Mass Transfer Coefficient in Multi-Stage Reformer/Membrane Modules for Hydrogen Production
Abstract
:1. Introduction
- The MR is mechanically complex and presents a large and unpractical heat transfer surface—in the MR, the concentric tube geometry yields an imbalance between the surfaces required for heat transfer (outer tube) and the available surface for mass transfer (of the inner membrane tube);
- RMM enables the de-coupling of separation and reaction operating temperatures, increasing the stability and the durability of the membranes and enabling independent optimization of the reforming temperature;
- RMM simplifies the mechanical design of membrane tubes compared with the one embedded in a catalyst tube, and a simple shell and tube geometry can be selected for the tubular separation module;
- RMM simplifies maintenance of the Pd/Ag membrane modules and catalyst replacement.
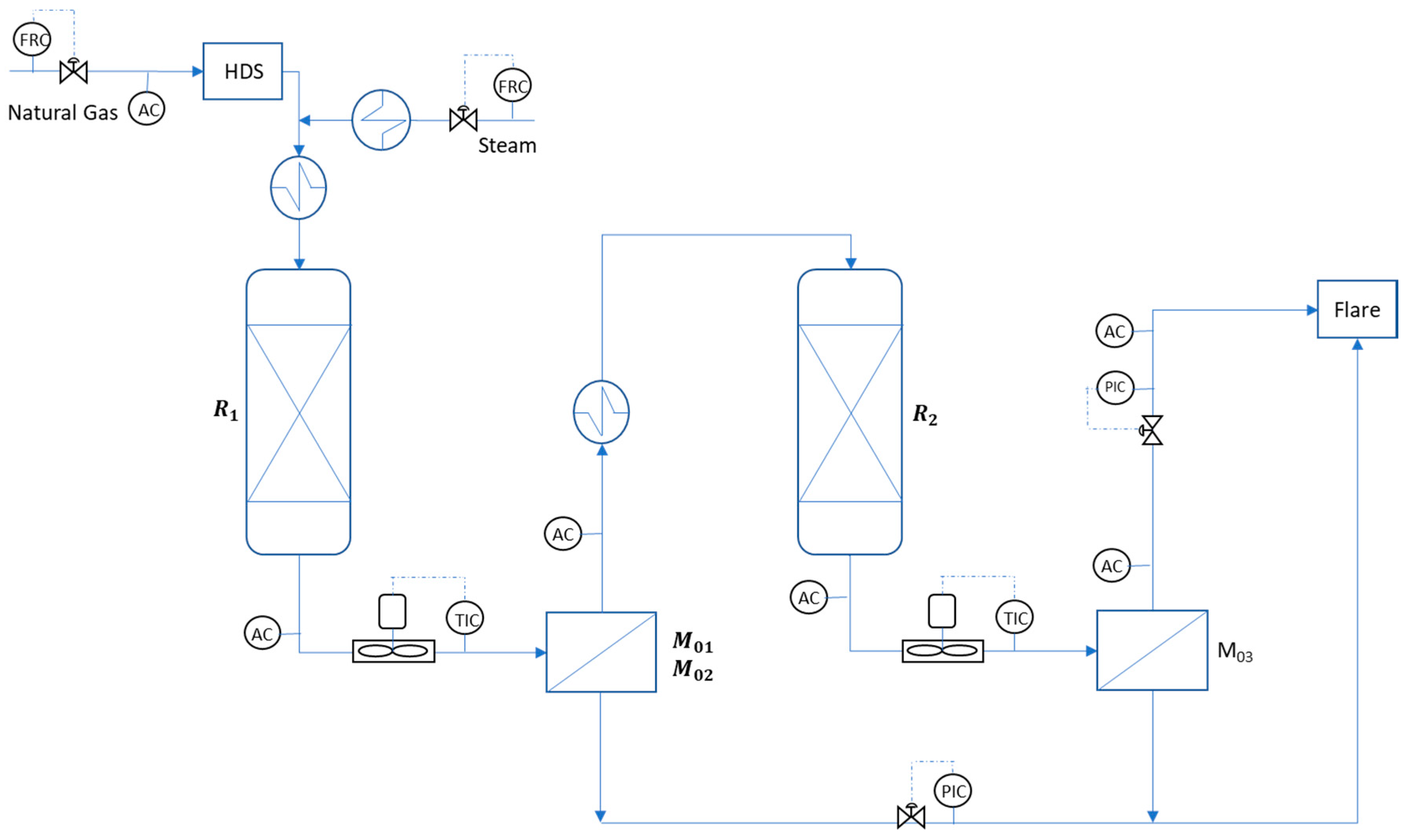
2. Materials and Methods
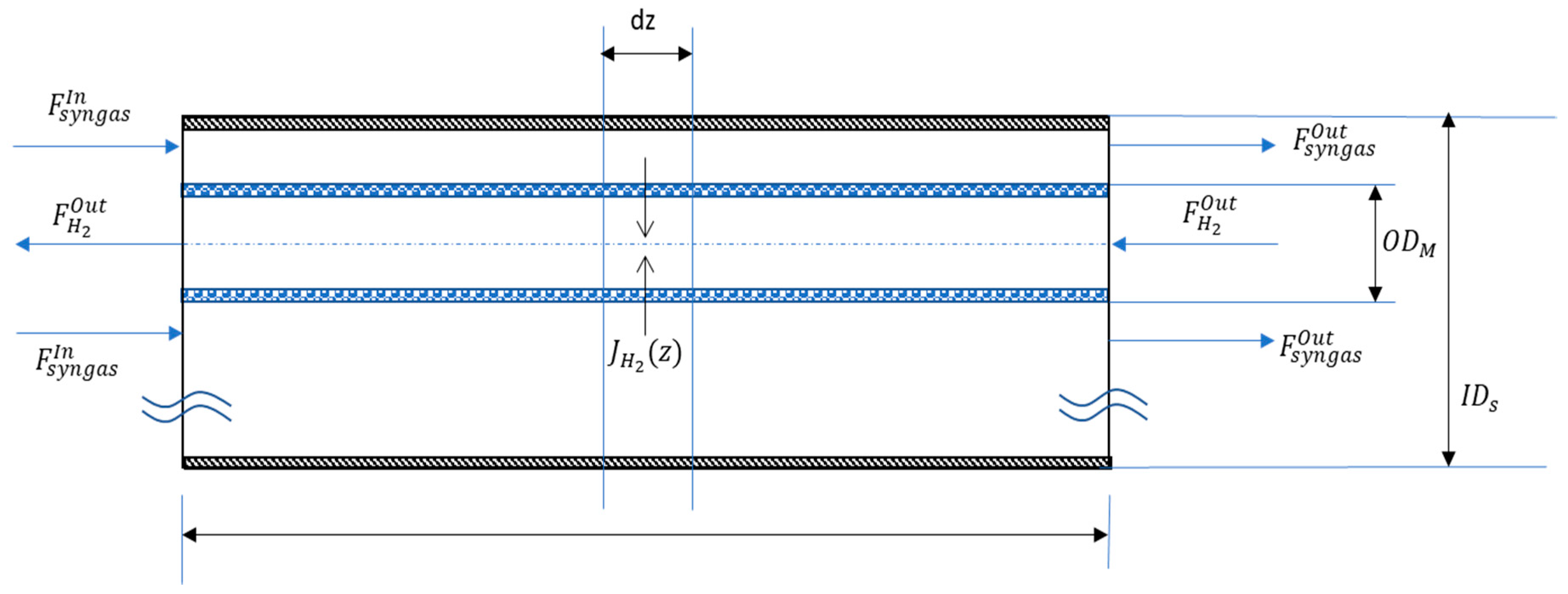
3. Mathematical Modelling
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| shell area of membranes (m2); | |
| total membrane surface (m2); | |
| total concentration (kmol·m−3); | |
| diffusion pre-exponential factor (m2·s−1); | |
| diffusivity of hydrogen in the mixture retentate side (m2·s−1); | |
| equivalent diameter of membranes (m); | |
| channel depth (m); | |
| activation energy for hydrogen permeation through metallic membranes (J·mol−1); | |
| activation energy for the diffusion of hydrogen atoms (J·mol−1); | |
| molar flow rate (kmol·h−1); | |
| mass transfer coefficient (kmol·h−1·m−2); | |
| overall mass transfer coefficient (kmol·h−1·m−2); | |
| hydrogen recovery factor; | |
| internal shell diameter (m); | |
| hydrogen flux (kmol·h−1·m−2); | |
| average hydrogen flux (kmol·h−1·m−2); | |
| mass transfer dimensional group; | |
| hydrogen permeance (kmol·h−1·m−1·bar−0.5); | |
| average hydrogen permeance (kmol·h−1·m−2·bar−0.5); | |
| membrane length (m); | |
| lower heating value (kJ/Nm3); | |
| natural gas (kg/h); | |
| number of membranes; | |
| outside tube diameter (m); | |
| outside membrane diameter (m); | |
| hydrogen permeability (kmol·h−1·m−1·bar−0.5); | |
| permeability pre-exponential factor (kmol·h−1·m−1·bar−0.5); | |
| tube pitch (m); | |
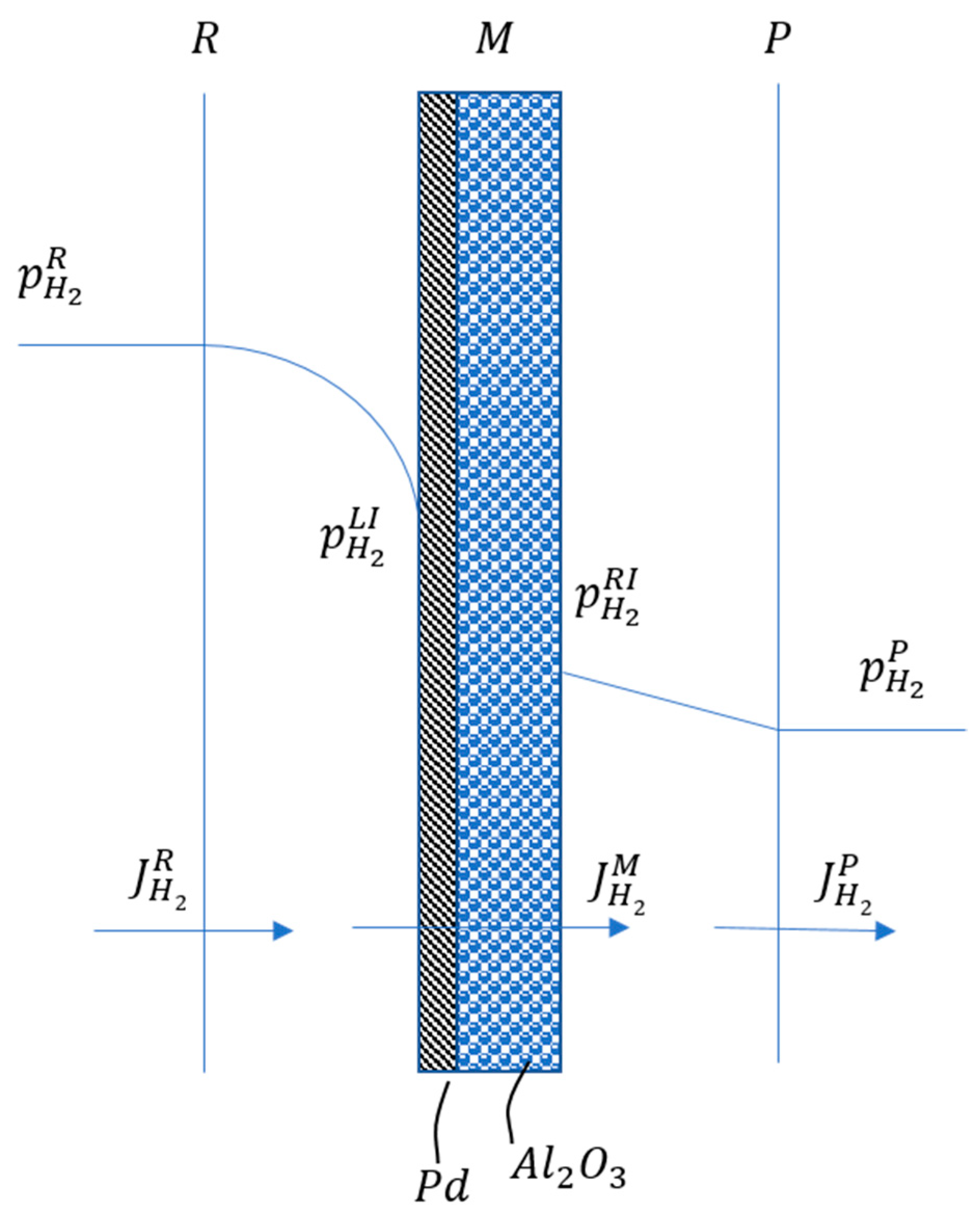
| hydrogen partial pressure on the right/left interface (bar); | |
| membrane internal radius (m); | |
| average Reynolds number; | |
| average velocity of the mixture, retentate side (m·s−1); | |
| average Schmidt number; | |
| channel width (m); | |
| hydrogen molar fraction on the retentate/lefth interface of i-esimo step; | |
| Apices and Subscripts | |
| H2O, CO, CO2, CH4; | |
| LI | relative to the left-interface in the film theory; |
| m | relative to the mixture; |
| cp | concentration polarization; |
| ML | logarithm mean; |
| relative to the permeate; | |
| relative to the retentate-side; | |
| relative to concentration polarization; | |
| M | relative to membrane; |
| relative to the right-interface in the film theory; | |
| relative to the shell-side; | |
| t | tube-side; |
| Greekletter | |
| δ | membrane thickness, (m); |
| viscosity of mixture, retentate side (Pa·s); | |
| density of mixture, retentate side (kg·m−3); | |
| standard enthalpy of the surface dissociation reaction (J·mol−1·K−1); | |
| entropy change of the dissociation reaction (J·mol−1); | |
References
- US Department of Energy. Available online: http://hydrogen.pnl.gov/hydrogen-data/hydrogen-production (accessed on 20 March 2018).
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- De Falco, M.; Piemonte, V. Solar enriched methane production by steam reforming process: Reactor design. Int. J. Hydrog. Energy 2011, 36, 7759–7762. [Google Scholar] [CrossRef]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen production with CO2capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbas, S.A. Decarbonization of fossil fuels as a strategy to control global warming. Renew. Sustain. Energy Rev. 2011, 15, 1828–1834. [Google Scholar] [CrossRef]
- Liguori, S.; Iulianelli, A.; Dalena, F.; Piemonte, V.; Huang, Y.; Basile, A. Methanol steam reforming in an Al2O3supported thin Pd-layer membrane reactor over Cu/ZnO/Al2O3catalyst. Int. J. Hydrogen Energy 2014, 39, 18702–18710. [Google Scholar] [CrossRef]
- Capocelli, M.; De Falco, M. Enriched methane: A ready solution for the transition towards the hydrogen economy. In Enriched Methane: The First Step Towards the Hydrogen Economy; de Falco, M., Basile, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–21. [Google Scholar]
- Erickson, W.; Tang, P.A. Vernon Reformation of hydrocarbon fuels. In Handbook of Hydrogenenergy; CRC Press, Taylor & Francis Group: Boca Rato, FL, USA, 2014. [Google Scholar]
- McDowall, W.; Eames, M. Forecasts, scenarios, visions, backcasts and roadmaps to thehydrogen economy: A review of the hydrogen futures literature. Energy Policy 2006, 34, 1236–1250. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen Storage Methods; Springer-Verlag: Berlin, Germany, 2004. [Google Scholar]
- Electric Vehicles Powered by Fuel-Cells Get a Second Look. Available online: https://www.economist.com/science-and-technology/2017/09/25/electric-vehicles-powered-by-fuel-cells-get-a-second-look (accessed on 11 March 2018).
- Tushar, K.G.; Mark, A.P. Energy Resources and Systems: Volume 2: Renewable Resources; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Murmura, M.A.; Cerbelli, S.; Annesini, M.C. An equilibrium theory for catalytic steam reforming in membrane reactors. Chem. Eng. Sci. 2017, 160, 291–303. [Google Scholar] [CrossRef]
- Murmura, M.A.; Cerbelli, S.; Annesini, M.C. Transport-reaction-permeation regimes in catalytic membrane reactorsfor hydrogen production. The steam reforming of methane as a casestudy. Chem. Eng. Sci. 2017, 162, 88–103. [Google Scholar] [CrossRef]
- Abir, H.; Sheintuch, M. Modeling H2 transport through a Pd or Pd/Ag membrane, and itsinhibition by co-adsorbates, from first principles. J. Membr. Sci. 2014, 466, 58–69. [Google Scholar] [CrossRef]
- Barba, D.; Giacobbe, F.; de Cesaris, A.; Farace, A.; Iaquaniello, G.; Pipino, A. Membrane reforming in converting naturalgas to hydrogen (part one). Int. J. Hydrogen Energy 2008, 33, 3700–3709. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Giacobbe, F.; Morico, B.; Cosenza, S.; Farace, A. Membrane reforming in converting natural gas to hydrogen: Production costs, Part II. Int. J. Hydrogen Energy 2008, 33, 6595–6601. [Google Scholar] [CrossRef]
- de Falco, M.; Barba, D.; Cosenza, S.; Iaquaniello, G.; Farace, A.; Giacobbe, G. Reformer and membrane modules plant to optimizenatural gas conversion to hydrogen. Asia-Pac. J. Chem. Eng. 2009, 4, 259–269. [Google Scholar] [CrossRef]
- De Falco, M.; Salladini, A.; Iaquaniello, G. Reformer and membrane modules for methane conversion: Experimental assessment and perspectives of an innovative architecture. ChemSusChem 2011, 4, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Iaquaniello, G.; Salladini, A.; Palo, E.; Centi, G. Catalytic partial oxidation coupled with membrane purification to improveresource and energy efficiency in syngas production. ChemSusChem 2015, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Palo, E.; Salladini, A.; Morico, B.; Palma, V.; Ricca, A.; Iaquaniello, G. Application of Pd-Based Membrane Reactors: An Industrial Perspective. Membranes 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- de Falco, M.; Salladini, A.; Palo, E.; Iaquaniello, G. Pd-Alloy Membrane Reactor for Natural Gas Steam Reforming: An Innovative Process Design for the Capture of CO2. Ind. Eng. Chem. Res. 2015, 54, 6950–6958. [Google Scholar] [CrossRef]
- Ward, T.L.; Dao, T. Model of hydrogen permeation behavior in palladium membranes. J. Membr. Sci. 1999, 153, 211–231. [Google Scholar] [CrossRef]
- Caravella, A.; Barbieri, G.; Drioli, E. Concentration polarization analysis in self-supported Pd-based membranes. Sep. Purif. Technol. 2009, 66, 613–624. [Google Scholar] [CrossRef]
- Caravella, A.; Scura, F.; Barbieri, G.; Drioli, E. Sieverts law empirical exponent for PD-based membranes: Critical analysis in pure H2 permeation. J. Phys. Chem. B 2010, 114, 6033–6047. [Google Scholar] [CrossRef] [PubMed]
- Caravella, A.; Scura, F.; Barbieri, G.; Drioli, E. Inhibition by CO and polarization in pd-based membranes: A novel permeation reduction coefficient. J. Phys. Chem. B 2010, 114, 12264–12276. [Google Scholar] [CrossRef] [PubMed]
- Caravella, A.; Hara, S.; Drioli, E.; Barbieri, G. Sieverts law pressure exponent for hydrogen permeation through Pd-based membranes: Coupled influence of non-ideal diffusion and multicomponent external mass transfer. Int. J. Hydrogen Energy 2013, 38, 16229–16244. [Google Scholar] [CrossRef]
- Catalano, J.; Giacinti Baschetti, M.; Sarti, G.C. Influence of the gas phase resistance on hydrogen flux through thin palladium-silver membranes. J. Membr. Sci. 2009, 339, 57–67. [Google Scholar] [CrossRef]
- Catalano, J.; Giacinti Baschetti, M.; Sarti, G.C. Hydrogen permeation in palladium-based membranes in the presence of carbon monoxide. J. Membr. Sci. 2010, 362, 221–233. [Google Scholar] [CrossRef]
- Catalano, J.; Giacinti Baschetti, M.; Sarti, G.C. Influence of water vapor on hydrogen permeation through 2.5 µm Pd-Ag membranes. Int. J. Hydrogen Energy 2011, 36, 8658–8673. [Google Scholar] [CrossRef]
- Pizzi, D.; Worth, R.; Giacinti Baschetti, M.; Sarti, G.C.; Noda, K. Hydrogen permeability of 2.5 μm palladium-silver membranes deposited on ceramic supports. J. Membr. Sci. 2008, 325, 446–453. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Wang, D. Exponents for the pressure dependence of hydrogen permeation through Pd and Pd-Ag alloy membranes. J. Phys. Chem. C 2010, 114, 14482–14488. [Google Scholar] [CrossRef]
- Barbieri, G.; Scura, F.; Lentini, F.; De Luca, G.; Drioli, E. A novel model equation for the permeation of hydrogen in mixture with carbon monoxide through Pd-Ag membranes. Sep. Purif. Technol. 2008, 61, 217–224. [Google Scholar] [CrossRef]
- Holleck, G.L. Diffusion and Solubility of Hydrogen in Palladium and Palladium-Silver Alloys. J. Phys. Chem. 1970, 74, 503–511. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations, 3rd ed.; McGraw-Hill Chemical Engineering Series; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]

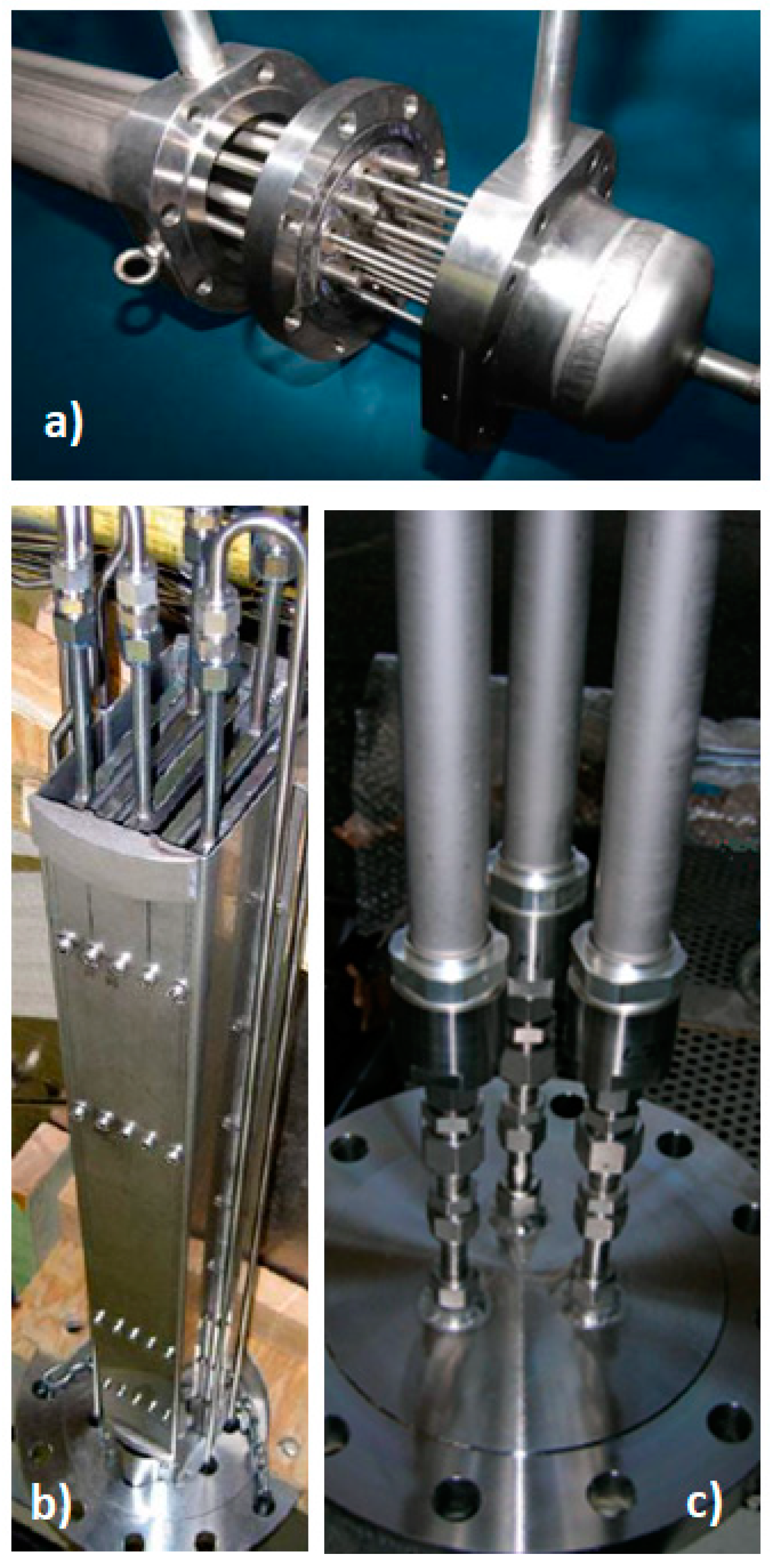
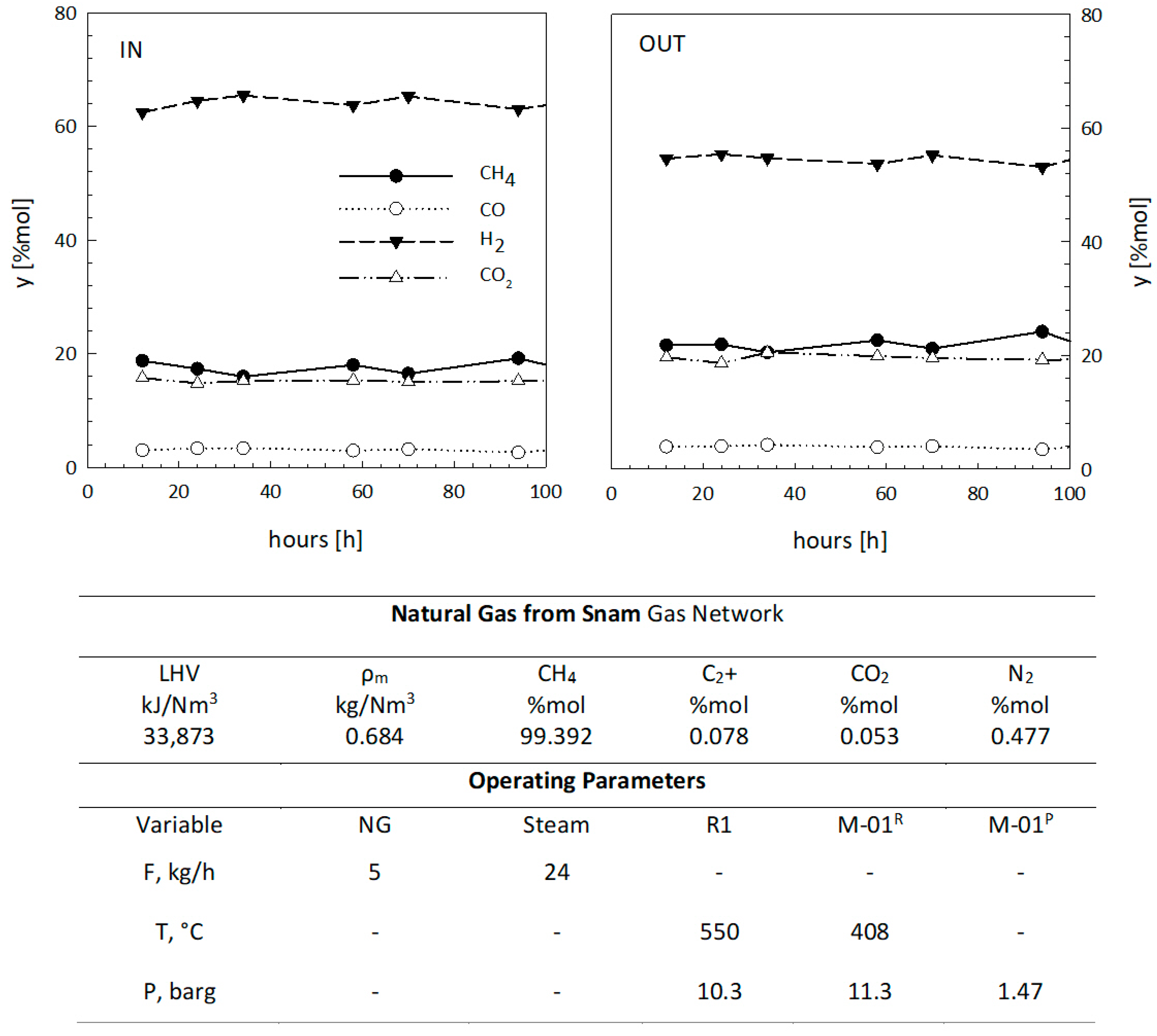
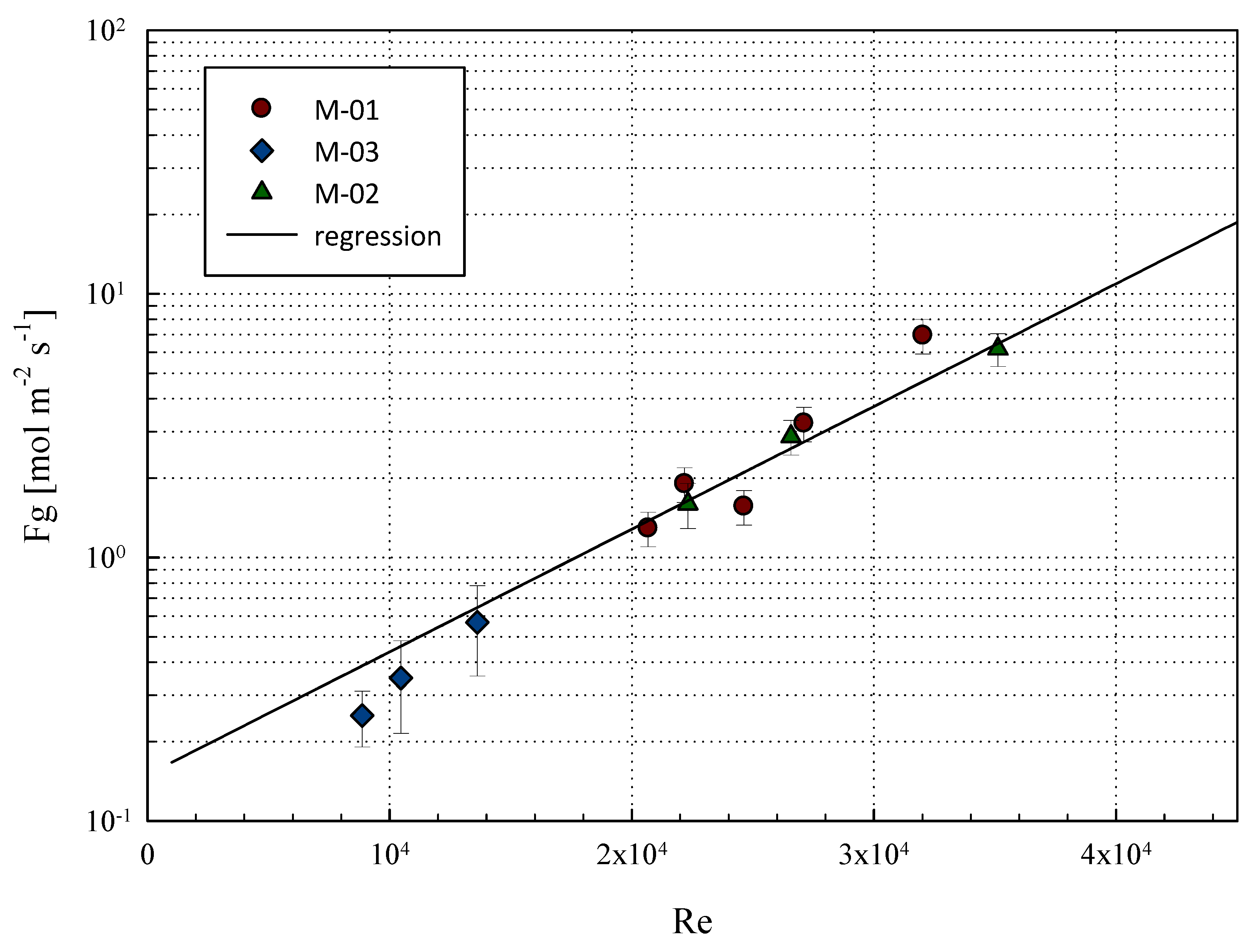
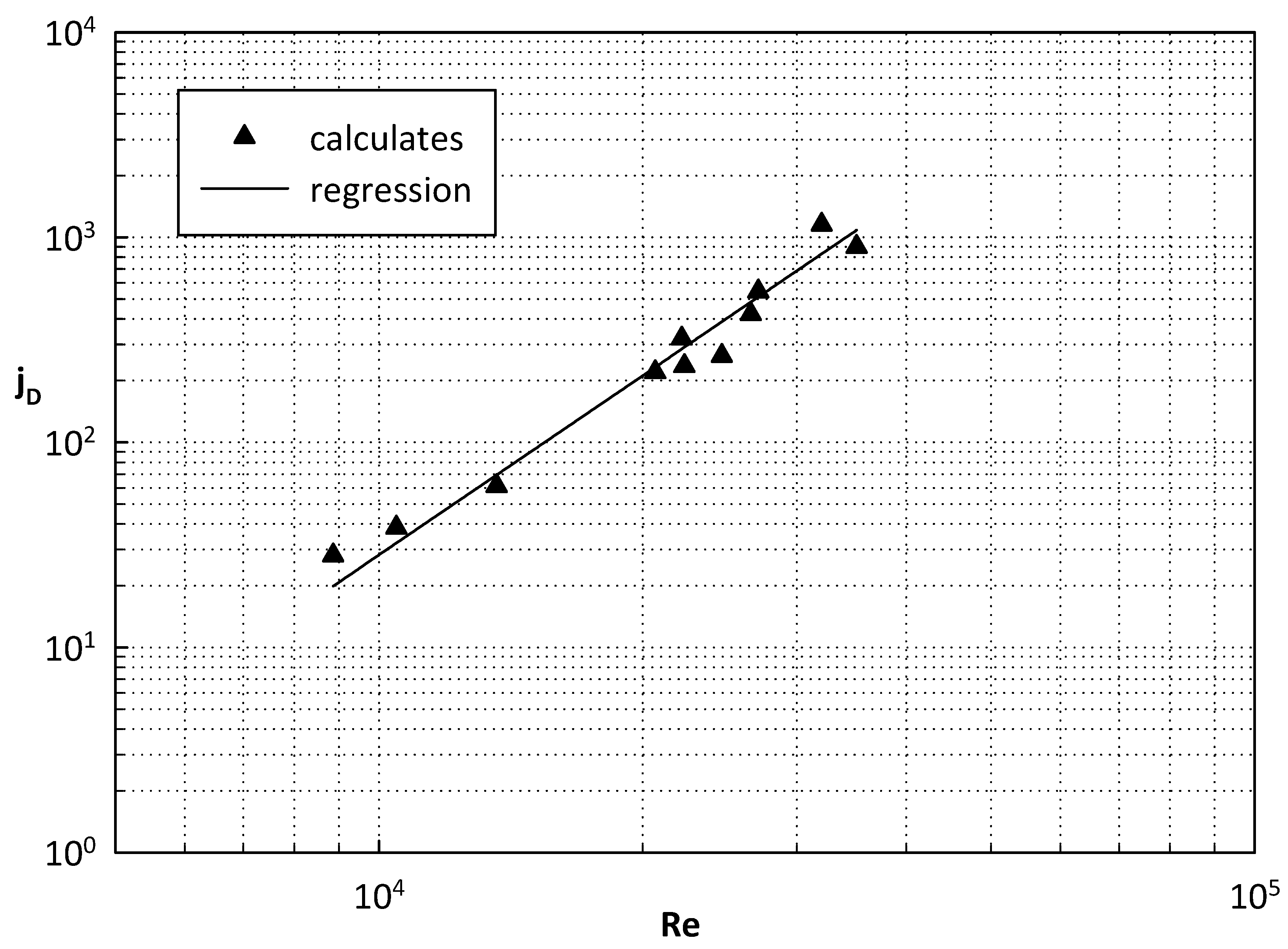
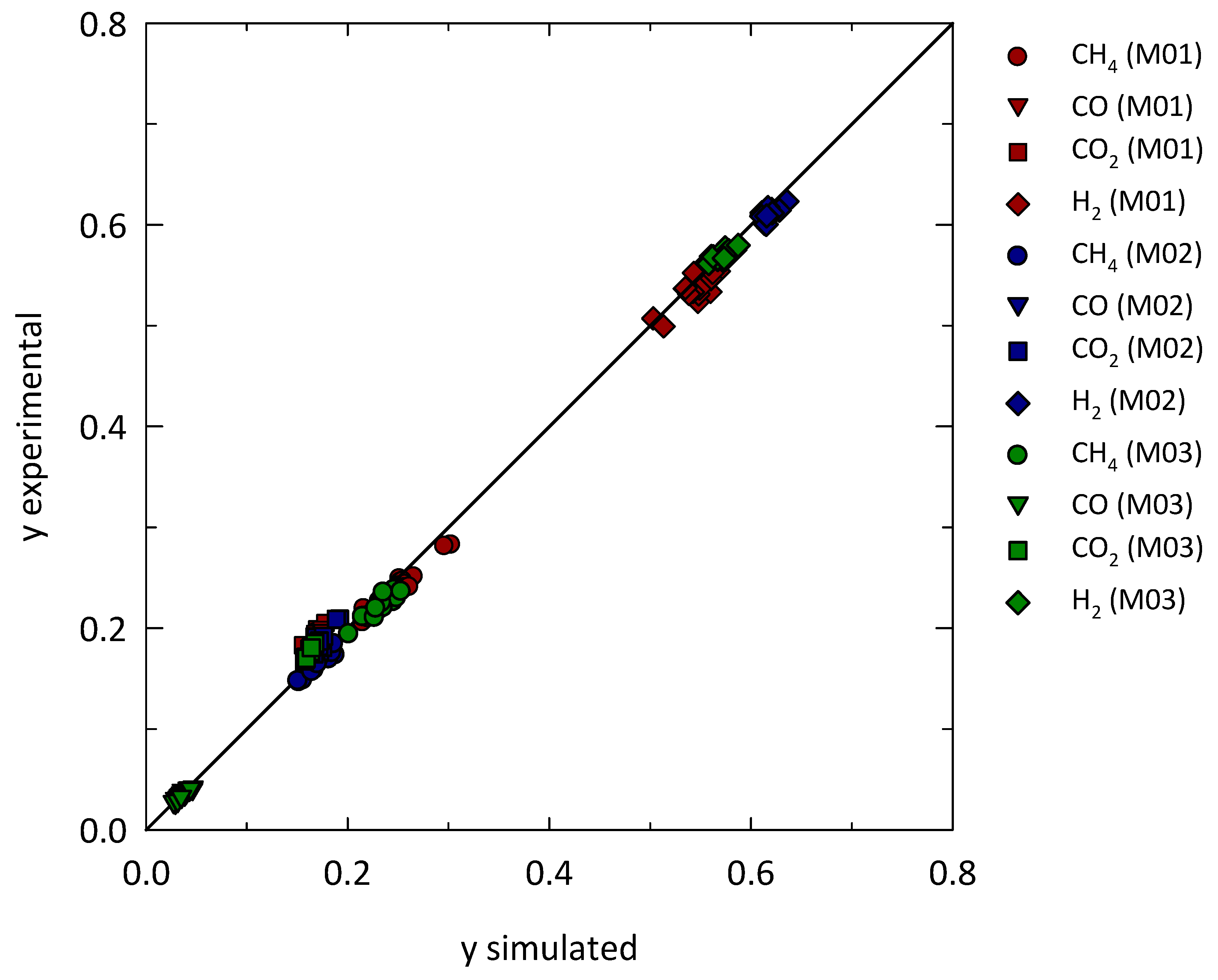
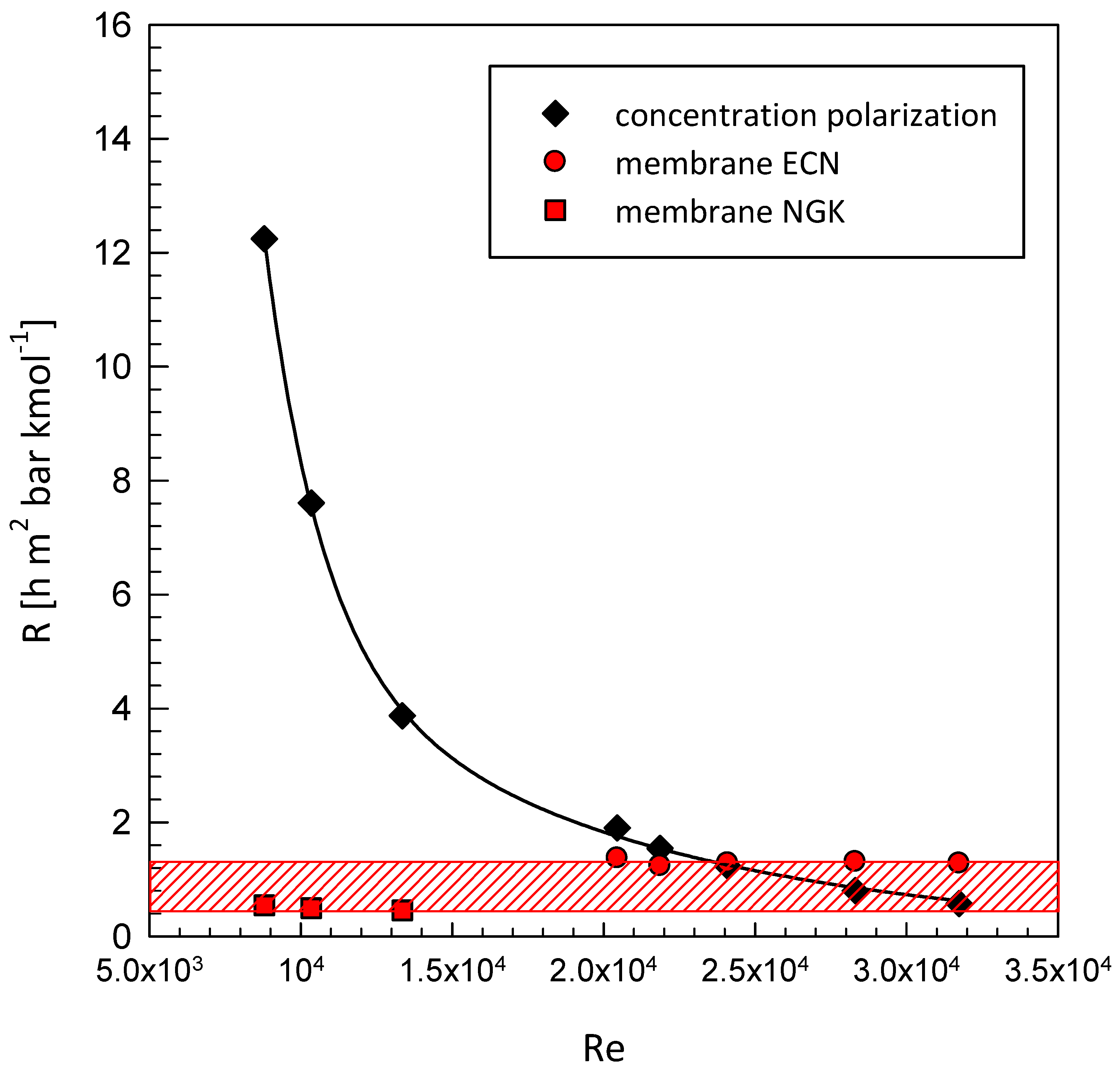
| Geometrical Features | Membrane Modules | ||
|---|---|---|---|
| M-01 | M-02 | M-03 | |
| IDS, in | 5 | 6 | 6 |
| Nm | 13 | 5 | 3 |
| ODt, mm | 14 | - | 30 |
| δ, μm | 2.5 | 25 | 2.5 |
| L, cm | 69 | 30 × 2 | 45 |
| AToT, m2 | 0.4 | 0.6 | 0.13 |
| T, °C | 408–438 | 402–424 | 397–455 |
| PR, bar | 11–11.5 | 11.5 | 11 |
| PP, bar | 1.4–1.6 | 1.4 | 1.3 |
| W, kg·h−1 | 29–46.4 | 29–46.4 | 29–46.4 |
| F, kmol·h−1 | 1.9–3.1 | 1.9–3.1 | 1.9–3.1 |
| kJ·mol−1 | 20.2 | 17.8 | 17.8 |
| , kmol·h−1·m−2·bar−0.5 | 1.69 × 10−4 | 2.67 × 10−4 | 2.67 × 10−4 |
| Variables | Tubular Membrane (M-01, M-02) | Flat Plat Module (M-03) |
|---|---|---|
| v, m/s | ||
| , m2 | ||
| , m | ||
| Parameter | Unit | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | ||
| F | kmol·h−1 | 1.94 | 1.74 | 2.10 | 1.91 | 2.30 | 2.12 | 2.57 | 2.34 | 3.06 | 2.78 |
| H2O | mol % | 56 | 60 | 54 | 58 | 57 | 61 | 57 | 62 | 57 | 62 |
| CO | mol % | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| CO2 | mol % | 6 | 7 | 6 | 7 | 6 | 7 | 6 | 7 | 6 | 7 |
| CH4 | mol % | 8 | 9 | 11 | 13 | 8 | 9 | 9 | 10 | 9 | 10 |
| H2 | mol % | 29 | 22 | 28 | 21 | 28 | 22 | 27 | 20 | 27 | 20 |
| HRF | % | 32 | 32 | 28 | 32 | 33 | |||||
| kmol·h−1·m−2 | 0.471 | 0.480 | 0.491 | 0.513 | 0.712 | ||||||
| kmol·h−1·m−2·bar0.5 | 1.92 | 2.13 | 2.01 | 2.10 | 2.16 | ||||||
| Parameter | Unit | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|---|
| IN | OUT | IN | OUT | IN | OUT | ||
| F | kmol·h−1 | 1.94 | 1.81 | 2.31 | 2.15 | 3.06 | 2.89 |
| H2O | mol % | 56 | 58 | 56 | 60 | 57 | 60 |
| CO | mol % | 1 | 2 | 1 | 1 | 1 | 1 |
| CO2 | mol % | 6 | 7 | 6 | 7 | 6 | 6 |
| CH4 | mol % | 8 | 9 | 9 | 9 | 9 | 10 |
| H2 | mol % | 29 | 24 | 28 | 23 | 27 | 23 |
| HRF | % | 23 | 24 | 20 | |||
| kmol·h−1·m−2 | 0.209 | 0.218 | 0.239 | ||||
| kmol·h−1·m−2·bar0.5 | 0.21 | 0.22 | 0.24 | ||||
| Parameter | Unit | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|---|
| IN | OUT | IN | OUT | IN | OUT | ||
| F | kmol·h–1 | 1.82 | 1.80 | 2.23 | 2.20 | 2.84 | 2.80 |
| H2O | mol % | 54 | 55 | 55 | 56 | 56 | 57 |
| CO | mol % | 2 | 2 | 2 | 2 | 1 | 1 |
| CO2 | mol % | 8 | 8 | 8 | 8 | 8 | 8 |
| CH4 | mol % | 7 | 7 | 7 | 7 | 7 | 7 |
| H2 | mol % | 29 | 28 | 28 | 27 | 28 | 27 |
| HRF | % | 3 | 4 | 5 | |||
| kmol·h−1·m−2 | 0.175 | 0.230 | 0.326 | ||||
| kmol·h−1·m−2·bar0.5 | 4.97 | 4.93 | 5.47 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba, D.; Capocelli, M.; De Falco, M.; Franchi, G.; Piemonte, V. Mass Transfer Coefficient in Multi-Stage Reformer/Membrane Modules for Hydrogen Production. Membranes 2018, 8, 109. https://doi.org/10.3390/membranes8040109
Barba D, Capocelli M, De Falco M, Franchi G, Piemonte V. Mass Transfer Coefficient in Multi-Stage Reformer/Membrane Modules for Hydrogen Production. Membranes. 2018; 8(4):109. https://doi.org/10.3390/membranes8040109
Chicago/Turabian StyleBarba, Diego, Mauro Capocelli, Marcello De Falco, Giovanni Franchi, and Vincenzo Piemonte. 2018. "Mass Transfer Coefficient in Multi-Stage Reformer/Membrane Modules for Hydrogen Production" Membranes 8, no. 4: 109. https://doi.org/10.3390/membranes8040109
APA StyleBarba, D., Capocelli, M., De Falco, M., Franchi, G., & Piemonte, V. (2018). Mass Transfer Coefficient in Multi-Stage Reformer/Membrane Modules for Hydrogen Production. Membranes, 8(4), 109. https://doi.org/10.3390/membranes8040109







