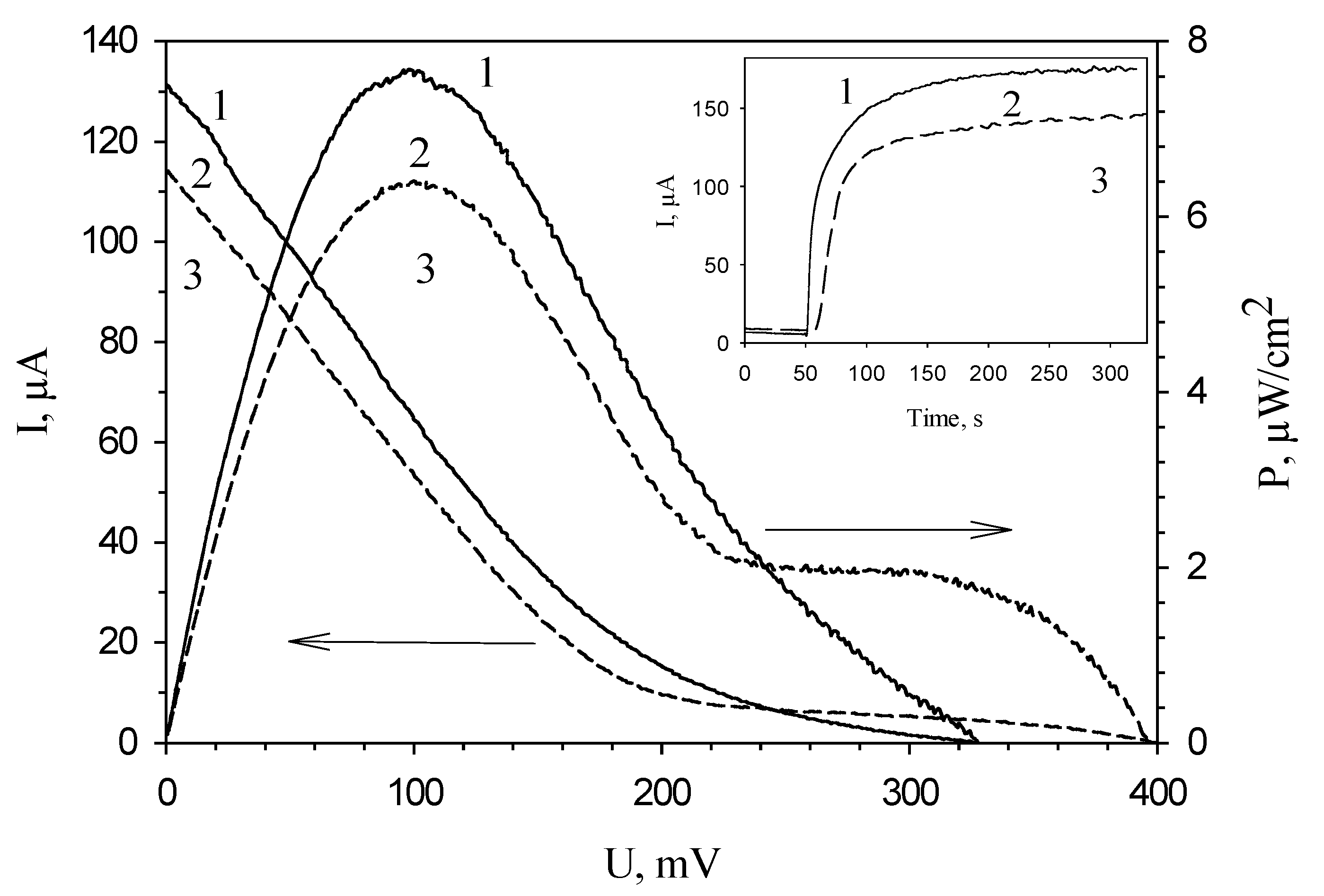
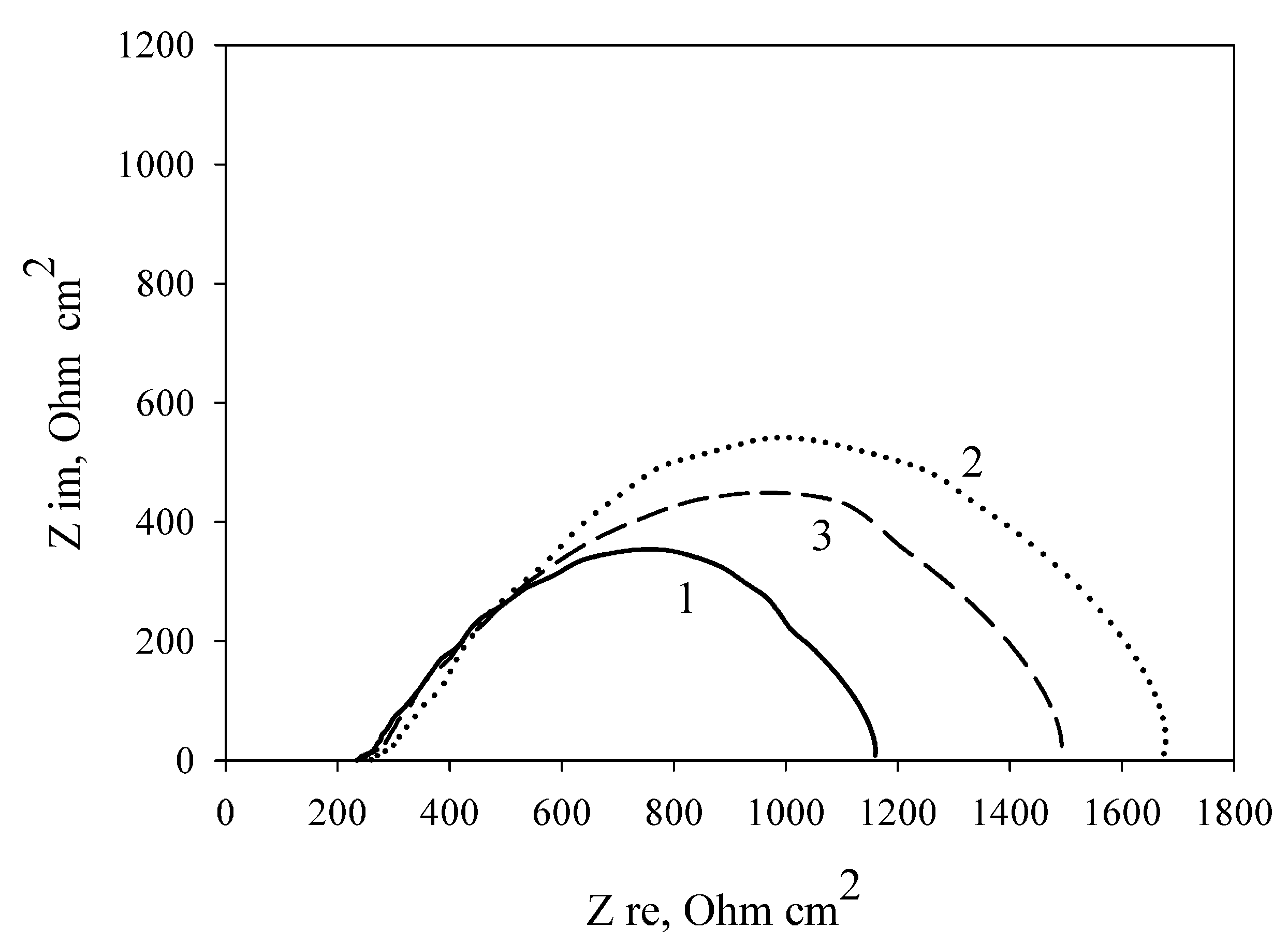
3.4. Modification of the Anode by Carbon Nanotubes
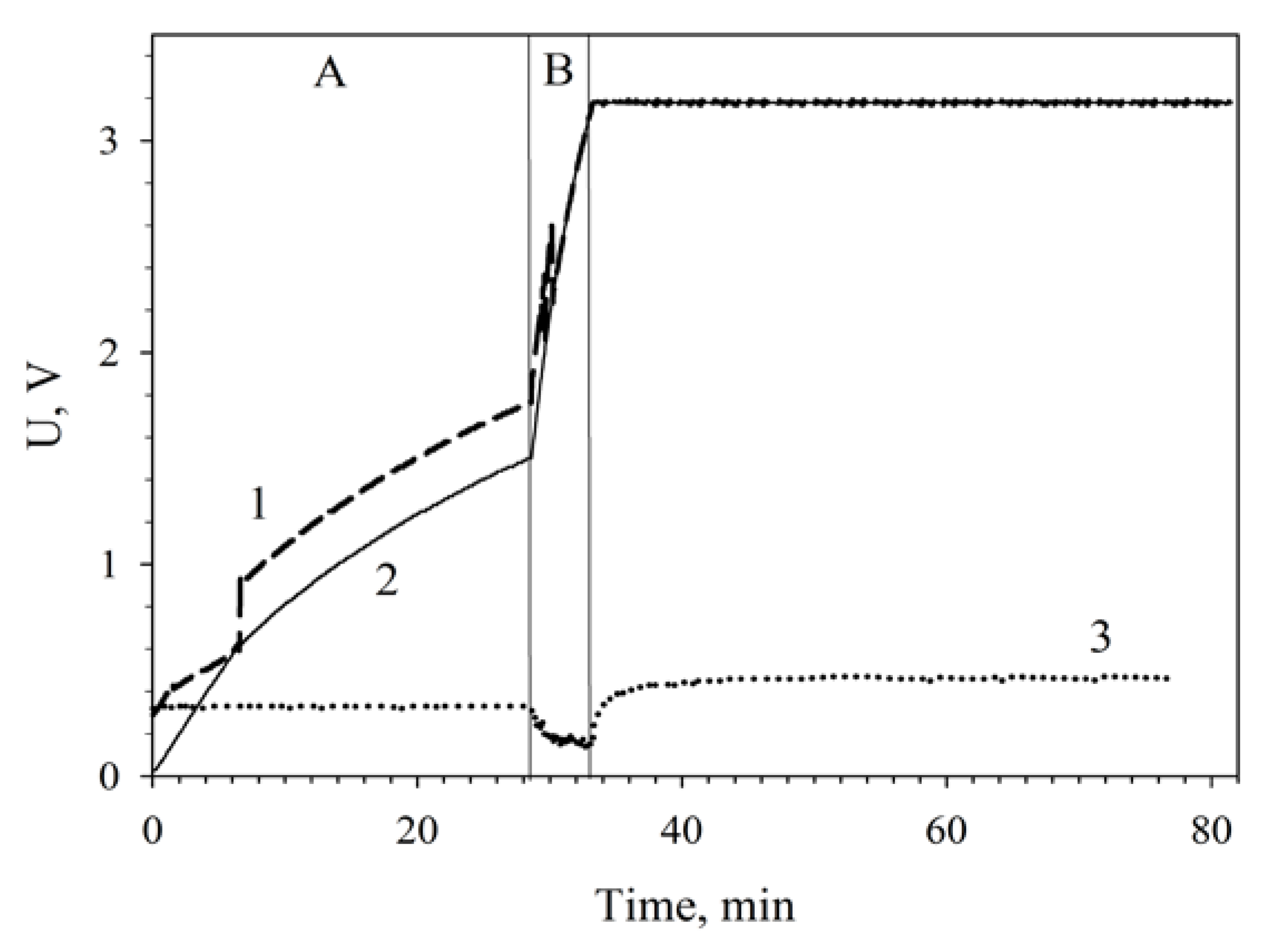
3.5. Applications of MWCNT/Chitosan/G. oxydans MFC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nikovskaya, G.N. The adhesive immobilization of microorganisms in water purification. Khim. Tekhnol. Vody 1989, 11, 158–169. [Google Scholar]
- Kozlyak, E.I.; Solomon, Z.G.; Yakimov, M.M. The sorption of Pseudomonas fluorescens 16n2 cells on cellulose triacetate fibers. Prikl. Biokhim. Mikrobiol. 1991, 27, 508–513. [Google Scholar]
- Kozlyak, E.I.; Solomon, Z.G.; Yakimov, M.M.; Fadyushina, T.V. The sorption of Pseudomonas fluorescens 16n2 cells on various adsorbents. Prikl. Biokhim. Mikrobiol. 1993, 29, 138–143. [Google Scholar]
- Górecka, E.; Jastrzębska, M. Immobilization techniques and biopolymer carriers. Biotechnol. Food Sci. 2011, 75, 65–86. [Google Scholar]
- Teles, F.R.R.; Fonseca, L.P. Applications of polymers for biomolecule immobilization in electrochemical biosensors. Mater. Sci. Eng. C 2008, 28, 1530–1533. [Google Scholar] [CrossRef]
- Odaci, D.; Timur, S.; Telefoncu, A. A microbial biosensor based on bacterial cells immobilized on chitosan matrix. Bioelectrochemistry 2009, 75, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Šefčovičová, J.; Filip, J.; Gemeiner, P.; Vikartovská, A.; Pätoprstý, V.; Tkac, J. High performance microbial 3-D bionanocomposite as a bioanode for a mediated biosensor device. Electrochem. Commun. 2011, 13, 966–968. [Google Scholar] [CrossRef]
- Higgins, S.R.; Foerster, D.; Cheung, A.; Lau, C.; Bretschger, O.; Minteer, S.D.; Nealson, K.; Atanassov, P.; Cooney, M.J. Fabrication of macroporous chitosan scaffolds doped with carbon nanotubes and their characterization in microbial fuel cell operation. Enzym. Microb. Technol. 2011, 48, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.; Ferrer, M.L.; Gutiérrez, M.C.; Jiménez, R.; Del Monte, F.; Leech, D. Three-dimensional microchanelled electrodes in flow-through configuration for bioanode formation and current generation. Energy Environ. Sci. 2011, 4, 4201–4210. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, X.F.; Huang, Y.X.; Sheng, G.P.; Wang, S.G.; Yu, H.Q. Carbon nanotube/chitosan nanocomposite as a biocompatible biocathode material to enhance the electricity generation of a microbial fuel cell. Energy Environ. Sci. 2011, 4, 1422–1427. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Berenjian, A.; Anarjan, N. Recent advances in application of chitosan in fuel cells. Sustain. Chem. Process. 2013, 1, 16. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzym. Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol-bacterial cellulose nanocomposite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90 B, 922–929. [Google Scholar] [CrossRef]
- Van Pham, D.; Tho Bach, L. Immobilized bacteria by using PVA (polyvinyl alcohol) crosslinked with sodium sulfate. Int. J. Sci. Eng. 2014, 7. [Google Scholar] [CrossRef][Green Version]
- Alenina, K.A.; Aleskerova, L.E.; Kascheyeva, P.B.; Ismailov, A.D. The poly(vinyl alcohol)-immobilized photobacteria for toxicology monitoring. Engineering 2012, 5, 118–120. [Google Scholar] [CrossRef]
- Alferov, V.A.; Filatova, N.M.; Asulyan, L.D.; Blokhin, I.V.; Goryacheva, A.A. Formation of a stable receptor element of the biosensor by immobilization of Gluconobacter oxydans bacterial cells into an N-vinylpyrrolidone-modified poly(vinyl alcohol) film, Izv. Nat. Sci. 2011, 1, 210–219. [Google Scholar]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Alferov, S.V.; Alferov, V.A.; Reshetilov, A.N. BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone. Enzym. Microb. Technol. 2013, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Demakov, V.A.; Maksimova, Y.G.; Maksimov, A.Y. Immobilization of microbial cells: Biotechnological aspects. Biotechnol. Russia 2008, 2, 40–62. [Google Scholar]
- Dayani, Y.; Malmstadt, N. Lipid bilayers covalently anchored to carbon nanotubes. Langmuir 2012, 28, 8174–8182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shleev, S.; Jarosz-Wilkolazka, A.; Khalunina, A.; Morozova, O.; Yaropolov, A.; Ruzgas, T.; Gorton, L. Direct electron transfer reactions of laccases from different origins on carbon electrodes. Bioelectrochem. 2005, 67, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Svitel, J.; Curilla, O.; Tkac, J. Microbial cell-based biosensor for sensing glucose, sucrose or lactose. Biotechnol. Appl. Biochem. 1998, 27 Pt 2, 153–158. [Google Scholar]
- Bertokova, A.; Bertok, T.; Filip, J.; Tkac, J. Gluconobacter sp. cells for manufacturing of effective electrochemical biosensors and biofuel cells. Chem. Pap. 2015, 69, 27–41. [Google Scholar] [CrossRef]
- Schenkmayerová, A.; Bertóková, A.; Šefčovičová, J.; Štefuca, V.; Bučko, M.; Vikartovská, A.; Gemeiner, P.; Tkáč, J.; Katrlík, J. Whole-cell Gluconobacter oxydans biosensor for 2-phenylethanol biooxidation monitoring. Anal. Chim. Acta 2015, 854, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, V.K.; Qazi, G.N.; Kumar, A. Gluconobacter oxydans: Its biotechnological applications. J. Mol. Microbiol. Biotechnol. 2001, 3, 445–456. [Google Scholar] [PubMed]
- Lusta, K.A.; Reshetilov, A.N. Physiologo-biochemical characteristics of Gluconobacter oxydans and prospects for its use in biotechnology and biosensor systems (review). Prikl. Biokhim. Mikrobiol. 1998, 34, 339–353. [Google Scholar] [PubMed]
- Švitel, J.; Tkáč, J.; Voštiar, I.; Navrátil, M.; Štefuca, V.; Bučko, M.; Gemeiner, P. Gluconobacter in biosensors: Applications of whole cells and enzymes isolated from Gluconobacter and Acetobacter to biosensor construction. Biotechnol. Lett. 2006, 28, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Deppenmeier, U.; Hoffmeister, M.; Prust, C. Biochemistry and biotechnological applications of Gluconobacter strains. Appl. Microbiol. Biotechnol. 2003, 60, 233–242. [Google Scholar] [CrossRef]
- Macauley, S.; McNeil, B.; Harvey, L.M. The genus Gluconobacter and its applications in biotechnology. Crit. Rev. Biotechnol. 2001, 21, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tkac, J.; Svitel, J.; Vostiar, I.; Navratil, M.; Gemeiner, P. Membrane-bound dehydrogenases from Gluconobacter sp.: Interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochemistry 2009, 76, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Reshetilov, A.N.; Plekhanova, Y.V.; Tarasov, S.E.; Arlyapov, V.A.; Kolesov, V.V.; Gutorov, M.A.; Gotovtsev, P.M.; Vasilov, R.G. Effect of some carbon nanomaterials on ethanol oxidation by Gluconobacter oxydans bacterial cells. Appl. Biochem. Microbiol. 2017, 53, 123–129. [Google Scholar] [CrossRef]
- Asulyan, L.D.; Filatova, N.M.; Arlyapov, V.A.; Alferov, S.V.; Alferov, V.A. A Composite for Production of a Polymer Film for Immobilization of Microorganisms in Biosensor Analyzers. RF Patent 2,461,625,C2, 30 December 2010. [Google Scholar]
- Wang, X.; Gu, H.; Yin, F.; Tu, Y. A glucose biosensor based on Prussian blue/chitosan hybrid film. Biosens. Bioelectron. 2009, 24, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470258590. [Google Scholar]
- Kumar, S.; Acharya, S.K. 2,6-Dichloro-phenol indophenol prevents switch-over of electrons between the cyanide-sensitive and -insensitive pathway of the mitochondrial electron transport chain in the presence of inhibitors. Anal. Biochem. 1999, 268, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.A.; Wisecarver, K.D. Cell immobilization using PVA crosslinked with boric acid. Biotechnol. Bioeng. 1992, 39, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Bhadra, C.M.; Christofferson, A.J.; Yarovsky, I.; Al Kobaisi, M.; Garvey, C.J.; Ponamoreva, O.N.; Alferov, S.V.; Alferov, V.A.; Tharushi Perera, P.G.; et al. Three-dimensional organization of self-encapsulating Gluconobacter oxydans bacterial cells. ACS Omega 2017, 2, 8099–8107. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.A.; D’Angelo, L.; Omer, N.; Windiasti, G.; Lu, X.; Xu, J. Carbon nanotube modification of microbial fuel cell electrodes. Biosens. Bioelectron. 2016, 85, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Oh, S.E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloys Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, Q.; Xie, W.; Zhang, Y. Effects of multi-walled carbon nanotubes with various diameters on bacterial cellular membranes: Cytotoxicity and adaptive mechanisms. Chemosphere 2017, 185, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ti.com/lit/ds/symlink/bq25504.pdf (accessed on 21 September 2018).
- Reshetilov, A.N.; Kitova, A.E.; Ivakhnenko, A.A.; Gotovtsev, P.M.; Vasilov, R.G.; Gutorov, M.A. Converter accumulation of electric energy generated by a microwatt-power microbial fuel cell. Vest. Biotekhnol. 2014, 10, 27–32. [Google Scholar]
- Futamata, H.; Bretschger, O.; Cheung, A.; Kan, J.; Owen, R.; Nealson, K.H. Adaptation of soil microbes during establishment of microbial fuel cell consortium fed with lactate. J. Biosci. Bioeng. 2013, 115, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Toczyłowska-Mamińska, R.; Szymona, K.; Król, P.; Gliniewicz, K.; Pielech-Przybylska, K.; Kloch, M.; Logan, B.E. Evolving microbial communities in cellulose-fed microbial fuel cell. Energies 2018, 11, 124. [Google Scholar] [CrossRef]
- Somov, A.; Gotovtsev, P.; Dyakov, A.; Alenicheva, A.; Plehanova, Y.; Tarasov, S.; Reshetilov, A. Bacteria to power the smart sensor applications: Biofuel cell for low-power IoT devices. In Proceedings of the IEEE World Forum on Internet of Things, WF-IoT 2018, Singapore, 5–8 February 2018; pp. 802–806. [Google Scholar]
- Stoll, Z.A.; Ma, Z.; Trivedi, C.B.; Spear, J.R.; Xu, P. Sacrificing power for more cost-effective treatment: A techno-economic approach for engineering microbial fuel cells. Chemosphere 2016, 161, 10–18. [Google Scholar] [CrossRef] [PubMed]
| Rct, Ohm cm2 | Rin, Ohm cm2 | Psp, μW/cm2 | ∆I, μА | |
|---|---|---|---|---|
| PVA | 1255 ± 213; 16.9% | 1251 ± 201; 16.1% | 6.56 ± 0.74; 11.3% | 134.7 ± 9.0; 6.7% |
| mPVA | 1294 ± 103; 8.0% | 1455 ± 192; 13.2% | 6.10 ± 0.32; 5.3% | 139.2 ± 7.5; 5.4% |
| Chitosan | 1069 ± 59; 5.5% | 1111 ± 71; 6.4% | 7.63 ± 0.28; 3.4% | 159.0 ± 5.9; 3.7% |
| Rct, Ohm cm2 | Rin, Ohm cm2 | Psp, μW/cm2 | ∆I, μА | |
|---|---|---|---|---|
| PVA | 908 ± 52; 5.7% | 1219 ± 43; 3.7% | 7.92 ± 0.51; 6.4% | 139.6 ± 4.8; 3.4% |
| mPVA | 1088 ± 70; 6.5% | 1277 ± 54; 4.2% | 7.23 ± 0.80; 11.0% | 147.2 ± 10.0; 6.8% |
| Chitosan | 722 ± 30; 4.2% | 977 ± 57; 5.9% | 10.56 ± 0.78; 7.8% | 194.0 ± 14.4; 7.4% |
| Cell | Power, μW | Internal Resistance, Ohm | Charging Time of 6800 μF Capacitor |
|---|---|---|---|
| Single MFC, nonmodified | 80 | 200 | Does not charge |
| Single MFC, MWCNT-modified | 110 | 160 | Does not charge |
| Series-connected nonmodified MFCs | 140 | 380 | 1st charge cycle, 80 min; 2nd charge cycle, 70 min |
| Series-connected MWCNT-modified MFCs | 198 | 290 | 1st cycle, 60 min; after 48 h, 32 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plekhanova, Y.; Tarasov, S.; Kolesov, V.; Kuznetsova, I.; Signore, M.; Quaranta, F.; Reshetilov, A. Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell. Membranes 2018, 8, 99. https://doi.org/10.3390/membranes8040099
Plekhanova Y, Tarasov S, Kolesov V, Kuznetsova I, Signore M, Quaranta F, Reshetilov A. Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell. Membranes. 2018; 8(4):99. https://doi.org/10.3390/membranes8040099
Chicago/Turabian StylePlekhanova, Yulia, Sergei Tarasov, Vladimir Kolesov, Iren Kuznetsova, Maria Signore, Fabio Quaranta, and Anatoly Reshetilov. 2018. "Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell" Membranes 8, no. 4: 99. https://doi.org/10.3390/membranes8040099
APA StylePlekhanova, Y., Tarasov, S., Kolesov, V., Kuznetsova, I., Signore, M., Quaranta, F., & Reshetilov, A. (2018). Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell. Membranes, 8(4), 99. https://doi.org/10.3390/membranes8040099