Application of Membrane Crystallization for Minerals’ Recovery from Produced Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Used
| Fiber Type | Thickness | Emod | Rm | Break | W | PMI Bubble Point | PMI Pore Size | Porosity | Membrane Area |
|---|---|---|---|---|---|---|---|---|---|
| (mm) | N/mm2 | N/mm2 | % | Nm | (bar) | (µm) | (%) | (m2) | |
| PP lab-made module | 0.45 | 103.75 | 4.16 | 174.40 | 1.11 | 0.76 | 0.2 | 73 | 0.0056 |
| PP Commercial modules | 0.45 | – | – | – | – | – | 0.2 | 73 | 0.2 |
| PVDF | 0.40 | 65.76 | 3.86 | 259.95 | 0.71 | 0.87 | 0.23 | 80.77 | 0.0021 |
2.2. Feed Composition
| Property | Value | |
|---|---|---|
| TDS | 248,000 | |
| Conductivity (mS/cm) | 228.2 | |
| pH | 6.15 | |
| TOC | 18.10 | |
| TC | 40.72 | |
| Sodium Na | 76,646 | |
| Calcium Ca | 6065 | |
| Magnesium Mg | 8361 | |
| Potassium K | 1396 | |
| Chloride Cl | 144,057 | |
| Phosphate | 1055 | |
| Sulphate SO4 | 1213 | |
| Nitrate NO3 | 613 | |
| Floride F | 472 |
2.3. Membrane Crystallization Tests
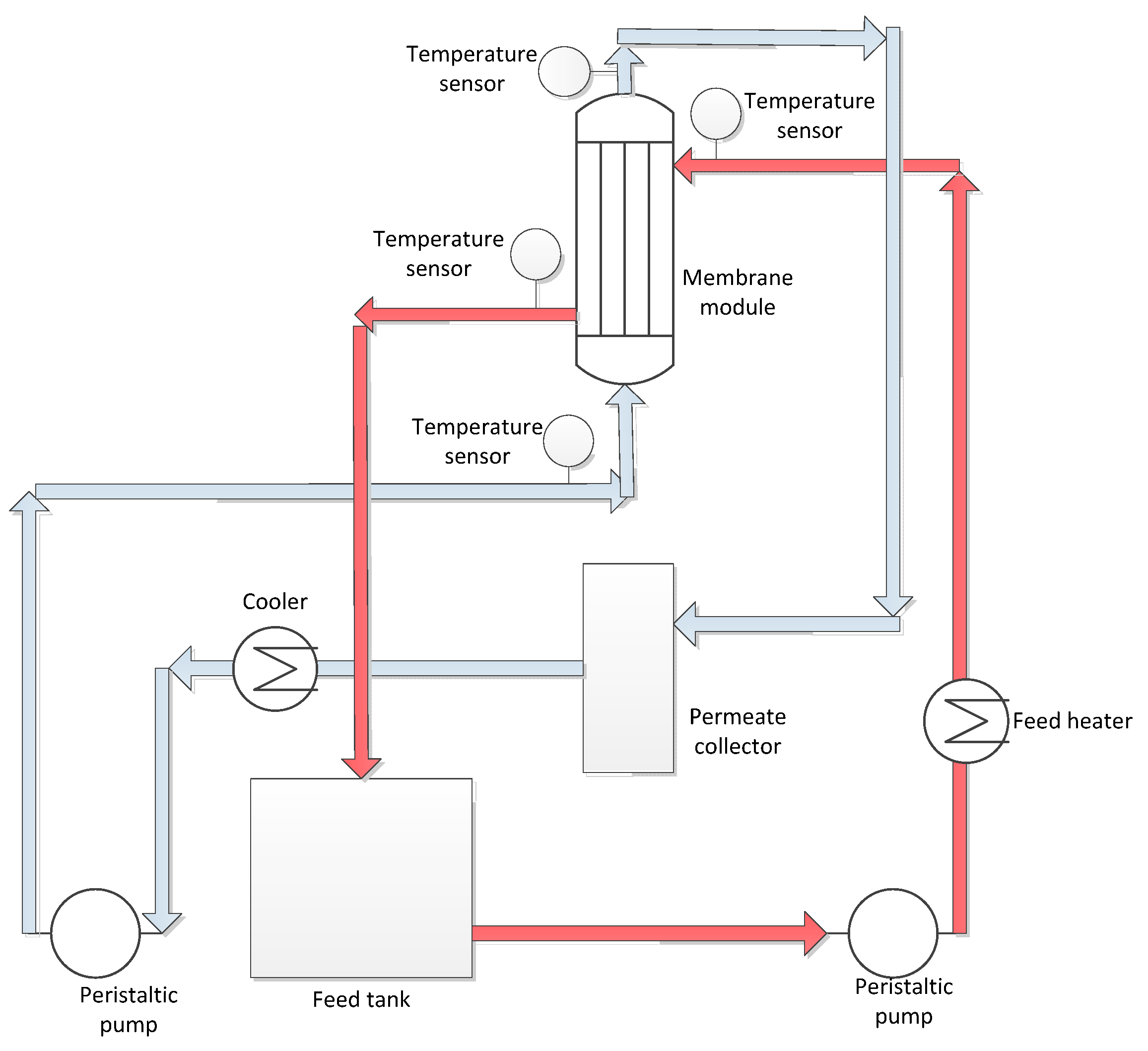
2.4. Crystals Characterization
2.5. Heat and Mass Transfer Analysis
3. Results and Discussion
3.1. Flux and Associated Resistances
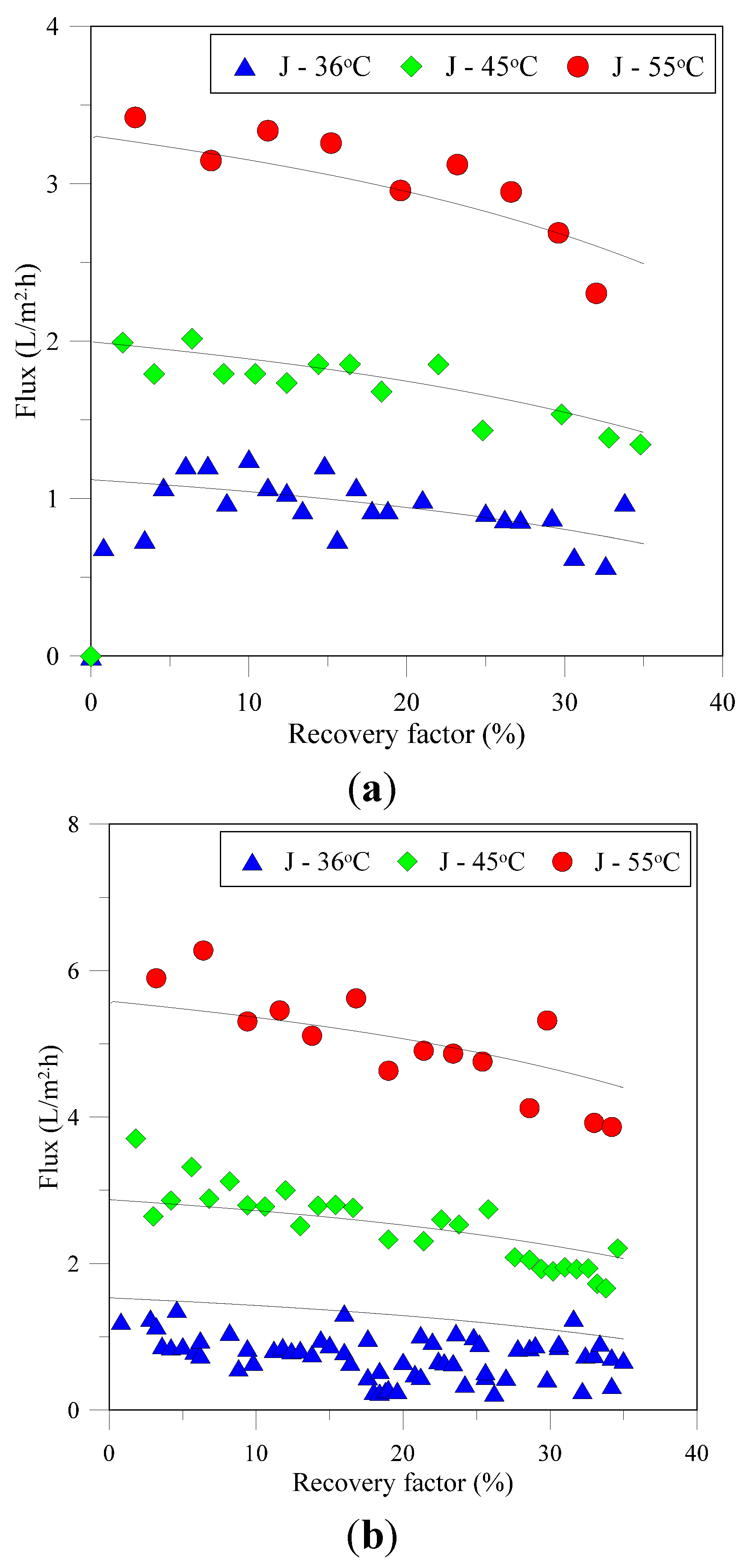
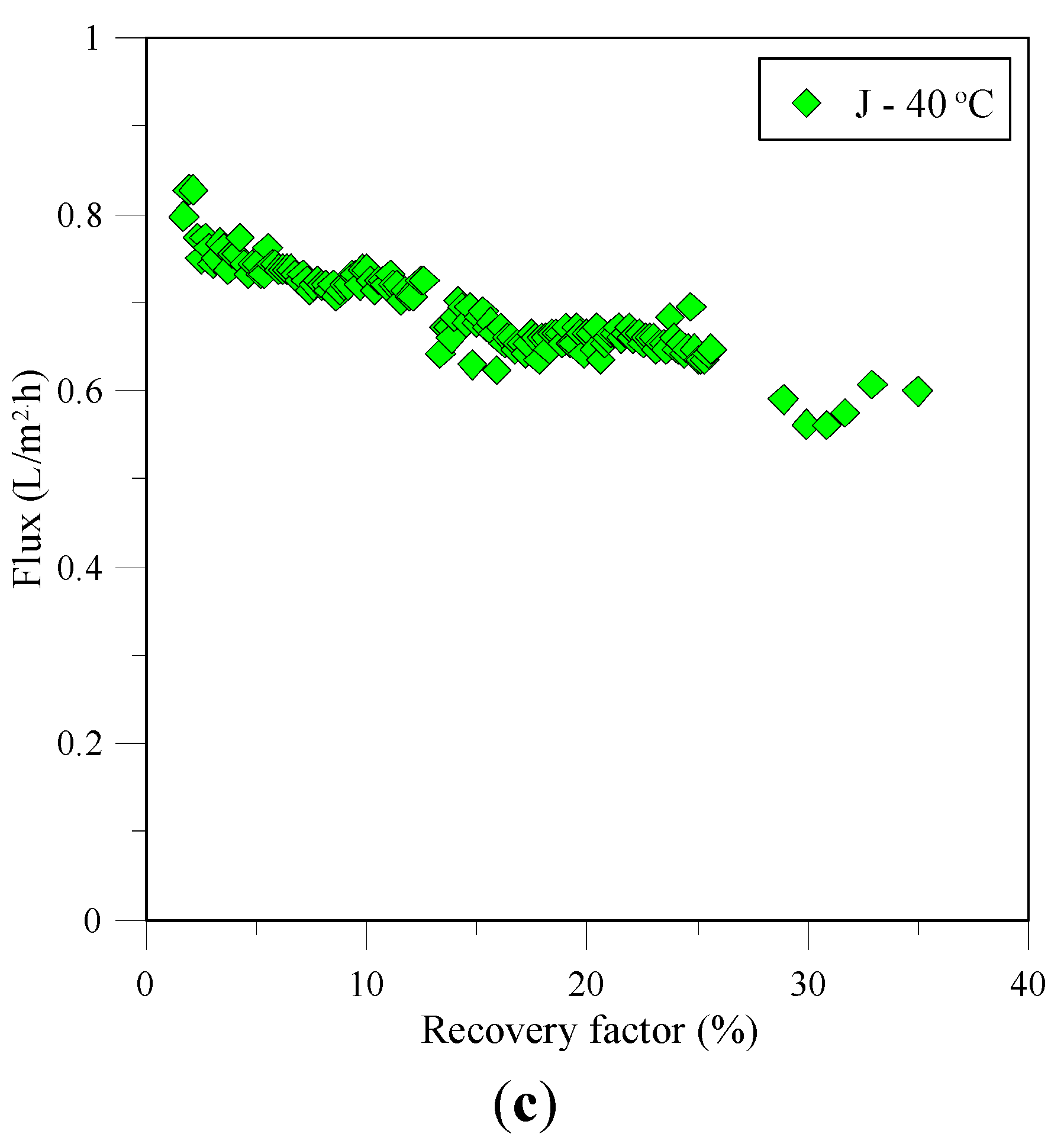

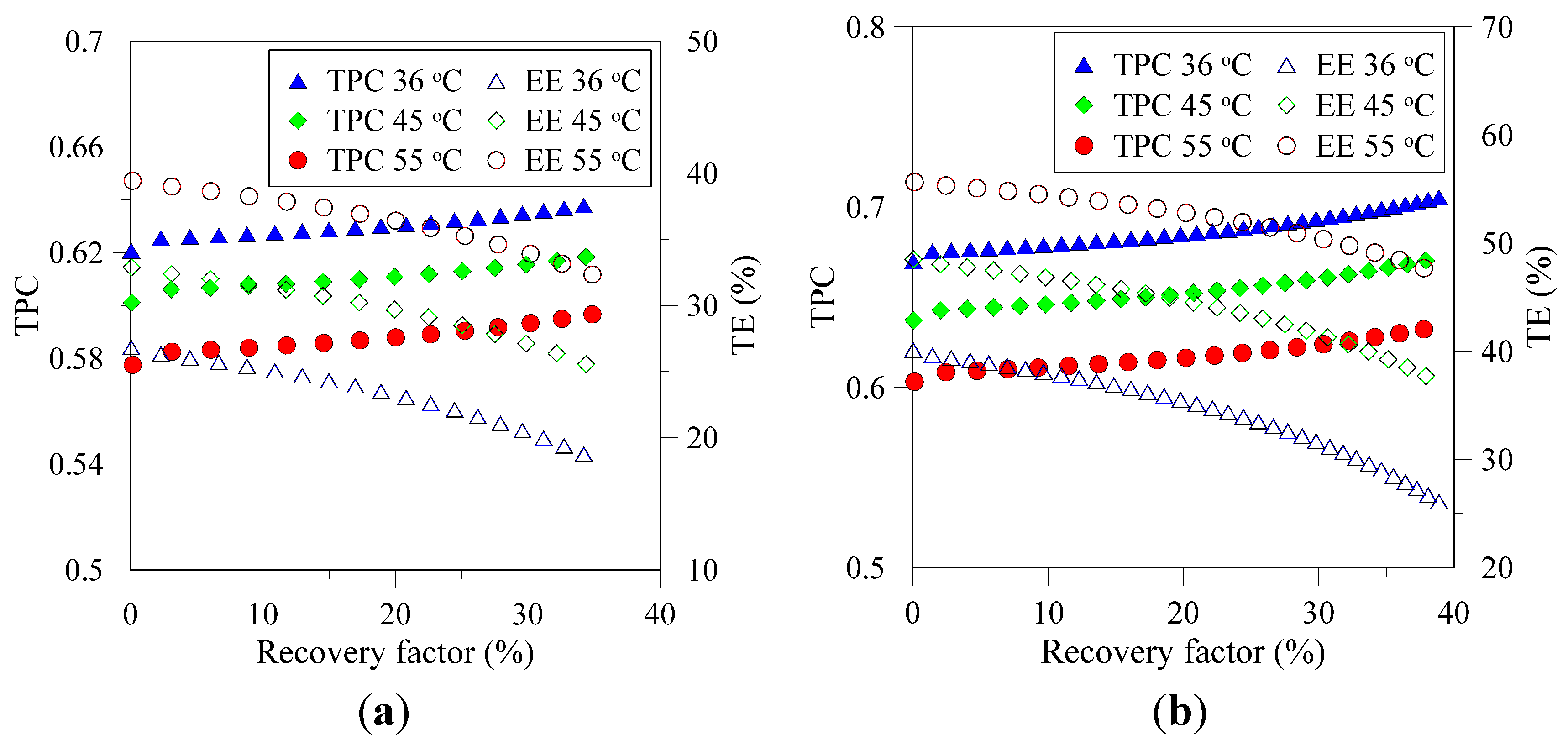
3.2. Characterization of Recovered Crystals

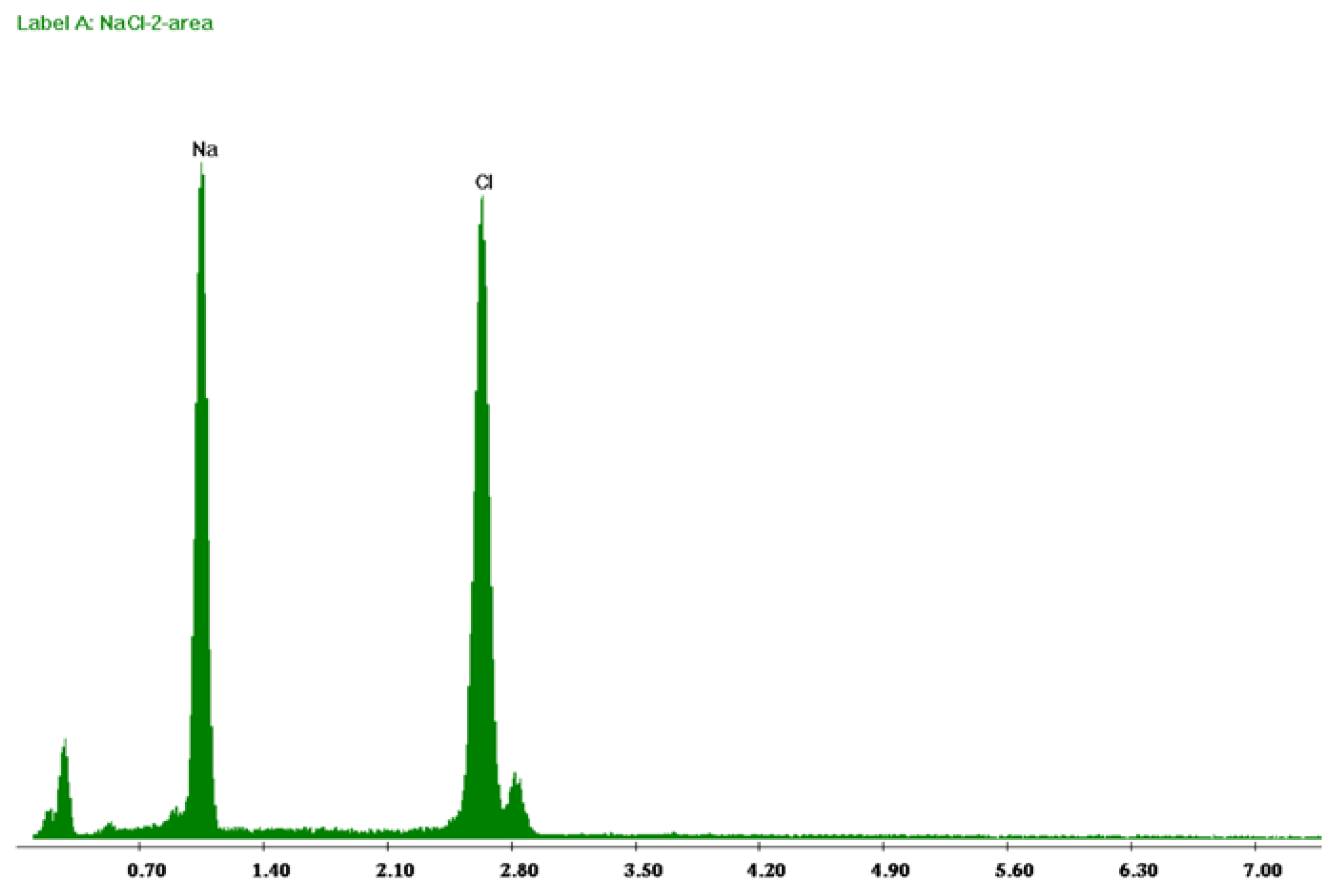


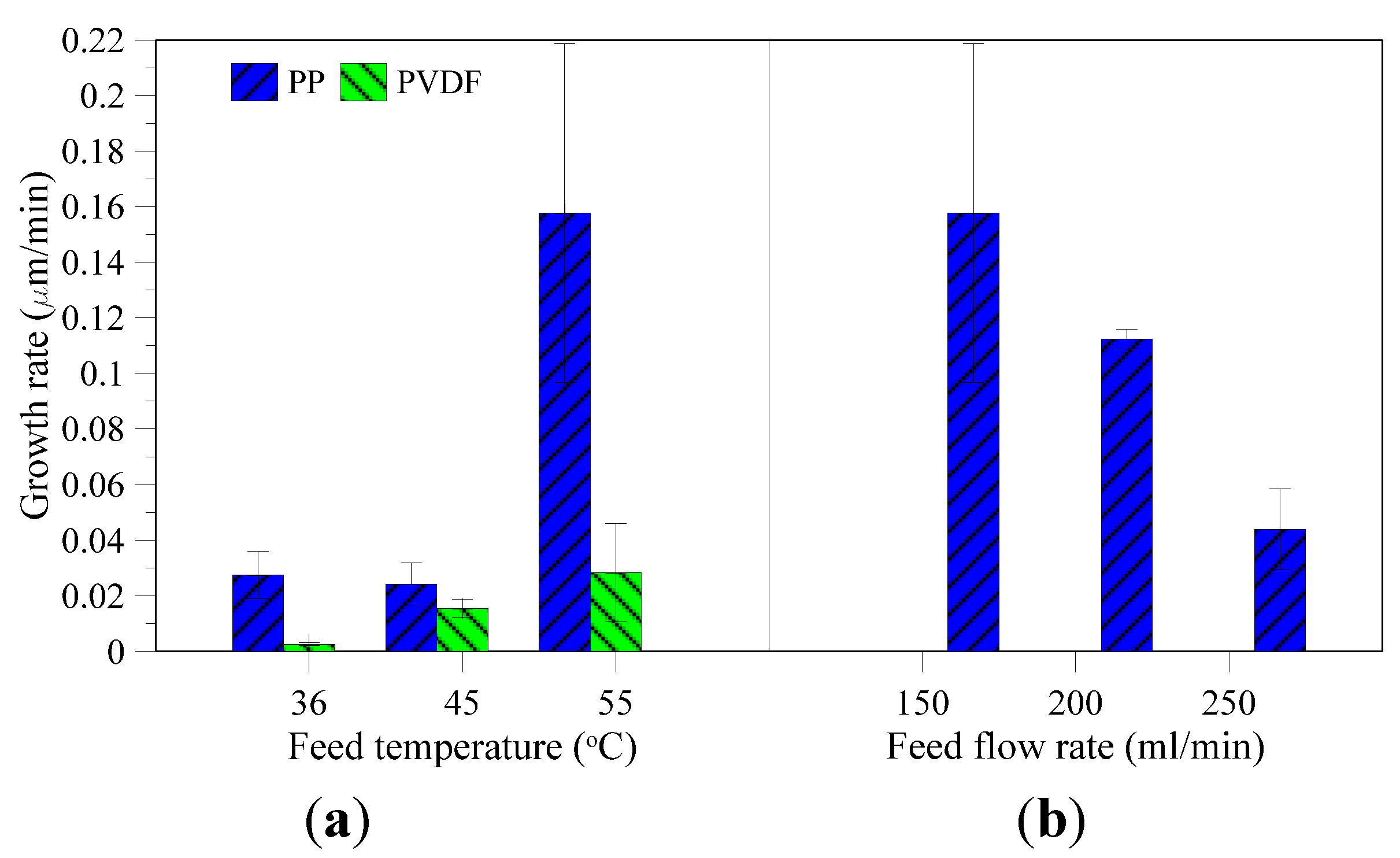
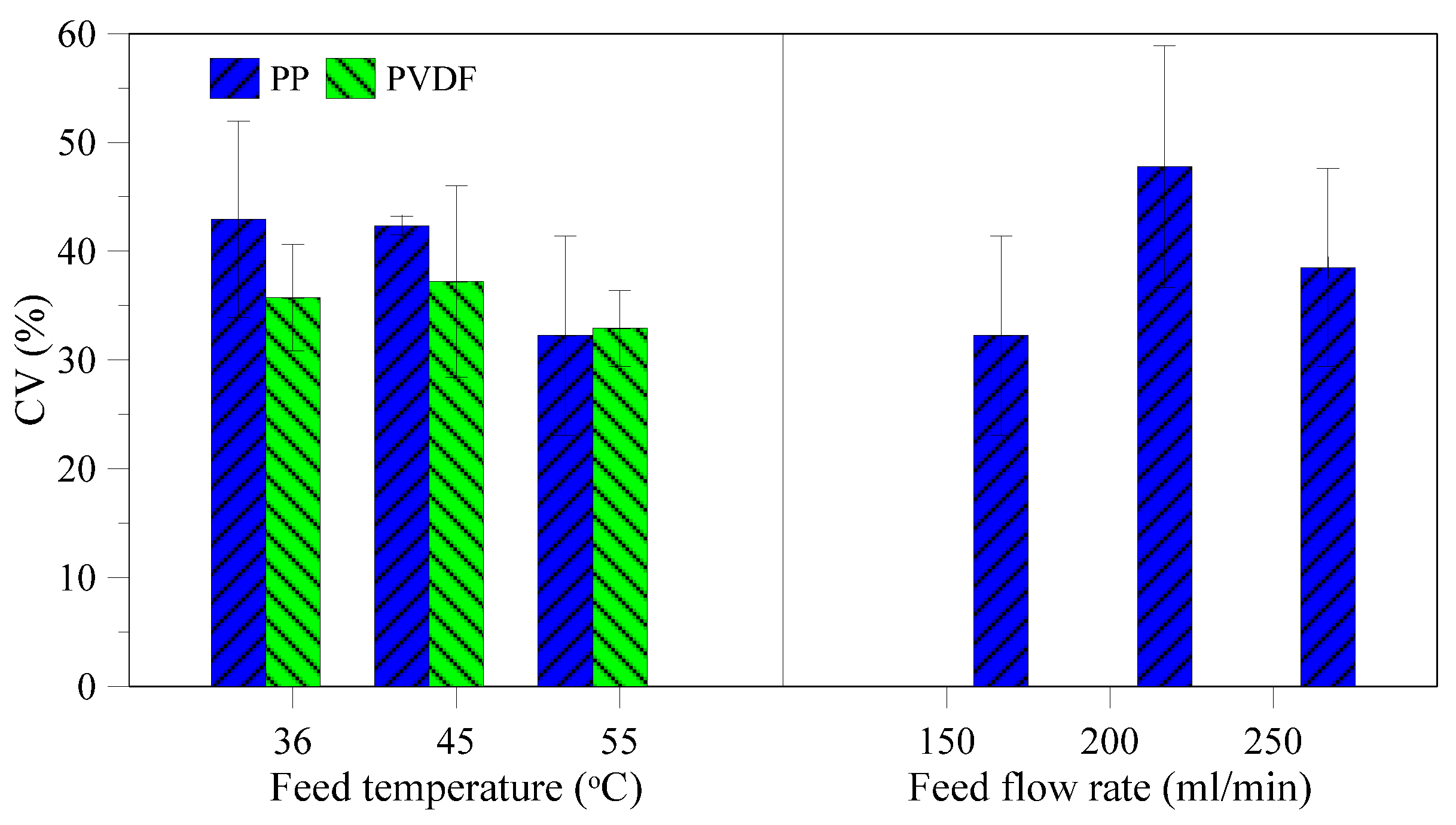
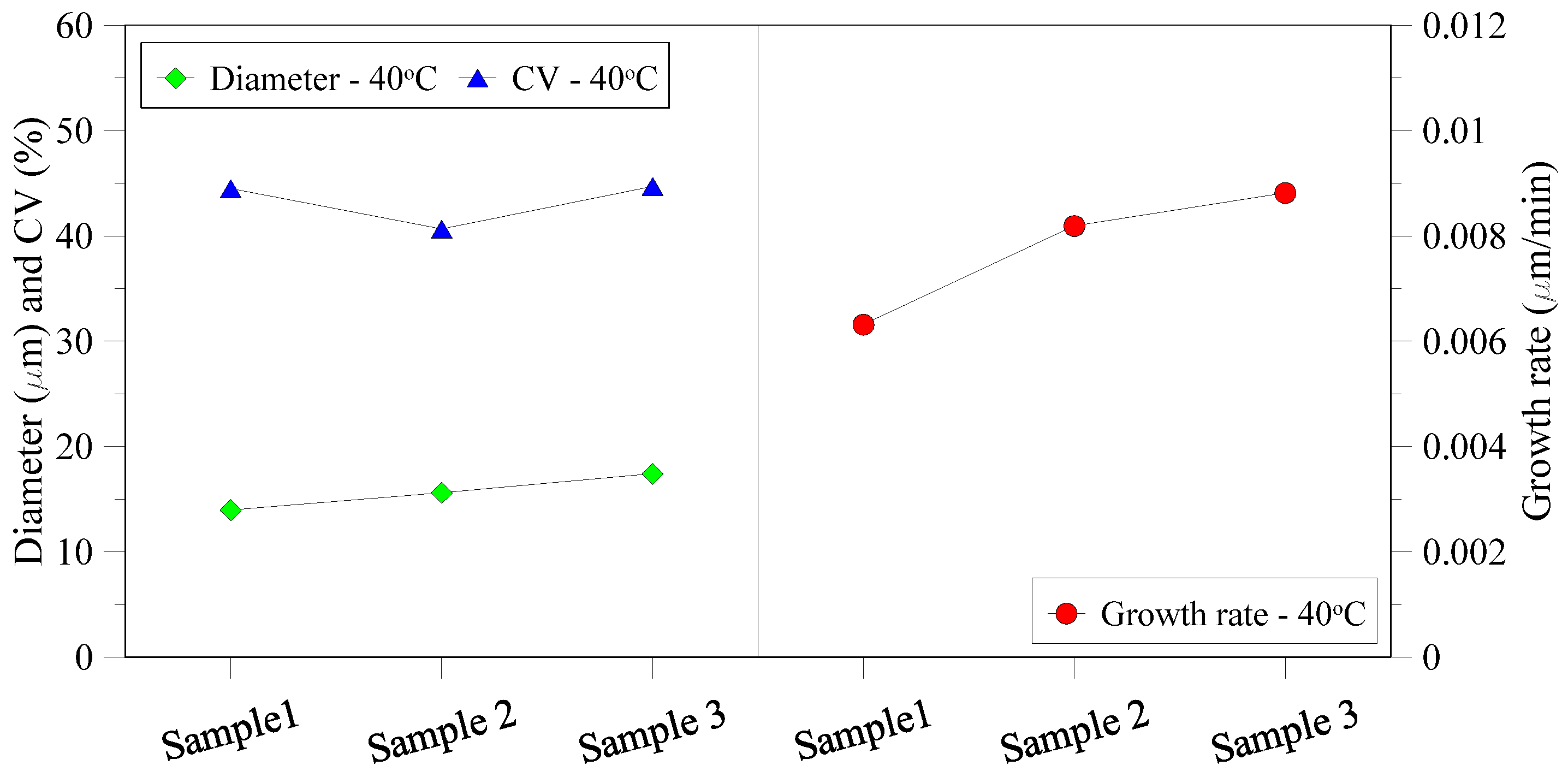

3.3. Membrane Morphology Analysis
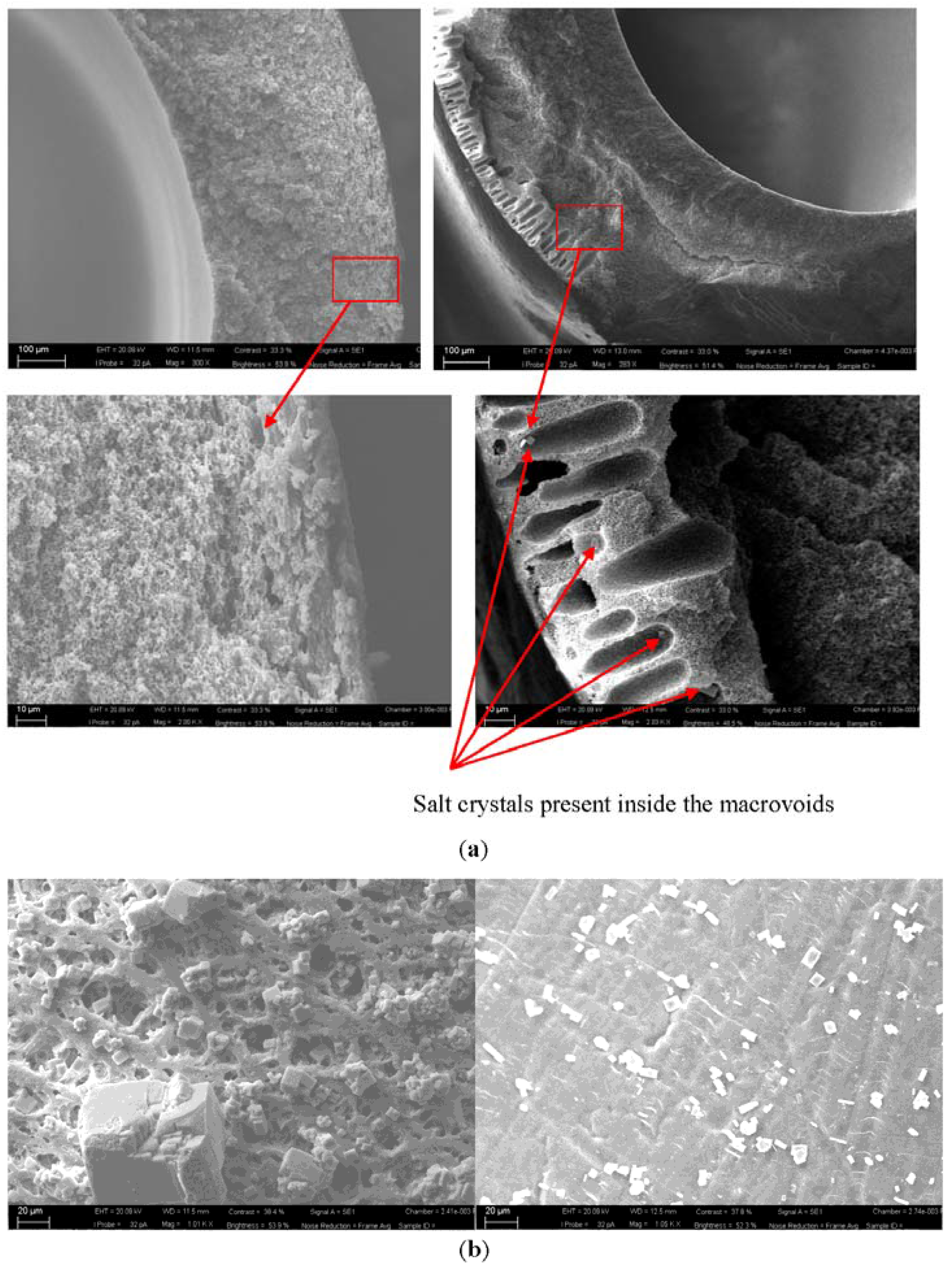
3.4. Cost Analysis
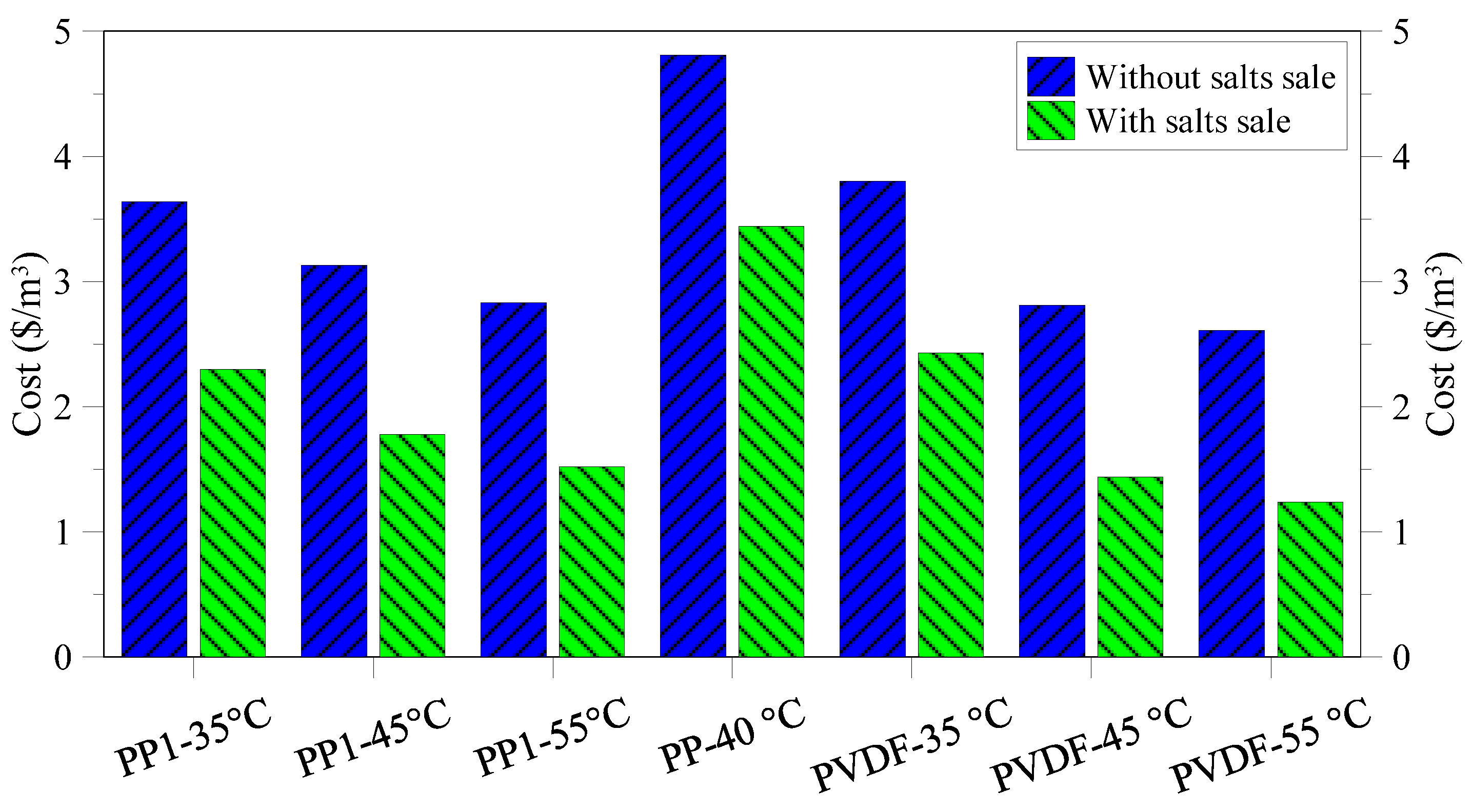

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Symbols and Abbreviations
| B | Membrane characteristics parameter |
| CV | Coefficient of variation (%) |
| D | Fiber inner diameter (m) |
| F | Cumulative percent function (µm) |
| G | Crystal growth rate (µm/min) |
| h | Heat transfer coefficient (w/K.m2) |
| J | Flux (kg/m2·s) |
| L | Crystal length (µm) |
| M | Molecular weight (kg/mol) |
| Nu | Nusselts number |
| n0 | Population density at length zero |
| P | Vapor pressure (Pa) |
| R | Resistance (Pa s m2/kg) |
| RF | Recovery factor (%) |
| T | Temperature (K) |
| TC | Total carbon (ppm) |
| TDS | Total dissolved solids (ppm) |
| TE | Thermal efficiency (%) |
| TOC | Total organic carbon (ppm) |
| x | Mole fraction |
| Suffix | |
| f | Feed |
| m | Membrane |
| p | Permeate |
| s | Solution |
| Greek symbols | |
| δm | Membrane thickness (m) |
| ε | Porosity (%) |
| r | Average pore size (m) |
| τ | Tortuosity factor |
References
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2012. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.T.; Nirmalakhandan, N.; Gardea-Torresdey, J.L. Performance evaluation of an activated sludge system for removing petroleum hydrocarbons from oilfield produced water. Adv. Environ. Res. 2002, 6, 455–470. [Google Scholar] [CrossRef]
- Dahm, K.; Guerra, K. Produced Water Management for Oil and Gas Operations Bureau of Reclamation. In Proceedings of the 19th International Petroleum Environmental Conference, Denver, CO, USA, 30 October–1 November 2012.
- Çakmakce, M.; Kayaalp, N.; Koyuncu, I. Desalination of produced water from oil production fields by membrane processes. Desalination 2008, 222, 176–186. [Google Scholar] [CrossRef]
- Kose, B.; Ozgun, H.; Ersahin, M.E.; Dizge, N.; Koseoglu-Imer, D.Y.; Atay, B.; Kaya, R.; Altınbas, M.; Sayılı, S.; Hoshan, P.; et al. Performance evaluation of a submerged membrane bioreactor for the treatment of brackish oil and natural gas field produced water. Desalination 2008, 285, 295–300. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Sharghi, E.A.; Bonakdarpour, B.; Roustazade, P.; Amoozegar, M.A.; Rabbani, A.R. The biological treatment of high salinity synthetic oilfield produced water in a submerged membrane bioreactor using a halophilic bacterial consortium. J. Chem. Technol. Biotechnol. 2013, 88, 2016–2026. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef]
- Simon, A.R.; Fujioka, T.; Price, W.; Nghiem, L. Sodium hydroxide production from sodium carbonate and bicarbonate solutions using membrane electrolysis: A feasibility study. Sep. Purif. Technol. 2014, 127, 70–76. [Google Scholar] [CrossRef]
- Xu, P.; Cath, T.; Drewes, J.E. Novel and Emerging Technologies for Produced Water Treatment. In Proceedings of the US EPA Technical Workshops for Hydraulic Fracturing, Arlington, VA, USA, 30 March 2011.
- Camacho, L.; Dumée, L.; Zhang, J.; Li, J.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Chavez, L.H.A.; Ben-Sasson, M.; Castrillón, S.R.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sirkar, K.K. Desalination of brine and produced water by direct contact membrane distillation at high temperatures and pressures. J. Membr. Sci. 2012, 389, 380–388. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced water treatment: Application of air gap membrane distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Curcio, E.; Criscuoli, A.; Drioli, E. Membrane crystallizers. Ind. Eng. Chem. Res. 2001, 40, 2679–2684. [Google Scholar] [CrossRef]
- Li, W.; van der Bruggen, B.; Luis, P. Integration of reverse osmosis and membrane crystallization for sodium sulphate recovery. Chem. Eng. Process. Process Intensif. 2014, 85, 57–68. [Google Scholar] [CrossRef]
- Drioli, E.; Curcio, E.; Criscuoli, A.; Di, G. Integrated system for recovery of CaCO3, NaCl and MgSO4·7H2O from nanofiltration retentate. J. Membr. Sci. 2004, 239, 27–38. [Google Scholar] [CrossRef]
- Di Profio, G.; Tucci, S.; Curcio, E.; Drioli, E. Controlling polymorphism with membrane-based crystallizers: Application to form I and II of paracetamol. Chem. Mater. 2007, 19, 2386–2388. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Thomas, R.; Mansoor, B.; Johnson, D.; Hilal, N. Effect of dry-out on the fouling of PVDF and PTFE membranes under conditions simulating intermittent seawater membrane distillation (SWMD). J. Membr. Sci. 2013, 438, 126–139. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Simone, S.; Macedonio, F.; L-Jlil, S.A.A.; al Shabonah, F.S.; Al-Romaih, H.S.; Al-Harbi, O.; Figoli, A.; Criscuoli, A. Novel PVDF hollow fiber membranes for vacuum and direct contact membrane distillation applications. Sep. Purif. Technol. 2013, 115, 27–38. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct contact membrane distillation for treatment of oilfield produced water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Macedonio, F.; Quist-Jensen, C.A.; Al-Harbi, O.; Alromaih, H.; Al-Jlil, S.A.; al Shabouna, F.; Drioli, E. Thermodynamic modeling of brine and its use in membrane crystallizer. Desalination 2013, 323, 83–92. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Sieder, E.N.; Tate, G. Heat transfer and pressure drop of liquid in tubes. Ind. Eng. Chem. 1936, 28, 1429–1435. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Morawski, A.W. Membrane distillation with laminar flow. Sep. Purif. Technol. 1997, 5866, 2–6. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M. Heat transport in the membrane distillation process. J. Membr. Sci. 1998, 144, 211–222. [Google Scholar] [CrossRef]
- Thomas, L. Heat Transfer; Prentice-Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Ali, A.; Aimar, P.; Drioli, E. Effect of module design and flow patterns on performance of membrane distillation process. Chem. Eng. J. 2015, 277, 368–377. [Google Scholar] [CrossRef]
- Yun, Y.; Ma, R.; Zhang, W.; Fane, A.G.; Li, J. Direct contact membrane distillation mechanism for high concentration NaCl solutions. Desalination 2006, 188, 251–262. [Google Scholar] [CrossRef]
- Ali, A.; Macedonio, F.; Drioli, E.; Aljlil, S.; Alharbi, O.A. Experimental and theoretical evaluation of temperature polarization phenomenon in direct contact membrane distillation. Chem. Eng. Res. Des. 2013, 91, 1966–1977. [Google Scholar] [CrossRef]
- Martinez-Diez, L.; Vazquez-Gonzalez, M.I.; Florido-Diaz, F.J. Study of membrane distillation using channel spacers. J. Membr. Sci. 1998, 144, 45–56. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Optimization of module length and membrane thickness for membrane distillation. In Proceedings of the 2nd International Workshop on Membrane Distillation and Innovating Membrane Operations in Desalination and Water Reuse, Ravello, Italy, 1–4 July 2015.
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- MartõÂnez-DõÂez, M.; VaÂzquez-GonzaÂlez, L. Temperature and concentration polarization in membrane distillation of aqueous salt solutions. J. Membr. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Wilcox, W.R. Crystallization flow. J. Cryst. Growth 1972, 12, 93–96. [Google Scholar] [CrossRef]
- Mersmann, A.; Eble, A.; Heyer, C. Crystal growth. In Crystallization Technology Handbook; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Myerson, A. Handbook of Industrial Crystallization; Butterworth Heinemann Elsevier Inc.: Boston, MA, USA, 2002. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Application of Membrane Crystallization for Minerals’ Recovery from Produced Water. Membranes 2015, 5, 772-792. https://doi.org/10.3390/membranes5040772
Ali A, Quist-Jensen CA, Macedonio F, Drioli E. Application of Membrane Crystallization for Minerals’ Recovery from Produced Water. Membranes. 2015; 5(4):772-792. https://doi.org/10.3390/membranes5040772
Chicago/Turabian StyleAli, Aamer, Cejna Anna Quist-Jensen, Francesca Macedonio, and Enrico Drioli. 2015. "Application of Membrane Crystallization for Minerals’ Recovery from Produced Water" Membranes 5, no. 4: 772-792. https://doi.org/10.3390/membranes5040772
APA StyleAli, A., Quist-Jensen, C. A., Macedonio, F., & Drioli, E. (2015). Application of Membrane Crystallization for Minerals’ Recovery from Produced Water. Membranes, 5(4), 772-792. https://doi.org/10.3390/membranes5040772








