Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology
Abstract
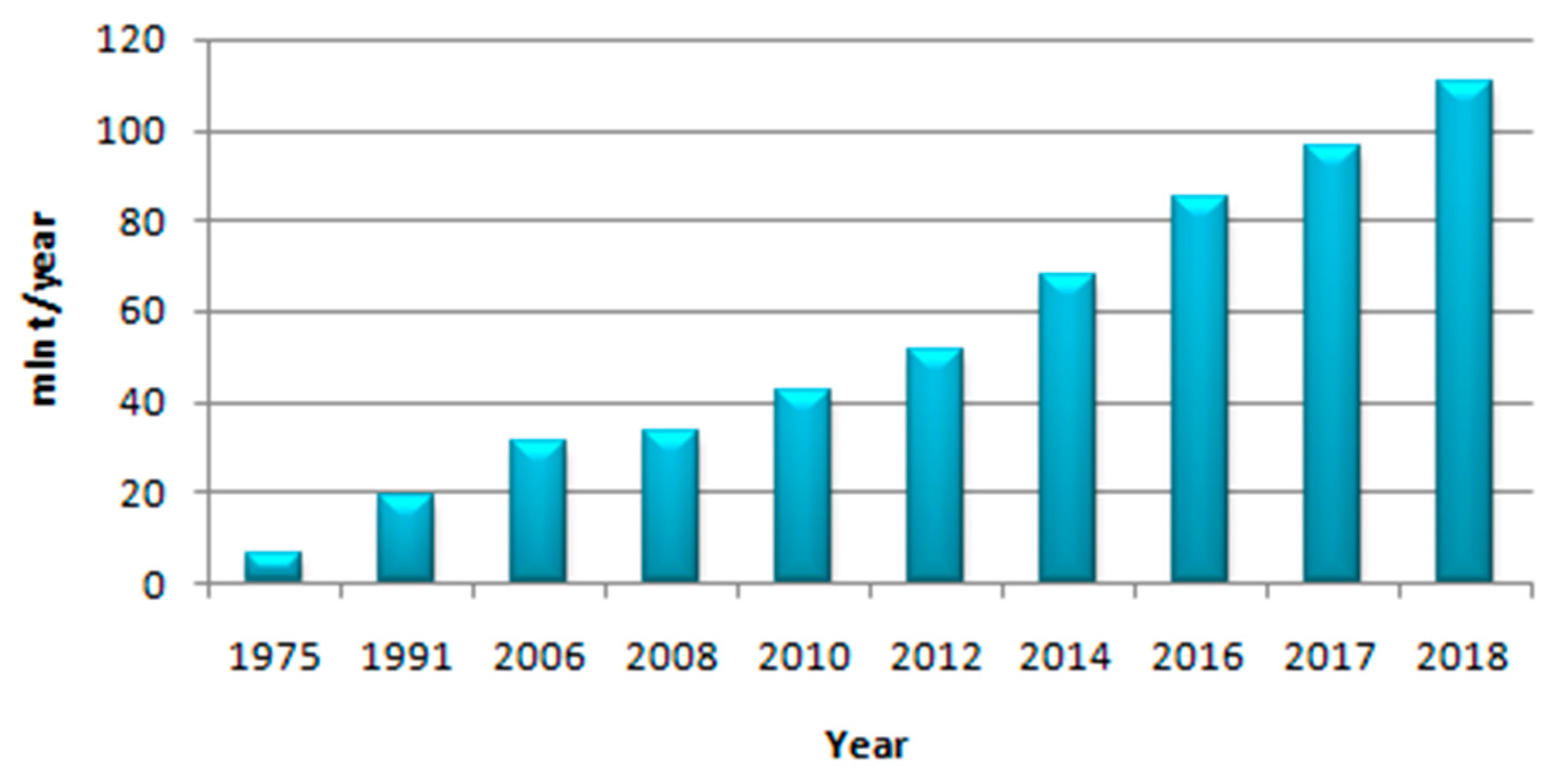
1. Introduction
2. Methanol Production
2.1. Natural Gas as a Primary Source of Methanol Production
2.1.1. Methanol Production through BASF Process
2.1.2. Methanol Production through ICI PROCESS
2.2. Biomass and Char as Feedstocks for Methanol Synthesis
2.3. CO2 Hydrogenation for Methanol Generation
- (a)
- Recycling of the unconverted synthesis gas after products separation by condensation;
- (b)
- in situ reaction products removal.
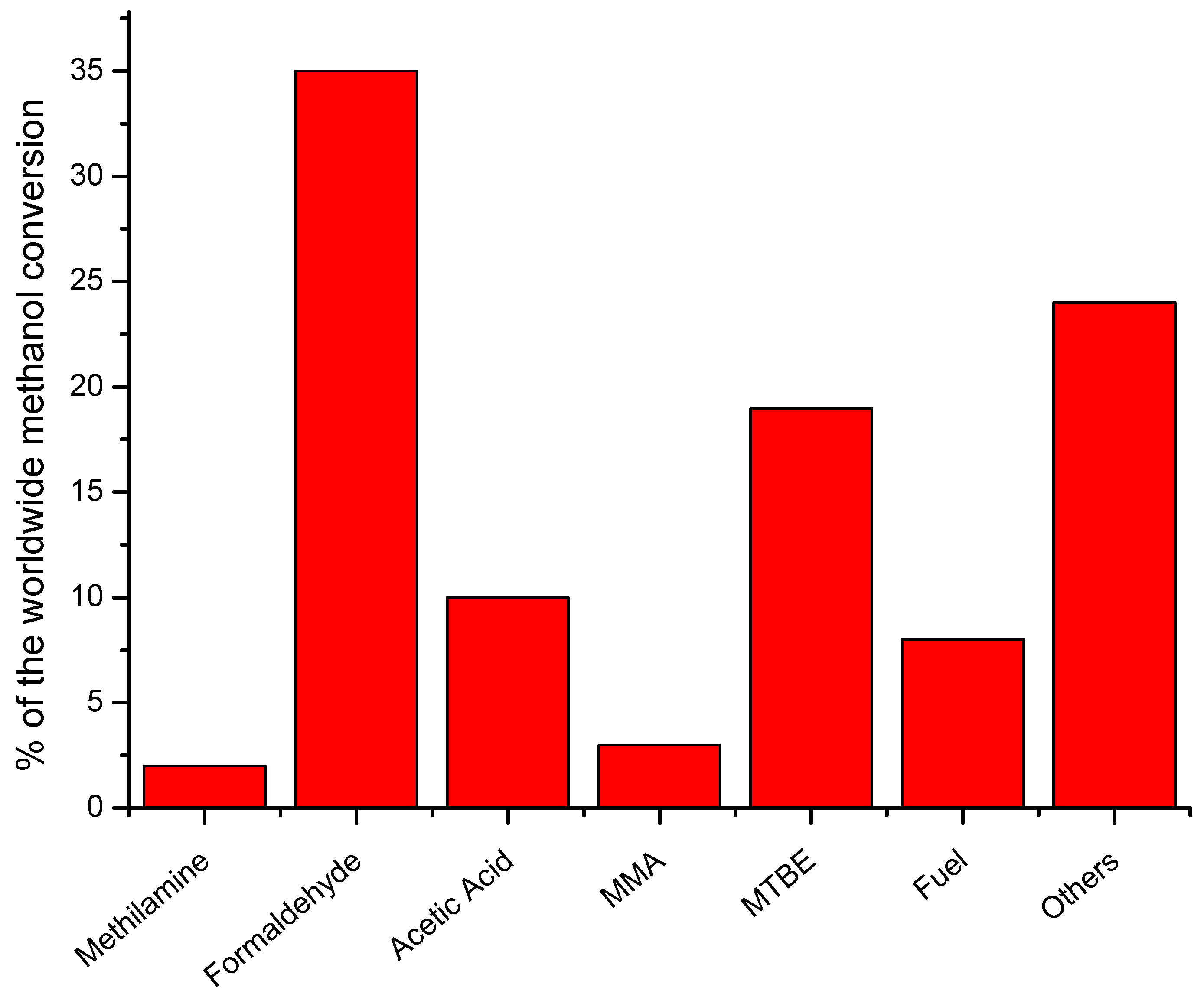
3. Methanol Utilization
3.1. Methanol Transformation into Dimethylether
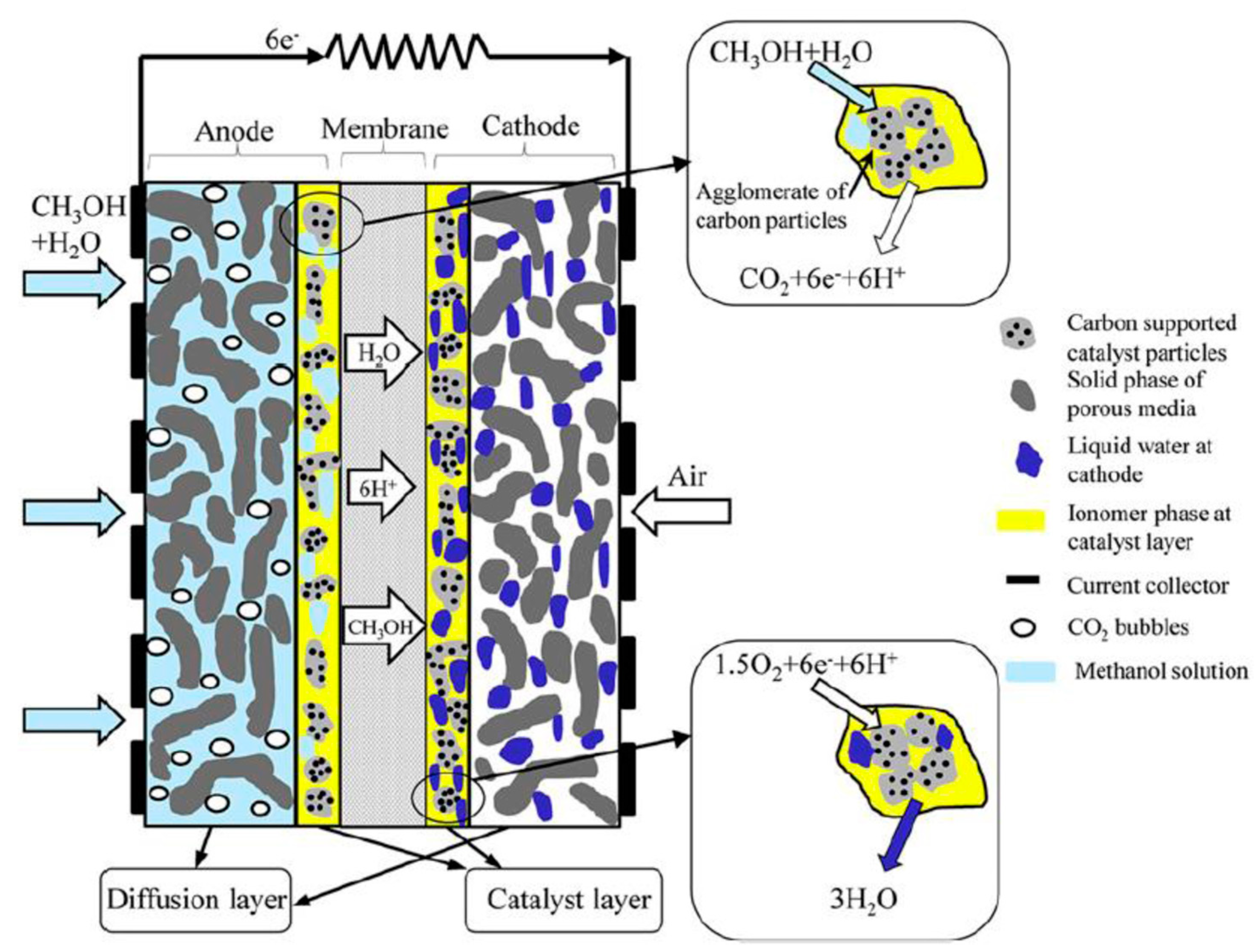
3.2. Methanol in Fuel Cells Applications: DMFCs
- (1)
- Anode gas diffusion layer (AGDL),
- (2)
- anode catalyst layer (ACL),
- (3)
- polymer electrolyte membrane,
- (4)
- cathode catalyst layer,
- (5)
- cathode gas diffusion layer.
4. Methanol Exploitation for Hydrogen Generation
4.1. Methanol Decomposition Reaction
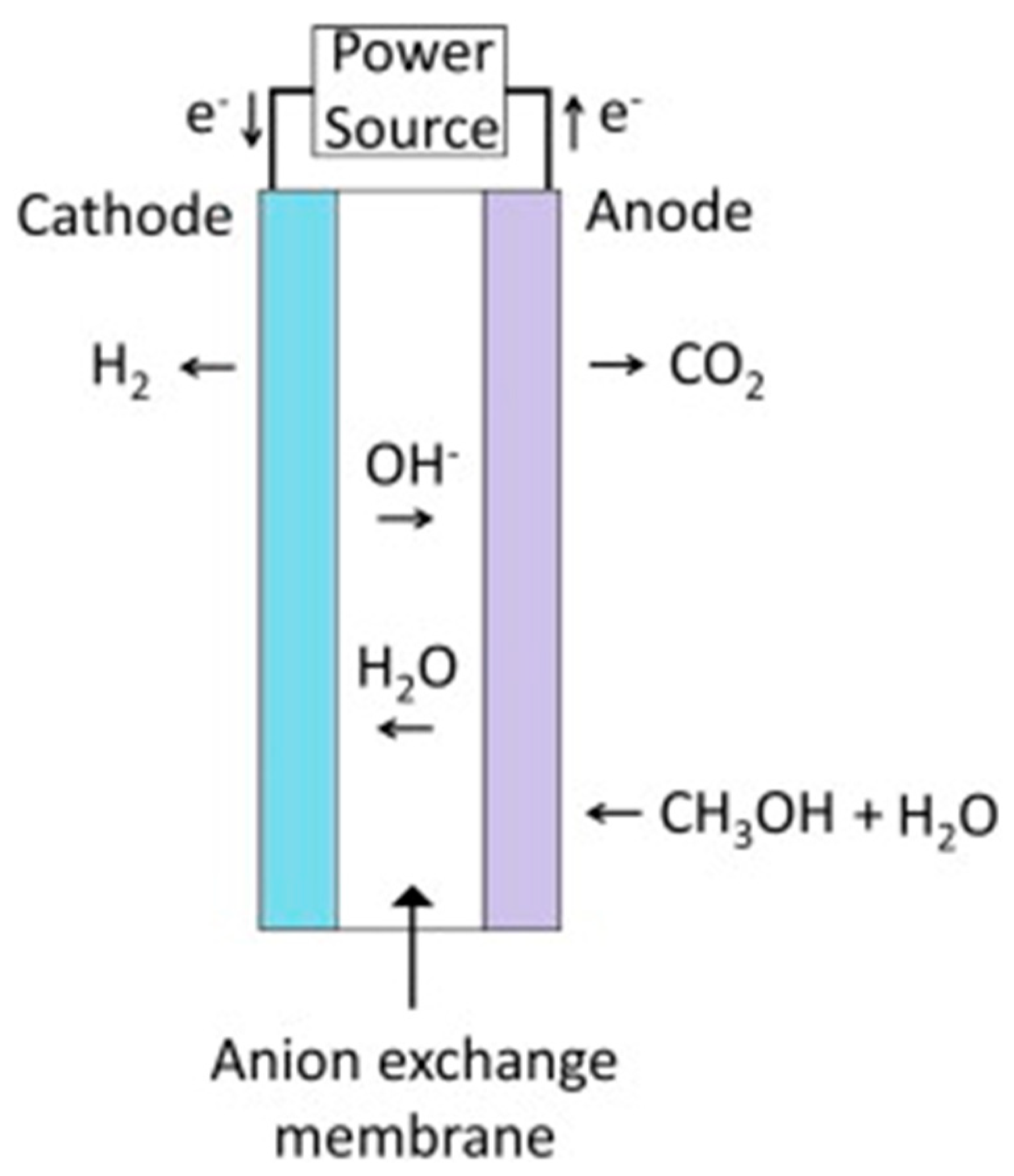
4.2. Methanol–Water Solution Electrolysis
4.3. Methanol Steam Reforming Reaction for Hydrogen Generation
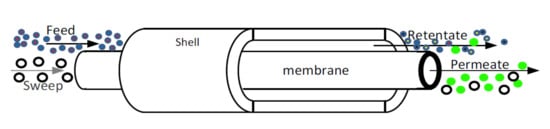
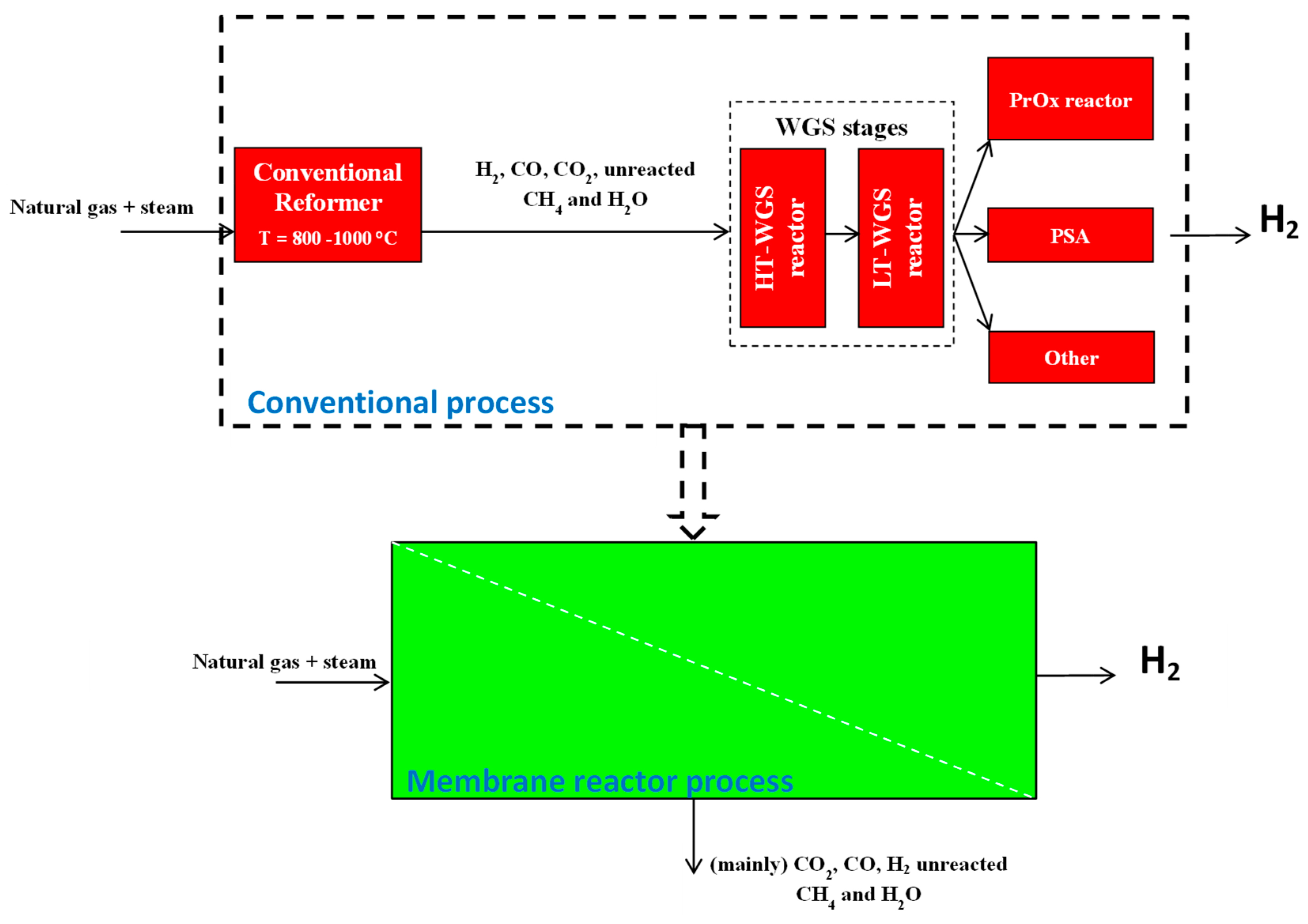
4.4. Process Intensification Strategy Applied to MSR Reactors
- Extractor modality: The membrane selectively removes hydrogen from the reaction mixture for permeation.
- Distributor modality: The membrane allows the controlled addition of hydrogen to the reaction mixture.
- Contactor modality: The membrane emphasizes the contact within reactants and catalyst.
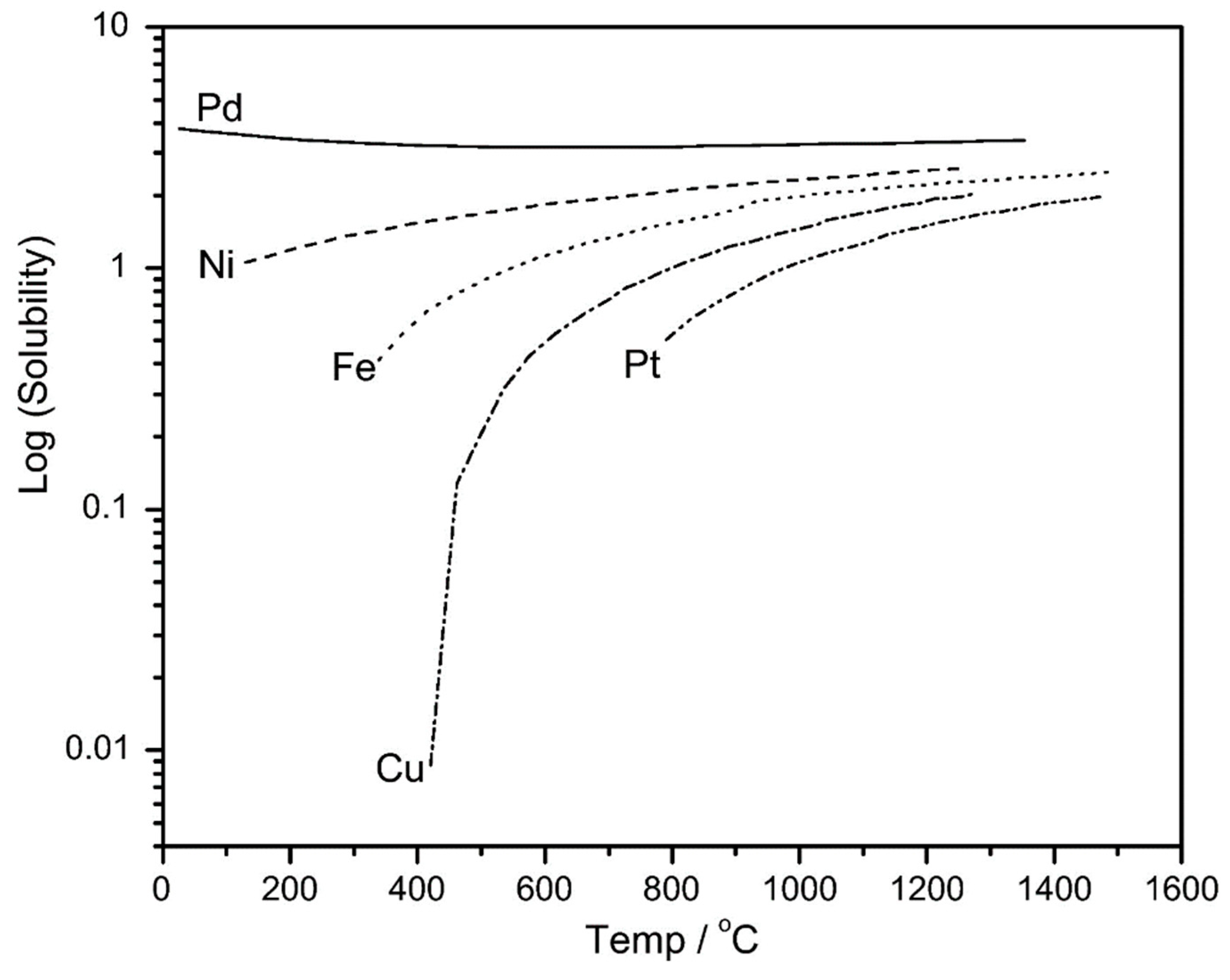
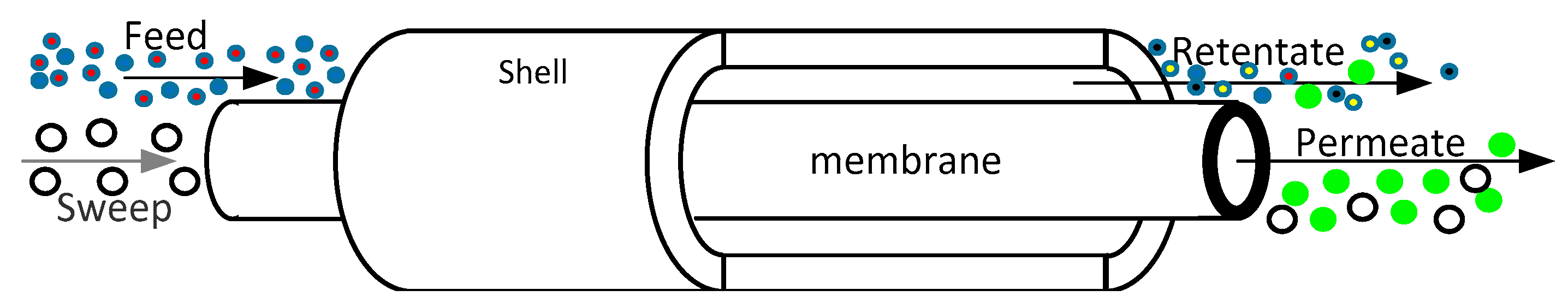
4.5. Applications of Pd-Based Membranes in Membrane Reactors
- Hydrogen molecules adsorption from the membrane;
- dissociation of hydrogen molecules on the membrane surface;
- reversible dissociative chemisorption of atomic hydrogen;
- reversible dissolution of atomic hydrogen in the metal lattice of the membrane;
- diffusion into the metal of atomic hydrogen proceeds from the higher hydrogen pressure to the lower hydrogen membrane side;
- desorption of recombined atomic hydrogen into molecular form.
4.6. Methanol Steam Reforming Reaction in Membrane Reactors for Hydrogen Generation
4.7. Photocatalytic Membrane Reactors: Methanol Production from CO2 Reduction
5. Recent European Projects Involving Methanol Production, Utilization, and Transformation
6. Conclusions and Future Trends
Funding
Conflicts of Interest
List of Acronyms
| ACL | Anode Catalyst Layer |
| AGDL | Anode Gas Diffusion Layer |
| AMR | Autothermal Methane Reforming |
| BASF | BadischeAnilin und Soda Fabrik |
| CR | Conventional Reactor |
| DME | DiMethyl Ether |
| DMFC | Direct Methanol Fuel Cell |
| ELP | Electroless Plating |
| GHG | Greenhouse Gases |
| HT | High Temperature |
| ICI | Imperial Chemical Industries |
| MD | Methanol Decomposition |
| MR | Membrane Reactor |
| MSR | Methanol Steam Reforming |
| MTBE | Methyl Tertiary Butyl Ether |
| NCCC | National Carbon Capture Center |
| PEM | Proton Exchange Membrane |
| PMR | Photocatalytic Membrane Reactor |
| POM | Partial Oxidation of Methanol |
| SMR | Steam Methane Reforming |
| WGS | Water-Gas Shift |
References
- Dalena, F.; Senatore, A.; Tursi, A.; Basile, A. Bioenergy production from second and third generation feedstocks. In Bioenergy Systems for the Future; Dalena, F., Basile, A., Rossi, C., Eds.; Prospects for Biofuels and Biohydrogen; Elseiver Publishing: London, UK, 2017. [Google Scholar]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol Production and Application. An overview. In The Science and Engineering of Methanol; Basile, A., Dalena, F., Eds.; Elsevier Publishing: London, UK, 2017; ISBN 978-0-444-63903-5. [Google Scholar]
- The Methanol Industry. Available online: https://www.methanol.org/the-methanol-industry/ (accessed on 17 October 2018).
- Cifre, P.G.; Badr, O. Renewable hydrogen utilisation for the production of methanol. Energy Convers. Manag. 2007, 48, 519–527. [Google Scholar] [CrossRef]
- Hughes, D.O. Methanol: The chemical of the future. Chemsa 1980, 6, 180–182. [Google Scholar]
- Ali, K.A.; Abdullah, A.Z.; Mohamed, A.R. Recent development in catalytic technologies for methanol synthesis from renewable sources: A critical review. Renew. Sustain. Energy Rev. 2015, 44, 508–518. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Kolesnichenko, N.V.; Ezhova, N.N. Slurry technology in methanol synthesis (Review). Pet. Chem. 2016, 56, 77–95. [Google Scholar] [CrossRef]
- Sabatier, P. How I have been led to the direct hydrogenation method by metallic catalysts. Ind. Eng. Chem. 1926, 18, 1005–1008. [Google Scholar] [CrossRef]
- Tijm, P.J.A.; Waller, F.J.; Browna, D.M. Methanol technology developments for the new millennium. Appl. Catal. A 2001, 221, 275–282. [Google Scholar] [CrossRef]
- Couper, J.R.; Beasley, T.; Penney, W.R. The Chemical Process Industries Infrastructure: Function and Economics; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–644. ISBN 9780824704353. [Google Scholar]
- Natta, G. Synthesis of Methanol, Ch. 8 of Catalysis; Emmett, P.H., Ed.; Reinhold Publishing Corporation: New York, NY, USA, 1955; Volume 3, pp. 1–363. [Google Scholar]
- Zhen, X.; Wang, Y. An Overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493. [Google Scholar] [CrossRef]
- Gumber, S.; Gurumoorthy, A.V.P. Methanol Economy Versus Hydrogen Economy, in Methanol: Science and Engineering; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 661–674. ISBN 978-044463903-5. [Google Scholar]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Kapran, A.Y.; Orlyk, S.M. Hydrogen production in methanol reforming on modified copper–zinc catalysts: A review. Theor. Exp. Chem. 2016, 53, 1–16. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Liguori, S.; Basile, A. Production of hydrogen via glycerol steam reforming in a Pd-Ag membrane reactor over Co-Al2O3 catalyst. Asia-Pac. J. Chem. Eng. 2010, 5, 138–145. [Google Scholar] [CrossRef]
- Madej-Lachowska, M.; Kulawska, M.; Słoczyński, J. Methanol as a high purity hydrogen source for fuel cells: A brief review of catalysts and rate expressions. Chem. Process Eng. 2017, 38, 147–162. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Y.; He, X. Theoretical study of the mechanism of methanol steam reforming over Pd/ZnO. Prog. Chem. 2012, 24, 873–878. [Google Scholar]
- Bertau, M.; Wernicke, H.J.; Schmidt, F. Methanol Utilization Technologies. In Methanol: The Basic Chemical and Energy Feedstock of the Future; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin, Germany, 2014; pp. 1–677. ISBN 978-3-642-39709-7. [Google Scholar]
- Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2000. [Google Scholar]
- Da Silva, M.J. Synthesis of methanol from methane: Challenges and advances on the multi-step (syngas) and one-step routes (DMTM). Fuel Process Technol. 2016, 145, 42–61. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Liu, H.; Zhang, X. Life cycle assessment and economic analysis of methanol production from coke oven gas compared with coal and natural gas routes. J. Clean. Prod. 2018, 185, 299–308. [Google Scholar] [CrossRef]
- Wilkinson, S.K.; Van De Water, L.G.A.; Miller, B.; Simmons, M.J.H.; Stitt, E.H.; Watson, M.J. Understanding the generation of methanol synthesis and water gas shift activity over copper-based catalysts—A spatially resolved experimental kinetic study using steady and non-steady state operation under CO/CO2/H2feeds. J. Catal. 2016, 337, 208–220. [Google Scholar] [CrossRef]
- Mittasch, A.; Pier, M. Synthetic Manufacture of Methanol. U.S. Patent 1569775, 12 January 1926. [Google Scholar]
- Brown, N.J.; García-Trenco, A.; Weiner, J.; White, E.R.; Allinson, M.; Chen, Y.; Peter, P.W.; Emma, K.G.; Klaus, H.; Milo, S.P.S.; et al. From organometallic zinc and copper complexes to highly active colloidal catalysts for the conversion of CO2 to methanol. ACS Catal. 2015, 5, 2895–2902. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The applications of morphology controlled ZnO in catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, L.; Guo, Q.; Fan, H.; Zheng, H.; Xie, K. Influence of the calcination on the activity and stability of the Cu/ZnO/Al2O3 catalyst in liquid phase methanol synthesis. Fuel 2010, 89, 1348–1352. [Google Scholar] [CrossRef]
- Van Bennekom, J.G.; Venderbosch, R.H.; Winkelman, J.G.M.; Wilbers, E.; Assink, D.; Lemmens, K.P.J.; Heeres, H.J. Methanol synthesis beyond chemical equilibrium. Chem. Eng. Sci. 2013, 87, 204–208. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A Review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Wilhelm, D.J.; Simbeck, D.R.; Karp, A.D.; Dickenson, R.L. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2011, 71, 139–148. [Google Scholar] [CrossRef]
- Zhao, Z.; Lakshminarayanan, N.; Swartz, S.L.; Arkenberg, G.B.; Felix, L.G.; Slimane, R.B.; Choi, C.C.; Ozkan, U.S. Characterization of olivine-supported nickel silicate as potential catalysts for tar removal from biomass gasification. Appl. Catal. A 2015, 489, 42–50. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol steam reforming on Cu/ZnO/Al2O3 catalysts. Part. 2. A comprehensive kinetic model. Appl. Catal. A 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Phongamwong, T.; Chantaprasertporn, U.; Witoon, T.; Numpilai, T.; Poo-Arporn, Y.; Limphirat, W.; Waleeporn, D.; Peerapan, D. Metta Chareonpanich, Limtrakul, J. CO2 hydrogenation to methanol over CuO–ZnO–ZrO2–SiO2 catalysts: Effects of SiO2 contents. Chem. Eng. J. 2017, 316, 692–703. [Google Scholar] [CrossRef]
- Tisseraud, C.; Comminges, C.; Belin, T.; Ahouari, H.; Soualah, A.; Pouilloux, Y.; Le Valant, A. The Cu–ZnO synergy in methanol synthesis from CO2, Part 2: Origin of the methanol and CO selectivities explained by experimental studies and a sphere contact quantification model in randomly packed binary mixtures on Cu–ZnO coprecipitate catalysts. J. Catal. 2015, 330, 533–544. [Google Scholar] [CrossRef]
- Tisseraud, C.; Comminges, C.; Habrioux, A.; Pronier, S.; Pouilloux, Y.; Le Valant, A. Cu-ZnO catalysts for CO2 hydrogenation to methanol: Morphology change induced by ZnO lixiviation and its impact on the active phase formation. Mol. Catal. 2018, 446, 98–105. [Google Scholar] [CrossRef]
- Liao, F.; Huang, Y.; Ge, J.; Zheng, W.; Tedsree, K.; Collier, P.; Hong, X.; Tsang, S.C. Morphology-Dependent Interactions of ZnO with Cu Nanoparticles at the Materials’ Interface in Selective Hydrogenation of CO2 to CH3OH. Angew. Chem. 2011, 50, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Shabangu, S.; Woolf, D.; Fisher, E.M.; Angenent, L.T.; Lehmann, J. Techno-economic assessment of biomass slow pyrolysis into different biochar and methanol concepts. Fuel 2017, 117, 742–748. [Google Scholar] [CrossRef]
- Kempegowda, R.S.; Pannir Selvam, P.V.; Skreiberg, Ø.; Tran, K.-Q. Process synthesis and economics of combined biomethanol and CHP energy production derived from biomass wastes. J. Chem. Technol. Biotechnol. 2012, 87, 897–902. [Google Scholar] [CrossRef]
- Marie-Rose, S.C.; Chornet, E.; Lynch, D.; Lavoie, J.-M. From biomass-rich residues into fuels and green chemicals via gasification and catalytic synthesis. WIT Trans. Ecol. Environ. 2011, 143, 123–132. [Google Scholar]
- Dalena, F.; Basile, A. Bioalcohols Production. In Alcohols and Bioalcohols: Characteristics, Production and Uses; Dalena, F., Basile, A., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 1–22. ISBN 978-1-63321-934-2. [Google Scholar]
- Dai, J.; Saayman, J.; Grace, J.R.; Ellis, N. Gasification of woody biomass. Ann. Rev. Chem. Biomol. Eng. 2015, 6, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Trop, P.; Anicic, B.; Goricanec, D. Production of methanol from a mixture of torrefied biomass and coal. Energy 2014, 77, 125–132. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Basile, A.; Blasi, A.; Fiorenza, G.; Iulianelli, A.; Longo, T.; Calabrò, V. Membrane and Membrane Reactor Technologies in the Treatment of Syngas Streams Produced from Gasification Processes. In Gasification: Chemistry, Processes and Applications; Baker, M.D., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 139–174. ISBN 978-1-61209-681-0. [Google Scholar]
- Korens, N.; Simbeck, D.R.; Wilhelm, D.J. Process Screening Analysis of Alternative Gas Treating and Sulphur Removal for Gasification; Final Report by SFA Pacific Inc. Engineering & Economic Consultants, DOE/NETL Task Order No. 739656-00100; SFA Pacific Inc.: Mountain View, CA, USA, 2002; pp. 1–104. Available online: https://www.netl.doe.gov/File%20Library/Research/Coal/energy%20systems/gasification/pubs/SFA-Pacific_Process-Screening-Analysis_Dec-2002.pdf (accessed on 17 October 2018).
- Stangeland, K.; Li, H.; Yu, Z. Thermodynamic analysis of chemical and phase equilibria in CO2 hydrogenation to methanol, dimethyl ether, and higher alcohols. Ind. Eng. Chem. Res. 2018, 57, 4081–4094. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Liu, J.; Jin, F.; Fan, M.; Zhu, L.; Tang, C.; Chang, R.; Jia, Q.; Li, Q. Production of high-pure hydrogen by an integrated catalytic process: Comparison of different lignocellulosic biomasses and three major components. Fuel 2018, 226, 322–330. [Google Scholar] [CrossRef]
- Global Methanol Demand Growth Driven by Methanol to Olefins as Chinese Thirst for chemical Supply Grows, IHS Markit Says, IHS Online Newsroom. Available online: http://news.ihsmarkit.com (accessed on 17 October 2018).
- Lerner, A.; Brear, M.J.; Lacey, J.S.; Gordon, R.L.; Webley, P.A. Life cycle analysis (LCA) of low emission methanol and di-methyl ether (DME) derived from natural gas. Fuel 2018, 220, 871–878. [Google Scholar] [CrossRef]
- Hosseininejad, S.; Afacan, A.; Hayes, R.E. Catalytic and kinetic study of methanol dehydration to dimethyl ether. Chem. Eng. Res. Des. 2012, 90, 825–833. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Ohno, Y.; Yoshida, M.; Shikada, T.; Inokoshi, O.; Ogawa, T.; Inoue, N. New Direct Synthesis Technology for DME (Dimethyl Ether) and its Application Technology; JFE Technical Report No. 8; JFE: Tokyo, Japan, 2006; pp. 34–40. Available online: http://www.jfe-steel.co.jp/en/research/report/008/pdf/008-06.pdf (accessed on 17 October 2018).
- Bahrami, H.; Faghri, A. Review and advances of direct methanol fuel cells: Part II: Modeling and numerical simulation. J. Power Sources 2013, 230, 303–320. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.H.; Eriksson, S.-G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhao, T.S.; Chen, R.; Wong, C.W. The effect of methanol concentration on the performance of a passive DMFC. Electrochem. Commun. 2005, 7, 288–294. [Google Scholar] [CrossRef]
- Toshiba Launches Direct Methanol Fuel Cell in Japan as External Power for Mobile Electronic Devices. Available online: https://www.toshiba.co.jp/about/press/2009_10/pr2201.htm (accessed on 17 October 2018).
- Iulianelli, A.; Gugliuzza, A.; Clarizia, G.; Ebrasu, D.; Bevilacqua, A.; Trotta, F.; Basile, A. Sulfonation of PEEK-WC polymer via chloro-sulfonic acid for potential PEM fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 12688–12695. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. J. Hydrogen Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Basile, A.; Iulianelli, A. Advances in Hydrogen Production, Storage and Distribution, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 1–574. ISBN 978-0-85709-768-2. [Google Scholar]
- International Energy Agency (IEA). Energy Technology Perspectives 2014. Available online: https://www.iea.org/publications/freepublications/publication/EnergyTechnologyPerspectives_ES.pdf (accessed on 17 October 2018).
- Usami, Y.; Kagawa, K.; Kawazoe, M.; Matsumura, Y.; Sakurai, H.; Haruta, M. Catalytic methanol decomposition at low temperatures over palladium supported on metal oxides. Appl. Catal. A 1998, 171, 123–130. [Google Scholar] [CrossRef]
- Menia, S.; Tebibel, H.; Lassouane, L.; Khellaf, A.; Nouicer, I. Hydrogen production by methanol aqueous electrolysis using photovoltaic energy: Algerian potential. Int. J. Hydrogen Energy 2017, 42, 8661–8669. [Google Scholar] [CrossRef]
- Take, T.; Tsurutani, K.; Umeda, M. Hydrogen production by methanol-water solution electrolysis. J. Power Sources 2007, 164, 9–16. [Google Scholar] [CrossRef]
- Tuomi, S.; Santasalo-Aarnio, A.; Kanninen, P.; Kallio, T. Hydrogen production by methanol–water solution electrolysis with an alkaline membrane cell. J. Power Sources 2013, 229, 32–35. [Google Scholar] [CrossRef]
- Mastalir, Á.; Patzkó, Á.; Frank, B.; Schomäcker, R.; Ressler, T.; Schlögl, R. Steam reforming of methanol over Cu/ZnO/Al2O3 modified with hydrotalcites. Catal. Commun. 2007, 8, 1684–1690. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Tsunoda, T.; Suzuki, K.; Hamakawa, S.; Murata, K.; Shiozaki, R.; Ishii, T.; Kumagai, M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts. Top. Catal. 2003, 22, 205–213. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Menon, P.G. Activity and Characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-Alumina for methanol reforming for fuel cell vehicles. Appl. Catal. A 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn addition to supported Pd catalyst in the steam reforming of methanol. Appl. Catal. A 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Yang, H.-M.; Liao, P.-H. Preparation and activity of Cu/ZnO–CNTs nano-catalyst on steam reforming of methanol. Appl. Catal. A 2007, 317, 226–233. [Google Scholar] [CrossRef]
- Vidal Vázquez, F.; Simell, P.; Pennanen, J.; Lehtonen, J. Reactor design and catalysts testing for hydrogen production by methanol steam reforming for fuel cells applications. Int. J. Hydrogen Energy 2016, 41, 924–935. [Google Scholar] [CrossRef]
- Venvik, H.J.; Yang, J. Catalysis in microstructured reactors: Short review on small-scale syngas production and further conversion into methanol, DME and Fischer-Tropsch products. Catal. Today 2017, 285, 135–146. [Google Scholar] [CrossRef]
- Hessel, V.; Hardt, S.; Lowe, H. Chemical Micro Processing Engeneering, Fundamentals, Modelling and Reactions; Wiley-VCH, Verlag GmbH: Weinheim, Germany, 2004. [Google Scholar]
- Iulianelli, A.; Basile, A. Advances on Inorganic Membrane Reactors for Production of Hydrogen; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2018; pp. 1–11. ISBN 978-1-4939-2493-6. [Google Scholar]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Piemonte, V.; Di Paola, L.; De Falco, M.; Iulianelli, A.; Basile, A. 11-Hydrogen production Using Inorganic Membrane Reactors. In Advances in Hydrogen Production, Storage and Distribution; Basile, A., Iulianelli, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 283–316. ISBN 978-0-85709-768-2. [Google Scholar]
- Gallucci, F.; Medrano, J.A.; Fernandez, E.; Melendez, J.; Van Sint Annaland, M.; Pacheco-Tanaka, D.A. Advances on high temperature Pd-based membranes and membrane reactors for hydrogen purification and production. J. Membr. Sci. Res. 2017, 3, 142–156. [Google Scholar]
- Makertihartha, I.G.B.N.; Zunita, M.; Rizki, Z.; Dharmawijaya, P.T. Advances of zeolite based membrane for hydrogen production via water gas shift reaction. J. Phys. Conf. Ser. 2017, 877, 012076. [Google Scholar] [CrossRef]
- Bakonyi, P.; Nemestóthy, N.; Simon, V.; Bélafi-Bakó, K. Fermentative hydrogen production in anaerobic membrane bioreactors: A review. Bioresour. Technol. 2014, 156, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Tsydenov, D.E.; Parmon, V.N.; Vorontsov, A.V. Toward the design of asymmetric photocatalytic membranes for hydrogen production: Preparation of TiO2-based membranes and their properties. Int. J. Hydrogen Energy 2012, 37, 11046–11060. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. Sci. Eng. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of supported pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yip, A.C.K. A review on the production and purification of biomass-derived hydrogen using emerging membrane technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Petriev, I.S.; Baryshev, M.G.; Yaroslavtsev, A.B. Methanol steam reforming over ZrO2-supported catalysts in conventional and membrane reactors. Petrol. Chem. 2017, 57, 1219–1227. [Google Scholar] [CrossRef]
- Saidi, M. Performance assessment and evaluation of catalytic membrane reactor for pure hydrogen production via steam reforming of methanol. Int. J. Hydrogen Energy 2017, 42, 16170–16185. [Google Scholar] [CrossRef]
- Liguori, S.; Iulianelli, A.; Dalena, F.; Piemonte, V.; Huang, Y.; Basile, A. Methanol steam reforming in an Al2O3 supported thin Pd-layer membrane reactor over Cu/ZnO/Al2O3 catalyst. Int. J. Hydrogen Energy 2014, 39, 18702–18710. [Google Scholar] [CrossRef]
- Basile, A.; Tosti, S.; Capannelli, G.; Vitulli, G.; Iulianelli, A.; Gallucci, F.; Drioli, E. Co-current and counter-current modes for methanol steam reforming membrane reactor: Experimental study. Catal. Today 2006, 118, 237–245. [Google Scholar] [CrossRef]
- Lin, Y.M.; Rei, M.H. Study on hydrogen production from methanol steam reforming in supported palladium membrane reactor. Catal. Today 2001, 67, 77–84. [Google Scholar] [CrossRef]
- Israni, S.; Harold, M.P. Methanol steam reforming in single-fiber bed Pd-Ag membrane reactor: Experiments and modeling. J. Membr. Sci. 2011, 369, 375–387. [Google Scholar] [CrossRef]
- Rei, M.H.; Yeh, G.T.; Tsai, Y.H.; Kao, Y.L.; Shiau, L.D. Catalysis-spillover-membrane. III: The effect of hydrogen spillover on the palladium membrane reactor in the steam reforming reactions. J. Membr. Sci. 2011, 369, 299–307. [Google Scholar] [CrossRef]
- Lin, Y.M.; Lee, G.L.; Rei, M.H. An integrated purification and production of hydrogen with a palladium membrane-catalytic reactor. Catal. Today 1998, 44, 343–349. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Basile, A. Methanol steam reforming in a dense Pd-Ag membrane reactor: The pressure and WHSV effects on CO-free H2 production. J. Membr. Sci. 2008, 323, 235–240. [Google Scholar] [CrossRef]
- Itoh, N.; Kaneko, Y.; Igarashi, A. Efficient hydrogen production via methanol steam reforming by preventing back-permeation of hydrogen in a palladium membrane reactor. Ind. Eng. Chem. Res. 2002, 41, 4702–4706. [Google Scholar] [CrossRef]
- Wieland, S.; Melin, T.; Lamm, A. Membrane reactors for hydrogen production. Chem. Eng. Sci. 2002, 57, 1571–1576. [Google Scholar] [CrossRef]
- Sà, S.; Sousa, J.M.; Mendes, A. Steam reforming of methanol over a CuO/ZnO/Al2O3 catalyst part II: A carbon membrane reactor. Chem. Eng. Sci. 2011, 66, 5523–5530. [Google Scholar] [CrossRef]
- Briceño, K.; Iulianelli, A.; Montanè, D.; Garcia-Valls, R.; Basile, A. Carbon molecular sieve membranes supported on non-modified ceramic tubes for hydrogen separation in membrane reactors. Int. J. Hydrogen Energy 2012, 37, 13536–13544. [Google Scholar] [CrossRef]
- Briceño, K.; Montané, D.; Garcia-Valls, R.; Iulianelli, A.; Basile, A. Fabrication variables affecting the structure and properties of supported carbon molecular sieve membranes for hydrogen separation. J. Membr. Sci. 2012, 415–416, 288–297. [Google Scholar]
- Zhang, X.; Hu, H.; Zhu, Y.; Zhu, S. Methanol steam reforming to hydrogen in a carbon membrane reactor system. Ind. Eng. Chem. Res. 2006, 45, 7997–8001. [Google Scholar] [CrossRef]
- Lee, D.W.; Nam, S.E.; Sea, B.; Ihm, S.K.; Lee, K.H. Preparation of Pt-loaded hydrogen selective membranes for methanol reforming. Catal. Today 2006, 118, 198–204. [Google Scholar] [CrossRef]
- Lee, D.W.; Park, S.J.; Yu, C.Y.; Ihm, S.K.; Lee, K.H. Study on methanol reforming-inorganic membrane reactors combined with water-gas shift reaction and relationship between membrane performance and methanol conversion. J. Membr. Sci. 2008, 316, 63–72. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Bellardita, M.; Palmisano, L. Photocatalytic Processes in Membrane Reactors. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; Volume 3, pp. 101–138. [Google Scholar]
- Sellaro, M.; Bellardita, M.; Brunetti, A.; Fontananova, E.; Palmisano, L.; Drioli, E.; Barbieri, G. CO2 conversion in a photocatalytic continuous membrane reactor. RSC Adv. 2016, 6, 67418–67427. [Google Scholar] [CrossRef]
- Pathak, P.; Meziani, M.J.; Li, Y.; Cureton, L.T.; Sun, Y.P. Improving photoreduction of CO2 with homogeneously dispersed nanoscale TiO2. Chem. Commun. 2014, 10, 1234–1235. [Google Scholar] [CrossRef]
- Pomilla, F.R.; Brunetti, A.; Marcì, G.; García-López, E.I.; Fontananova, E.; Palmisano, L.; Barbieri, G. CO2 to liquid fuels: Photocatalytic conversion in a continuous membrane reactor. ACS Sustain. Chem. Eng. 2018, 6, 8743–8753. [Google Scholar] [CrossRef]
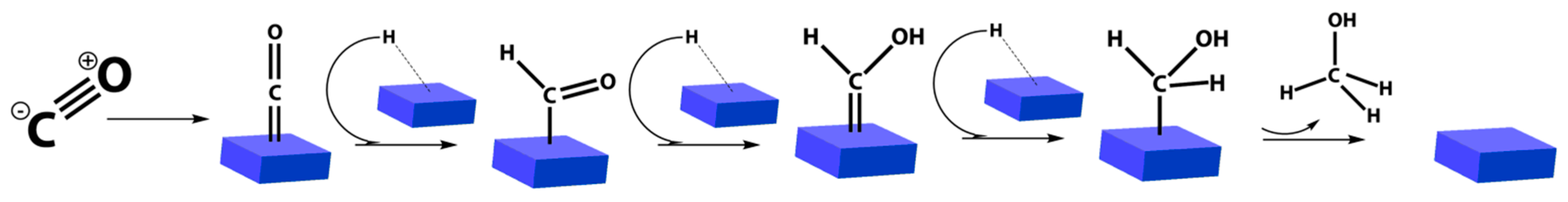
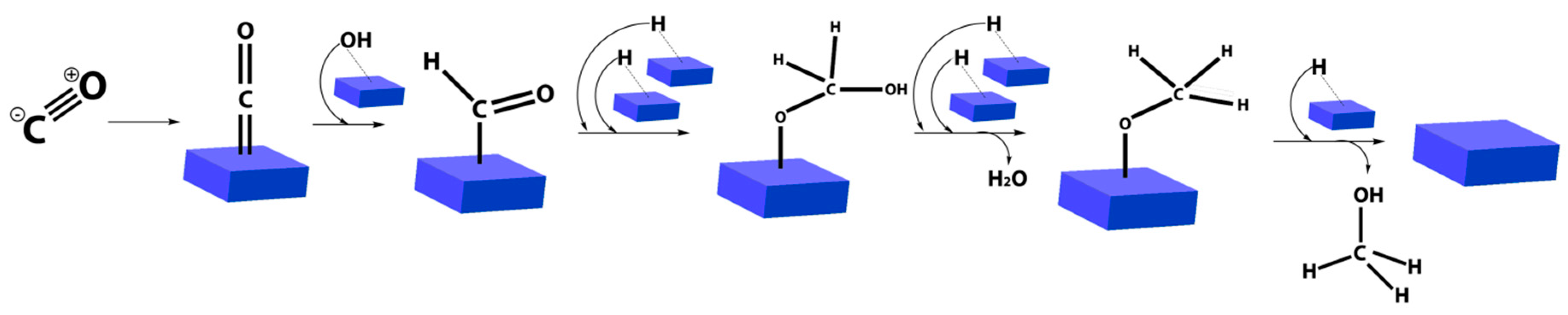
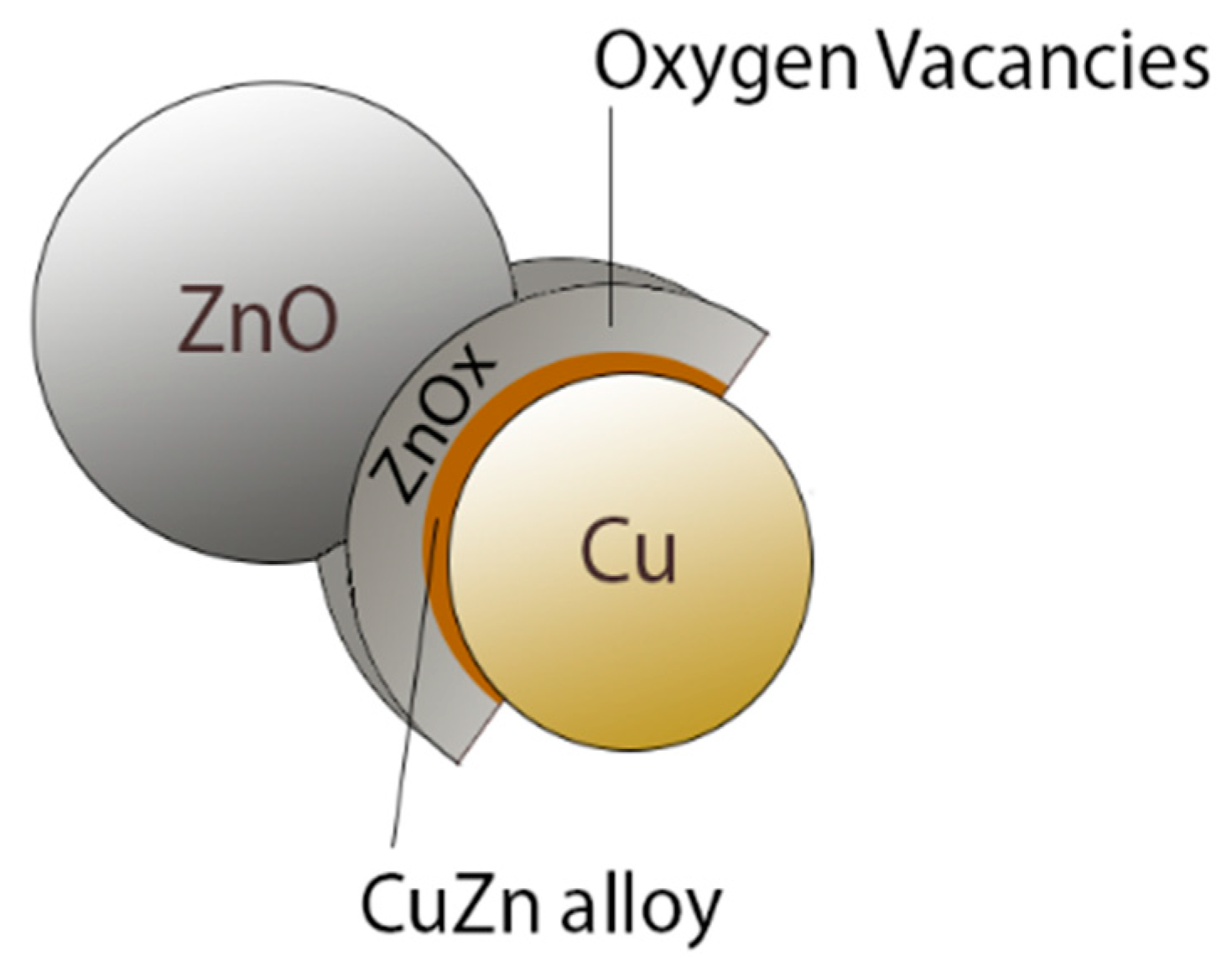
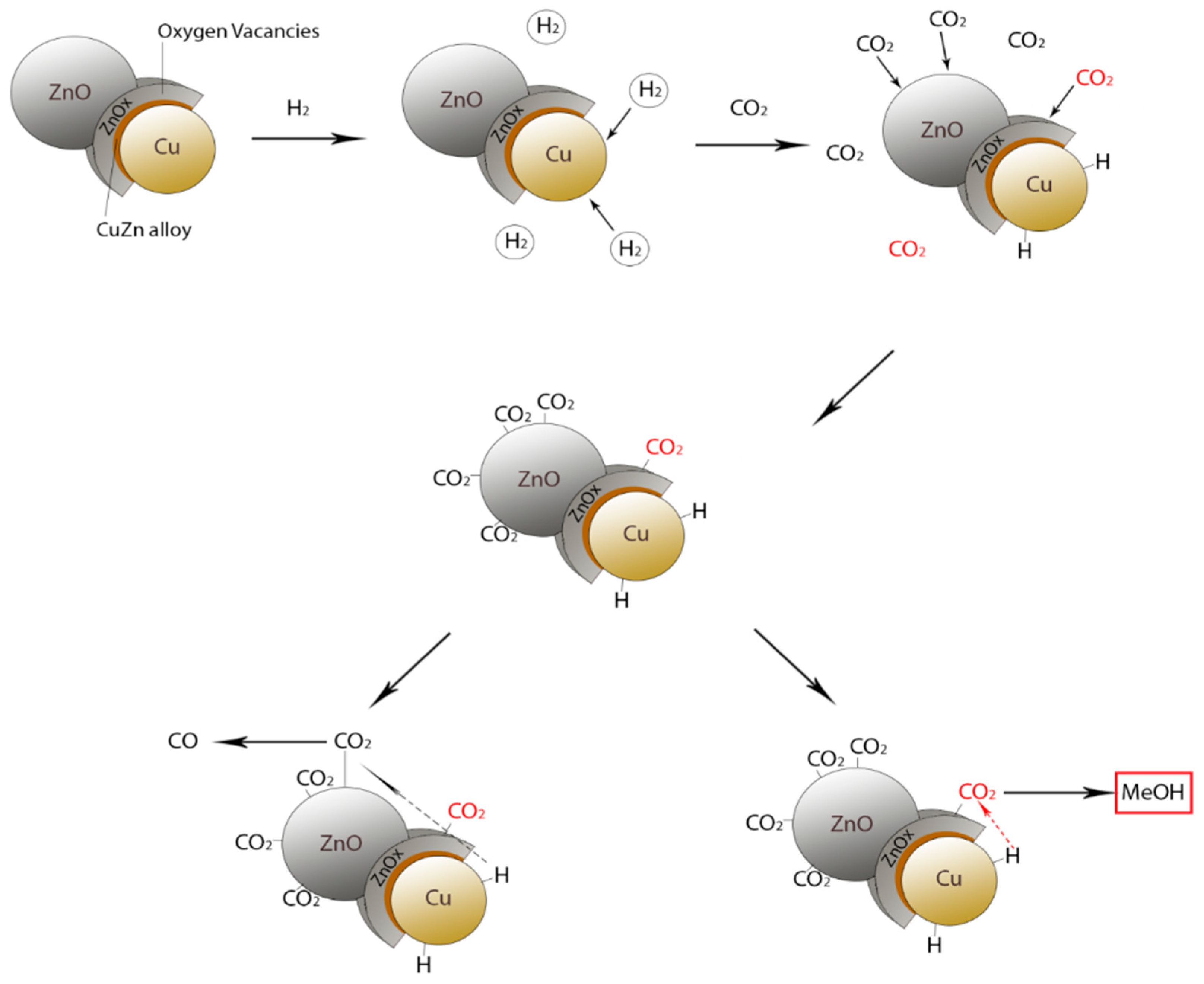
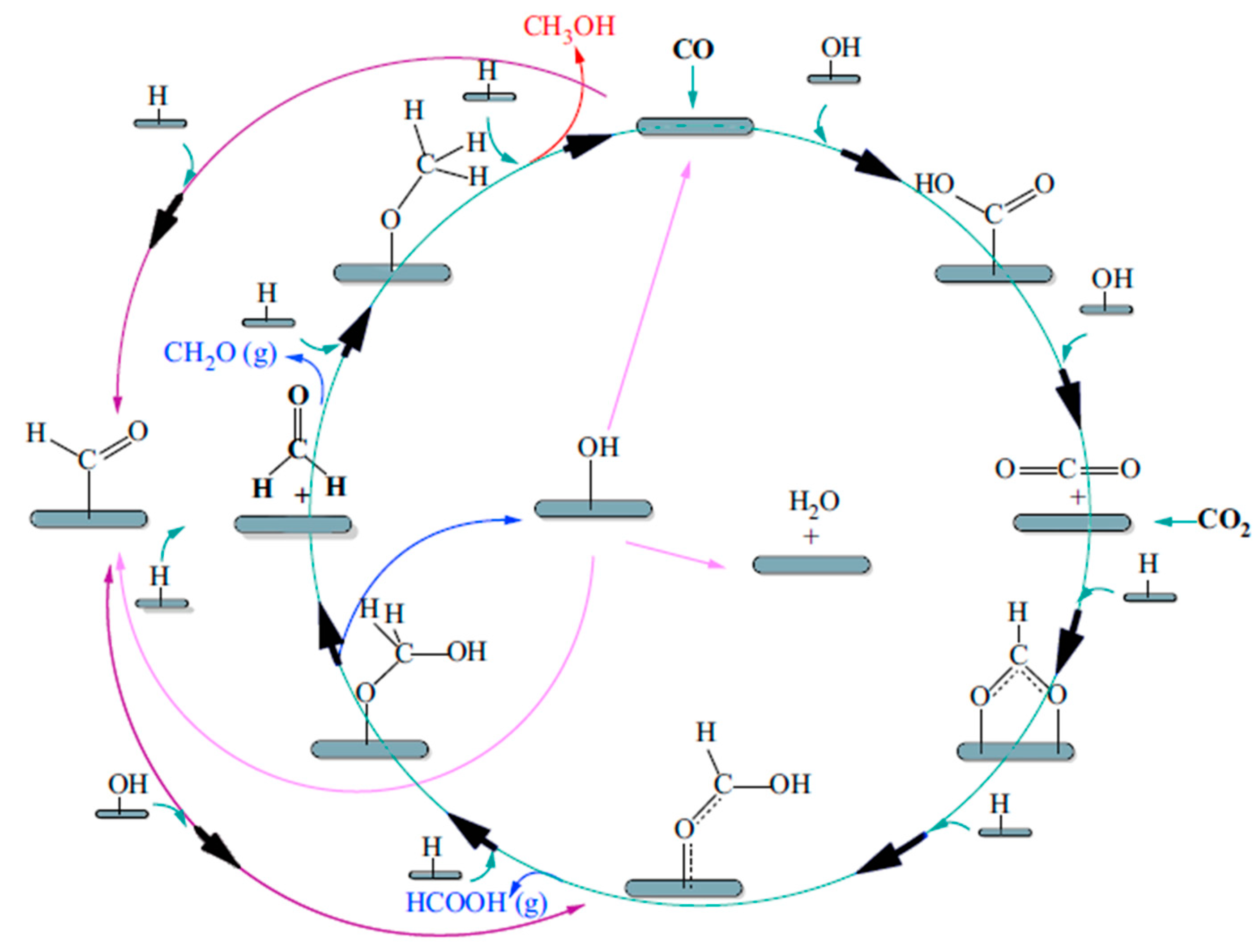
| Long-chain alcohols | nCO + 2nH2 = CnH2n+1OH + (n − 1)H2O |
| Aldehydes Ketons | RCH2CH2OH = RCH2CHO + H2 |
| 2RCH2CHO = RCH2COCHRCH3 + Oads | |
| Hydrocarbons | CO + 3H2 = CH4 + H2O |
| CO2 + 4H2 = CH4 + 2H2O | |
| nCO + (2n − 1)H2 = CnH2n+2 + nH2O | |
| Dimethyl ether | 2CO + 4H2 = CH3OCH3 + H2O |
| Productive Process | Feedstock | CH3OH Yield (t/Day) |
|---|---|---|
| BASF | Syngas | 7.9 × 10−2 |
| Dupont | Syngas | 1.1·× 10−1 |
| Haldor Topsoe | Syngas | 2.4·× 103 |
| ICI | Carbonaceous | 2.5·× 103 |
| Membranes | Membrane Preparation | Metallic Layer (μm) | Catalyst | H2O/CH3OH | T (°C) | p (bar) | Conv. (%) | H2 Recovery (%) | H2 Purity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Pd-Ag, Pd-Ru | Magnetron sputtering | 60, 12 | Ru/Rh/ZrO2 | 1/1 | 300 | - | - | 38, 18 | ≈100 | [90] |
| Pd-Ag | - | 6 | Cu/ZnO/Al2O3 | 1/1 | 300 | 2 | 98 | 64 | - | [91] |
| Pd/Al2O3 | ELP | 7 | CuO/ZnO/Al2O3 | 2.5/1 | 330 | 2.5 | 85 | >40 | ≈100 | [92] |
| Pd-Ag/TiO2-Al2O3 | ELP | - | Ru-Al2O3 | 4.5/1 | 550 | 1.3 | 65 | - | ≈72 | [93] |
| Pd/PSS | ELP | 20 | Cu/ZnO/Al2O3 | 1.2/1 | 350 | 6 | ≈95 | 97 | 99.9 | [94] |
| Pd-Ag/α-Al2O3 | ELP | ~4 | CuO/ZnO/Al2O3 | 1/1 | 250 | 3 10 | 100 | 45 95 | ≈100 | [95] |
| Pd-Ag/PSS | ELP | 20–25 | CuO/ZnO/Al2O3 | 1.2/1 | 240 | 10 | 36.1 | 18 | - | [96] |
| Pd/PSS | ELP | ~20–25 | Cu-based | 1.2/1 | 350 | - | 99 | - | ≈100 | [97] |
| Pd-Ag | Cold-rolling | 50 | CuO/Al2O3/ZnO MgO | 3/1 | 300 | 3 | - | 80 | ≈100 | [98] |
| Pd-Ru-In | - | 200 | Cu/ZnO/Al2O3 | 1.2/1 | 200 | 7 | ≈90 | ≈24 | ≈100 | [99] |
| Pd-Cu | - | 25 | Cu-Zn based | - | 300 | 10 | >90 | ≈38 | ≈100 | [100] |
| Carbon molecular sieve | Pyrolysis | - | CuO/ZnO/Al2O3 | 4/1 | 200 | 1 | ≈95 | ≈84 | - | [101] |
| Carbon supported | Pyrolysis | - | CuO/Al2O3/ZnO MgO | 3/1 | 250 | 2 | 55 | - | ≈80 | [102,103] |
| Carbon supported | - | - | Cu/ZnO/Al2O3 | 1.5/1 | 250 | 2 | ≈99 | - | 97 | [104] |
| SiO2/γ-Al2O3/Pt-SiO2/PSS | Soaking-rolling | - | Cu-Zn based | 1.3/1 | 230 | - | 100 | ~10 | - | [105] |
| SiO2/γ-Al2O3 | Soaking-rolling | - | Cu-Zn based | 3/1 | 260 | - | 42 | 5 | 98 | [106] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalena, F.; Senatore, A.; Basile, M.; Knani, S.; Basile, A.; Iulianelli, A. Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes 2018, 8, 98. https://doi.org/10.3390/membranes8040098
Dalena F, Senatore A, Basile M, Knani S, Basile A, Iulianelli A. Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes. 2018; 8(4):98. https://doi.org/10.3390/membranes8040098
Chicago/Turabian StyleDalena, Francesco, Alessandro Senatore, Marco Basile, Sarra Knani, Angelo Basile, and Adolfo Iulianelli. 2018. "Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology" Membranes 8, no. 4: 98. https://doi.org/10.3390/membranes8040098
APA StyleDalena, F., Senatore, A., Basile, M., Knani, S., Basile, A., & Iulianelli, A. (2018). Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes, 8(4), 98. https://doi.org/10.3390/membranes8040098