Zn2+-Mediated Co-Deposition of Dopamine/Tannic Acid/ZIF-8 on PVDF Hollow Fiber Membranes for Enhanced Antifouling Performance and Protein Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Materials
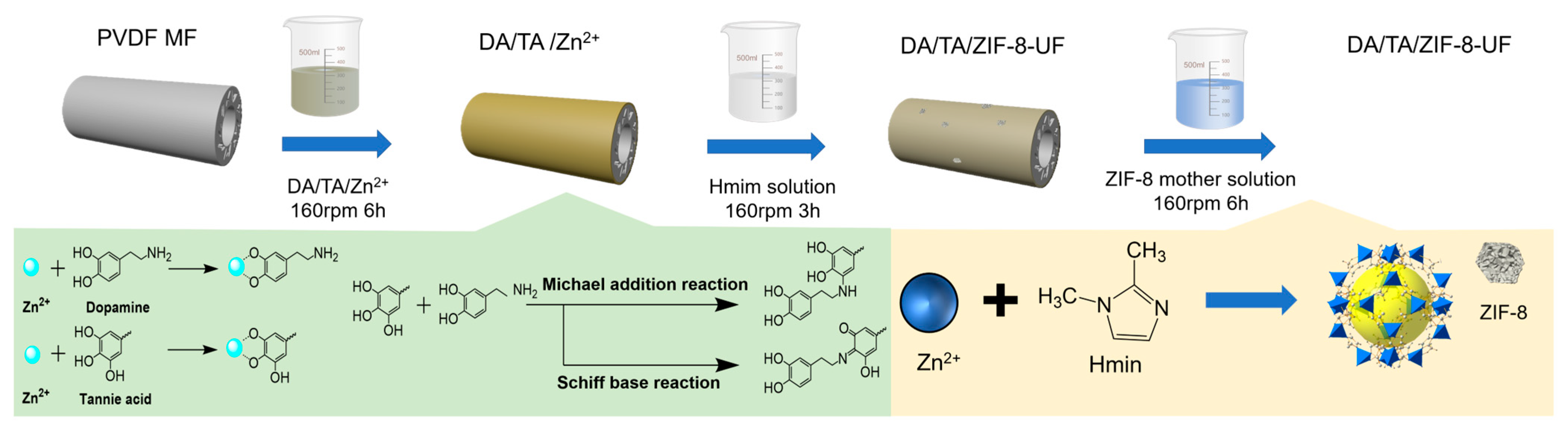
2.2. Membrane Modification
2.3. Characterization of Membrane Samples
2.4. Measurement of Membrane Permeability and Protein Rejection
2.5. Organic Fouling Tests
2.6. Analysis of Membrane Surface Anti-Protein Fouling Mechanism
3. Results and Discussion
3.1. Membrane Characterization
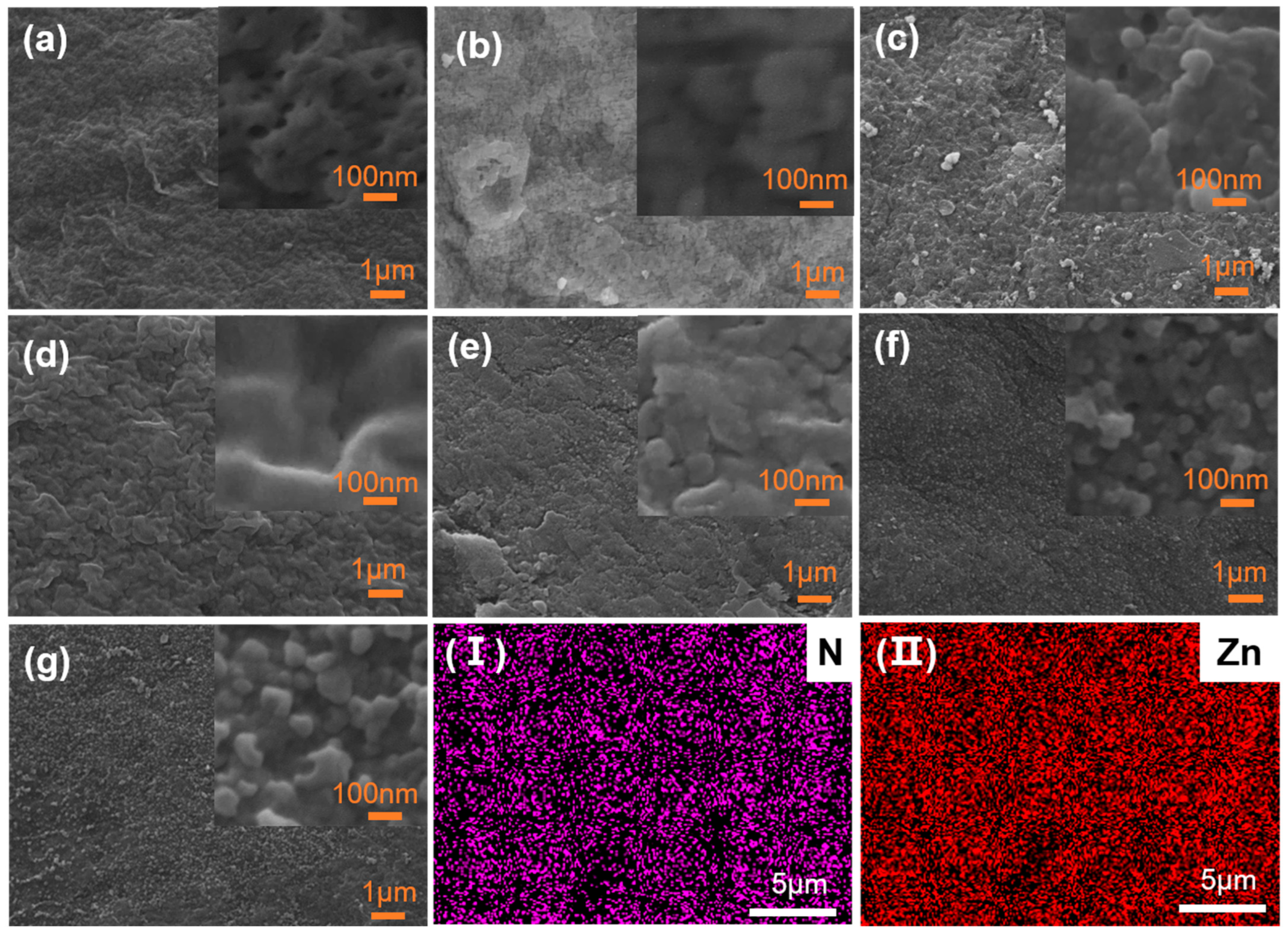
3.1.1. Morphologies of the Membranes
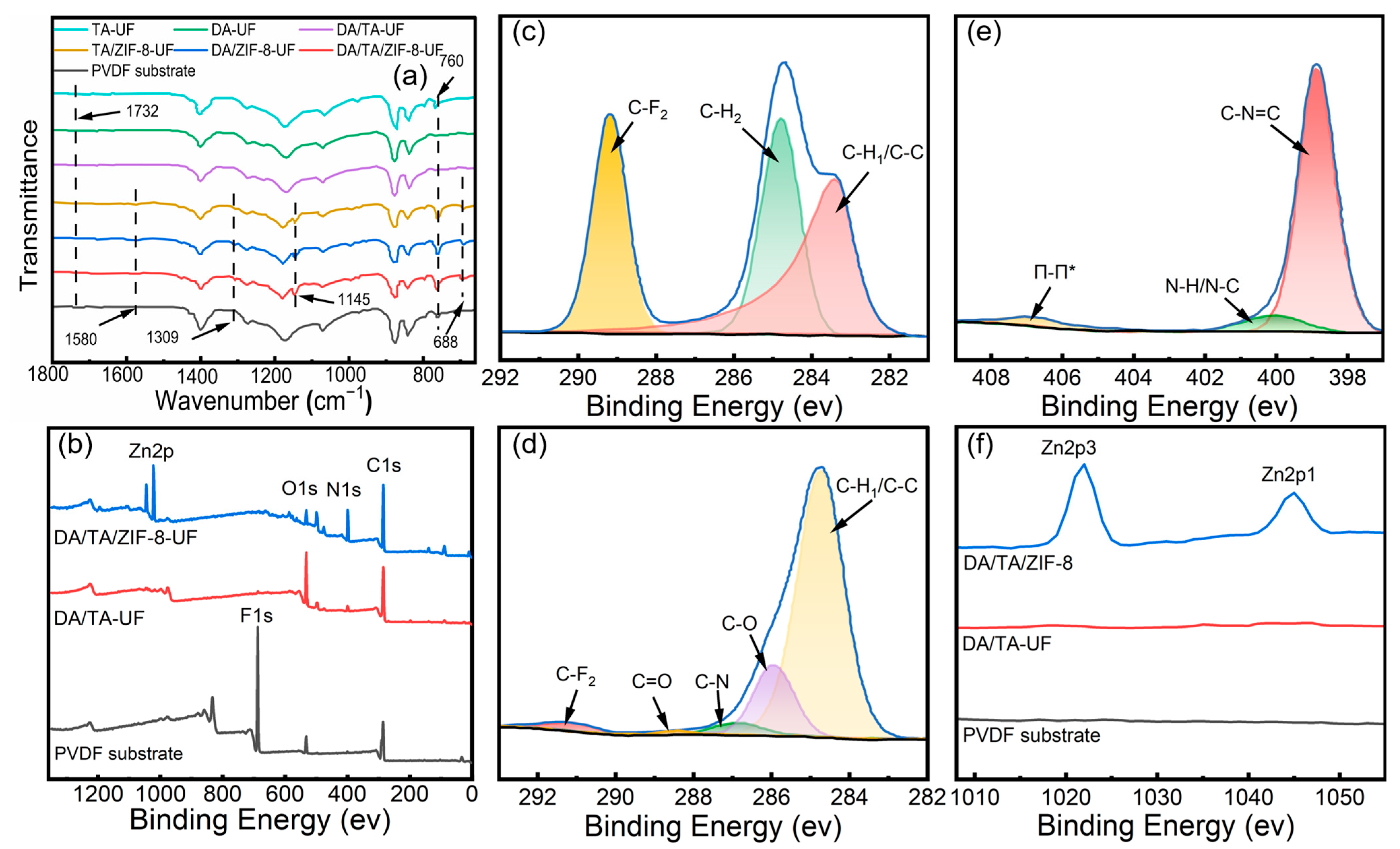
3.1.2. Surface Chemical Compositions
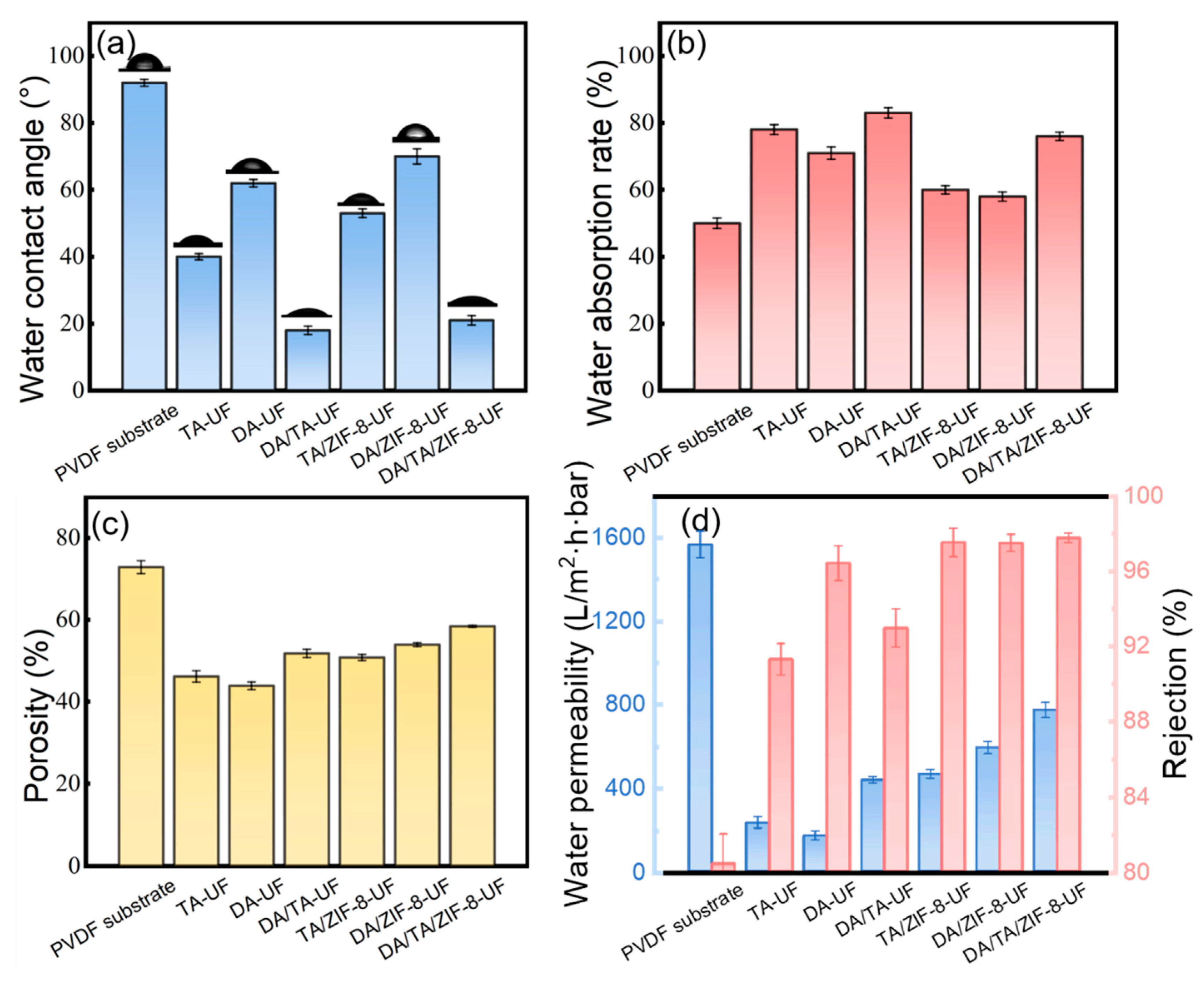
3.1.3. Membrane Hydrophilicity and Porosity
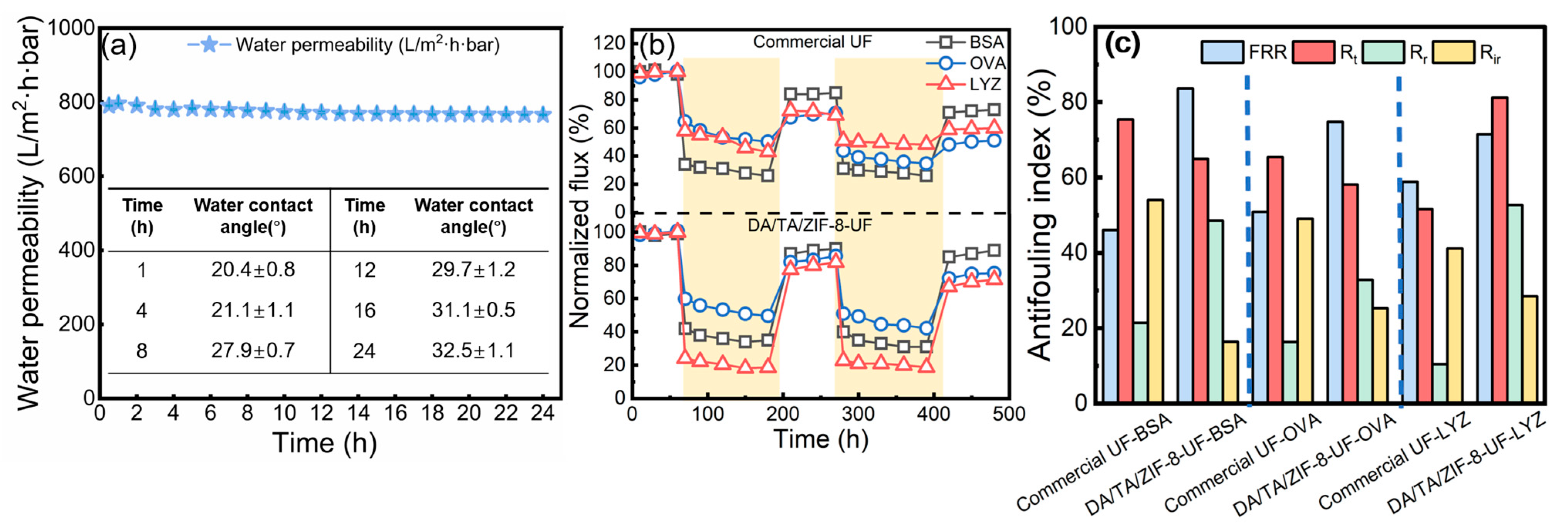
3.2. Separation Performance and Stability of Membranes
3.3. Antifouling Properties of UF Membranes
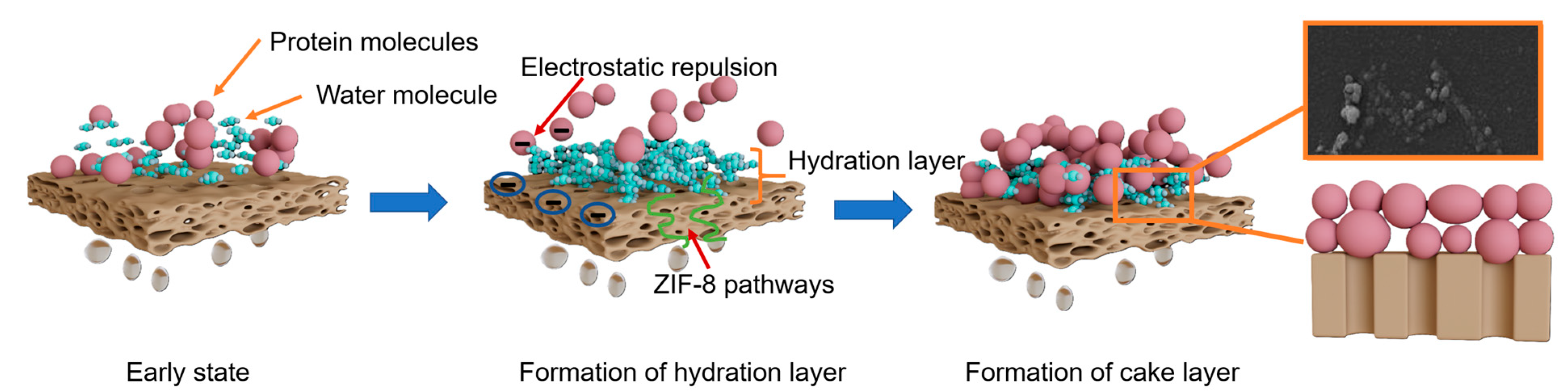
3.4. Anti-Fouling Mechanisms from xDLVO Theory
- (1)
- Hydration layer formation: The hydrophilic surface, rich in amino and phenolic hydroxyl groups, rapidly binds water molecules to form a dense hydration layer, preventing direct contact between proteins and the membrane surface and reducing initial adhesion.
- (2)
- Pore size optimization: The metal-polyphenol networks (Zn2+-TA coordination) and DA/TA copolymerization create a tailored surface morphology that reduces effective pore size without significant permeability loss. This structure minimizes internal pore blockage by protein molecules while maintaining rejection performance.
- (3)
- Enhanced water transport: The incorporated ZIF-8 crystals provide additional nanoporous pathways for water molecules, mitigating the permeability-selectivity trade-off and maintaining high flux during operation.
- (4)
- Reversible fouling layer: As protein concentration increases during filtration, foulants primarily form a loose cake layer on the membrane surface rather than penetrating pores. This external fouling is more easily removed through hydraulic cleaning due to the hydration layer’s lubricating effect and electrostatic repulsion from the negatively charged surface.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Gong, Y.; Jin, Y.; Zhou, J.; Wang, Y. Additive manufacturing of ultrafiltration membranes from block copolymer nanowires. Sep. Purif. Technol. 2025, 368, 132980. [Google Scholar] [CrossRef]
- Tai, Z.S.; Othman, M.H.D.; Tajul Arifin, N.D.; Shazana, N.A.; Iqbal, R.M.; Ab Rahman, M.; Wan Mustapa, W.N.F.; Kadirkhan, F.; Puteh, M.H.; Jaafar, J.; et al. Modification of PVDF hollow fiber membrane with ZnO structure via hydrothermal for enhanced antifouling property in membrane distillation. J. Membr. Sci. 2024, 692, 122294. [Google Scholar] [CrossRef]
- Liu, W.-M.; Chen, X.-R.; Mao, H.; Li, S.-H.; Zhang, Y.-M.; Xu, L.-H.; Zhao, Z.-P. Construction of PDMS@ZIF-8 composite membrane on PVDF hollow fiber inner surface for ultrafast ethanol/water separation. J. Membr. Sci. 2024, 704, 122889. [Google Scholar] [CrossRef]
- Hou, C.; Du, L.; Chen, D.; Zhao, X.; Zhou, J.; Qiao, S. The preparation of electrically-enhanced and hydrophilic CNTs-PVDF composite hollow fiber membranes for fouling resistance. Sep. Purif. Technol. 2024, 351, 127968. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L.; Ooi, B.S. Construction of wetting resistance surface of highly hydrophobic PVDF-HFP/rGO coated supported PVDF hollow fiber composite membrane for membrane distillation. Chem. Eng. J. 2024, 493, 152565. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, C.; Jiang, X.; Zhou, Y.; Lin, X.; Zhao, X.; He, Q.; Chai, H.; Pang, X.; Ma, J. Dopamine-triggered one-step codeposition of zwitterionic surfactants for anti-fouling polyethersulfone ultrafiltration membrane modification. Appl. Surf. Sci. 2022, 598, 153871. [Google Scholar] [CrossRef]
- Wijers, M.C.; Jin, M.; Wessling, M.; Strathmann, H. Supported liquid membranes modification with sulphonated poly(ether ether ketone)—Permeability, selectivity and stability. J. Membr. Sci. 1998, 147, 117–130. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Xu, Q.; Wu, S.; Chen, Y. Regenerated cellulose strongly adhered by a supramolecular adhesive onto the PVDF membrane for a highly efficient oil/water separation. Green Chem. 2021, 23, 5633–5646. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, X.; Kaneda, M.; Zhang, W.; Bernstein, R.; Ma, J.; Elimelech, M. Graphene Oxide-Functionalized Membranes: The Importance of Nanosheet Surface Exposure for Biofouling Resistance. Environ. Sci. Technol. 2020, 54, 517–526. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Zhang, X.; Lu, X.; Liu, Q.; Zhang, T.; Cheng, W.; Ma, J. Utilization of Bidirectional Cation Transport in a Thin Film Composite Membrane: Selective Removal and Reclamation of Ammonium from Synthetic Digested Sludge Centrate via an Osmosis–Distillation Hybrid Membrane Process. Environ. Sci. Technol. 2020, 54, 10313–10322. [Google Scholar] [CrossRef]
- Cheng, W.; Xu, H.; Wang, P.; Wang, L.; Szymczyk, A.; Croué, J.-P.; Zhang, T. Modification Mechanism of Polyamide Reverse Osmosis Membrane by Persulfate: Roles of Hydroxyl and Sulfate Radicals. Environ. Sci. Technol. 2022, 56, 8864–8874. [Google Scholar] [CrossRef]
- Wang, P.; Jin, J.; Hou, R.; Jin, G.; Qin, L.; Chen, X.; Wang, H.; Zhou, Y.; Gao, C. From synergistic integration to advanced substitution: MOF-assisted biocatalytic membranes for emerging contaminants remediation. J. Environ. Manage 2025, 390, 126261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Wang, M.; Shen, Q.; Cong, S.; Zhao, Y.; Peng, J.; Pang, H. Metal-organic framework (MOF) membranes for Mg2+/Li+ separation. Sep. Purif. Technol. 2025, 374, 133737. [Google Scholar] [CrossRef]
- Ahmed, E.; Farghali, A.A.; Hmamm, M.F.M. Development of Cu-MOF@PVDF–PS hybrid membranes for high-temperature proton exchange membranes: Electrospinning, characterization, and fuel cell performance. RSC Adv. 2025, 15, 29777–29798. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Devadiga, V.; Mohan, S.; Balakrishna, R.G. Europium MOFs-enabled fluorescent membranes with optimized flux, filtration, and fouling resistance for wastewater treatment: A strategy to probe MOF distribution in membranes. J. Environ. Chem. Eng. 2025, 13, 118679. [Google Scholar] [CrossRef]
- Karimi, A.; Vatanpour, V.; Khataee, A.; Safarpour, M. Contra-diffusion synthesis of ZIF-8 layer on polyvinylidene fluoride ultrafiltration membranes for improved water purification. J. Ind. Eng. Chem. 2019, 73, 95–105. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Hu, P.; Peng, X. ZIF-8 coated polyvinylidenefluoride (PVDF) hollow fiber for highly efficient separation of small dye molecules. Appl. Mater. Today 2016, 5, 103–110. [Google Scholar] [CrossRef]
- Li, F.; Bhushan, B.; Pan, Y.; Zhao, X. Bioinspired superoleophobic/superhydrophilic functionalized cotton for efficient separation of immiscible oil-water mixtures and oil-water emulsions. J. Colloid. Interface Sci. 2019, 548, 123–130. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-Inspired Surface Engineering for Water-Remediation Materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef]
- Huang, S.; McDonald, J.A.; Kuchel, R.P.; Khan, S.J.; Leslie, G.; Tang, C.Y.; Mansouri, J.; Fane, A.G. Surface modification of nanofiltration membranes to improve the removal of organic micropollutants: Linking membrane characteristics to solute transmission. Water Res. 2021, 203, 117520. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Zhang, C.; Wang, Z.X.; Shao, L. Tailoring nanofiltration membrane performance for highly-efficient antibiotics removal by mussel-inspired modification. J. Membr. Sci. 2016, 499, 326–334. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, A.; Luo, X.; Lin, J.; Zheng, R.; Fan, H.; Selasie, S.V.; Lin, H. A facile method to modify polypropylene membrane by polydopamine coating via inkjet printing technique for superior performance. J. Colloid. Interface Sci. 2019, 552, 719–727. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Wen, S.; Wang, L.; Wang, S.; Wang, Y.; Lu, J.; Ma, J.; Cheng, W. Rapid co-deposition of dopamine and polyethyleneimine triggered by CuSO4/H2O2 oxidation to fabricate nanofiltration membranes with high selectivity and antifouling ability. Sep. Purif. Technol. 2023, 305, 122409. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Wang, Q.; Zhang, H.; Wang, Z.; Wu, Z. Evaluating of the performance of natural mineral vermiculite modified PVDF membrane for oil/water separation by membrane fouling model and XDLVO theory. J. Membr. Sci. 2022, 641, 119886. [Google Scholar] [CrossRef]
- Li, B.; Hou, D.; Li, C.; Yun, Y. Mussels-inspired design a carbon nanotube based underwater superoleophobic/hydrophobic Janus membrane with robust anti-oil-fouling for direct contact membrane distillation. Sep. Purif. Technol. 2022, 294, 121163. [Google Scholar] [CrossRef]
- Pz, A.; Wl, A.; Sr, A.; Yj, A.; Qin, S.A.; Cf, B.; Nk, A.; Hm, A. Modification of PVDF hollow fiber membrane by co-deposition of PDA/MPC-co-AEMA for membrane distillation application with anti-fouling and anti-scaling properties. J. Membr. Sci. 2021, 636, 119596. [Google Scholar] [CrossRef]
- Ye, W.; Liu, H.; Lin, F.; Lin, J.; Zhao, S.; Yang, S.; Hou, J.; Zhou, S.; Van der Bruggenf, B. High-flux nanofiltration membranes tailored by bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets for enhanced selectivity towards organics and salts. Environ. Sci. Nano 2019, 6, 2958–2967. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, Z.; Yang, H.; Wang, G.; Zhu, L. Mussel-inspired hydrophilic modification of polypropylene membrane for oil-in-water emulsion separation. Surf. Coat. Techol. 2020, 403, 126375. [Google Scholar] [CrossRef]
- Varol, H.S.; Herberger, T.; Kirsch, M.; Mikolei, J.; Veith, L.; Kannan-Sampathkumar, V.; Brand, R.D.; Synatschke, C.V.; Weil, T.; Andrieu-Brunsen, A. Electropolymerization of Polydopamine at Electrode-Supported Insulating Mesoporous Films. Chem. Mater. 2023, 35, 9192–9207. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid. Interface Sci. 2022, 305, 102689. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rezzadori, K.; Petrus, J.; Luccio, M.; Li], Q. Modification of hydrophobic commercial PVDF microfiltration membranes into superhydrophilic membranes by the mussel-inspired method with dopamine and polyethyleneimine. Sep. Purif. Technol. 2019, 212, 641–649. [Google Scholar] [CrossRef]
- Ren, J.; Xia, W.; Feng, X.; Zhao, Y. Surface modification of PVDF membrane by sulfonated chitosan for enhanced anti-fouling property via PDA coating layer. Mater. Lett. 2021, 307, 130981. [Google Scholar] [CrossRef]
- Zhan, K.; Kim, C.; Sung, K.; Ejima, H.; Yoshie, N. Tunicate-inspired gallol polymers for underwater adhesive: A comparative study of catechol and gallol. Biomacromolecules 2017, 18, 2959–2966. [Google Scholar] [CrossRef]
- Xie, H.; Shen, L.; Xu, Y.; Hong, H.; Yang, L.; Li, R.; Lin, H. Tannic Acid (Ta)-Based Coating Modified Membrane Enhanced by Successive Inkjet Printing of Fe3+ and Sodium Periodate (Sp) for Efficient Oil-Water Separation. J. Membr. Sci. 2022, 660, 120873. [Google Scholar] [CrossRef]
- Song, Y.Z.; Kong, X.; Yin, X.; Zhang, Y.; Sun, C.C.; Yuan, J.J.; Zhu, B.; Zhu, L.P. Tannin-inspired superhydrophilic and underwater superoleophobic polypropylene membrane for effective oil/water emulsions separation. Colloids Surf. Physicochem. Eng. Aspects 2017, 522, 585–592. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Zhang, W.; Lin, H.; Liao, B.Q. Plant polyphenol intermediated metal-organic framework (MOF) membranes for efficient desalination. J. Membr. Sci. 2021, 618, 118726. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, Z.; Wang, H.; Wang, L.; Qian, Y.; Long, X.; Ma, C.; Zhang, Z.; Li, J.; Zhang, H. Enhanced water permeance of a polyamide thin-film composite nanofiltration membrane with a metal-organic framework interlayer. J. Membr. Sci. 2021, 625, 119154. [Google Scholar] [CrossRef]
- Ye, L.; Liu, X.; Chen, Y.; Xu, L.; Fu, S.; Wu, X. Fe3+/tannic acid assisted preparation of dynamic assembled hollow fiber nanofiltration membrane with salt tolerance. J. Environ. Chem. Eng. 2025, 13, 118378. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, W.; Zhao, W.; Yang, Y.; Shen, H.; Chang, Y.; Zhao, S. Surface-functionalized composite hollow fiber membrane for extracorporeal membrane oxygenation. J. Membr. Sci. 2025, 733, 124377. [Google Scholar] [CrossRef]
- Mohmed, A.H.; Isawi, H.; Elbehary, S.A.; El-Aassar, A.H.M.; Shawky, H.A.; Mehany, M.A.S.; Khalil, M.M.H. Fabrication and characterization of nanocomposite hollow fiber membranes for wastewater treatment. Mater. Sci. Eng. B 2025, 321, 118557. [Google Scholar] [CrossRef]
- Varol, H.S.; Seeger, S. Fluorescent Staining of Silicone Micro- and Nanopatterns for Their Optical Imaging. Langmuir 2022, 38, 231–243. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, B.; Zhu, T.; Shi, J.; Xu, Z.; Hu, C.; Wang, J. Facile and low-cost approach towards a PVDF ultrafiltration membrane with enhanced hydrophilicity and antifouling performance via graphene oxide/water-bath coagulation. RSC Adv. 2015, 5, 7880–7889. [Google Scholar] [CrossRef]
- Li, M.-P.; Zhang, X.; Zhang, H.; Liu, W.-L.; Huang, Z.-H.; Xie, F.; Ma, X.-H.; Xu, Z.-L. Hydrophilic yolk-shell ZIF-8 modified polyamide thin-film nanocomposite membrane with improved permeability and selectivity. Sep. Purif. Technol. 2020, 247, 116990. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Yu, X.; Lin, T.; Xu, H.; Tao, H.; Chen, W. Ultrafiltration of up-flow biological activated carbon effluent: Extracellular polymer biofouling mechanism and mitigation using pre-ozonation with H2O2 backwashing. Water Res. 2020, 186, 116391. [Google Scholar] [CrossRef]
- Yuan, X.-T.; Wu, L.; Geng, H.-Z.; Wang, L.; Wang, W.; Yuan, X.-S.; He, B.; Jiang, Y.-X.; Ning, Y.-J.; Zhu, Z.-R.; et al. Polyaniline/polysulfone ultrafiltration membranes with improved permeability and anti-fouling behavior. J. Water Process Eng. 2021, 40, 101903. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, H.; Huang, D.; Gao, X.; Zhang, B.; Shen, Y.; Shi, W. Effect of pH on anionic polyacrylamide adhesion: New insights into membrane fouling based on XDLVO analysis. J. Mol. Liq. 2020, 320, 114463. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Liu, Y.; Cao, K.; Zhu, M.; Liu, H.; Tang, Y.; Wang, Q. Self-cleaning PDA/TA/FeOOH-PVDF membrane for efficient separation of volatile oil from water of traditional Chinese medicine. J. Membr. Sci. 2025, 734, 124467. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Zhao, Z.; Wang, P.; Wang, S.; Ma, J.; Cheng, W. High-stable bimetallic AgCu nanoalloys with core-shell structures for sustainable antibacterial and biofouling mitigation in nanofiltration. Water Res. 2025, 271, 122986. [Google Scholar] [CrossRef]
- Xiao, F.; Ge, H.; Wang, Y.; Bian, S.; Tong, Y.; Gao, C.; Zhu, G. Novel thin-film composite membrane with polydopamine-modified polyethylene support and tannic acid-Fe3+ interlayer for forward osmosis applications. J. Membr. Sci. 2022, 642, 119976. [Google Scholar] [CrossRef]
- Thekkayil, R.; John, H.; Gopinath, P. Grafting of self assembled polyaniline nanorods on reduced graphene oxide for nonlinear optical application. Synth. Met. 2013, 185–186, 38–44. [Google Scholar] [CrossRef]
- Jang, H.; Song, D.H.; Kim, I.C.; Kwon, Y.N. Fouling control through the hydrophilic surface modification of poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2014, 132, 41712. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Tang, J.; Yang, Y.W. Surface Immobilization of pH-Responsive Polymer Brushes on Mesoporous Silica Nanoparticles by Enzyme Mimetic Catalytic ATRP for Controlled Cargo Release. Polymers 2016, 8, 277. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Liu, Y.; Liu, M.; Zhu, C.; Zhang, L.; Zhang, H. A novel lithium ion-imprinted membrane with robust adsorption capacity and anti-fouling property based on the uniform multilayered interlayer. Desalination 2022, 539, 115973. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, J.; Liu, Y.; Li, Y.; Zhao, C.; Yang, Z.; Cai, L.; Yu, J.; Chen, Z.; Hu, Z. Tannic acid/ethylenediamine/succinic acid graft modified PVDF anti-pollution membrane and its application in the field of organic pollutant separation. J. Mater. Sci. 2022, 57, 20156–20173. [Google Scholar] [CrossRef]
- Chen, F.; Dong, S.; Wang, Z.; Xu, J.; Xu, R.; Wang, J. Preparation of mixed matrix composite membrane for hydrogen purification by incorporating ZIF-8 nanoparticles modified with tannic acid. Int. J. Hydrogen Energy 2020, 45, 7444–7454. [Google Scholar] [CrossRef]
- Battistoni, C.; Dormann, J.L.; Fiorani, D.; Paparazzo, E.; Viticoli, S. An XPS and Mössbauer study of the electronic properties of ZnCrxGa2−xO4 spinel solid solutions. Solid. State Commun. 1981, 39, 581–585. [Google Scholar] [CrossRef]
- Tan, H.; Tang, Y.; Hou, Z.; Yang, P.; Liu, C.; Xie, Z.; Li, S. Antimicrobial polymer-based zeolite imidazolate framework composite membranes for uranium extraction from wastewater and seawater. J. Colloid. Interface Sci. 2025, 677, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.; Sun, J.; Sun, J.; Wang, Y. Preparation and characterization of superior hydrophilic PVDF/DA membranes by the self-polymerization approach of dopamine. Front. Chem. 2023, 11, 1162348. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Zhang, Z.; Ren, L.; Han, J.; Zhang, H.; Li, J.; Wu, H.; Chen, J. Hydrophilic modification of PVDF membranes for oily water separation with enhanced anti-fouling performance. J. Appl. Polym. Sci. 2023, 140, e53738. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, J.; Zhang, J.; Li, J.; Qin, S.; Wu, X.; Cui, Z. Removal of heavy metal ions from acidic wastewater by constructing positively charged hollow fiber nanofiltration separating-layer based on Fe (III)/co deposition-quaternization. J. Water Process Eng. 2023, 56, 104450. [Google Scholar] [CrossRef]
- Yang, J.; Sun, W.; Ju, J.; Tan, Y.; Yuan, H. Facile Fabrication of Superwetting PVDF Membrane for Highly Efficient Oil/Water Separation. Polymers 2023, 15, 327. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Wang, W.; Wang, P.; Ni, L.; Wang, S.; Ma, J.; Cheng, W. Amine-Modified ZIF Composite Membranes: Regulated Nanochannel Interactions for Enhanced Cation Transport and Precise Separation. Environ. Sci. Technol. 2025, 59, 4199–4209. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Sun, Z.; Jiang, H.; Sun, X.; Ma, J.; He, M.; Zhang, W. Thin and defect-free ZIF-8 layer assisted enhancement of the monovalent perm-selectivity for cation exchange membrane. Desalination 2022, 529, 115637. [Google Scholar] [CrossRef]
- Bi, Q.; Li, Q.; Tian, Y.; Lin, Y.; Wang, X. Hydrophilic modification of poly(vinylidene fluoride) membrane with poly(vinyl pyrrolidone) via a cross-linking reaction. J. Appl. Polym. Sci. 2012, 127, 394–401. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Zhang, J. Zeolite imidazolate framework hybrid nanofiltration (NF) membranes with enhanced permselectivity for dye removal. J. Membr. Sci. 2017, 532, 76–86. [Google Scholar] [CrossRef]
- Liu, C.; Song, D.; Zhang, W.; He, Q.; Huangfu, X.; Sun, S.; Sun, Z.; Cheng, W.; Ma, J. Constructing zwitterionic polymer brush layer to enhance gravity-driven membrane performance by governing biofilm formation. Water Res. 2020, 168, 115181. [Google Scholar] [CrossRef]
| Membrane | DA (g/L) | TA (g/L) | Zn2+ (mol/L) | Hmim (mmol/L) | Deposition Time (h) |
|---|---|---|---|---|---|
| DA-UF | 1 | - | - | - | 6 |
| TA-UF | - | 4 | - | - | 6 |
| DA/TA-UF | 1 | 4 | - | - | 6 |
| DA/ZIF-8-UF | 1 | - | 0.015 | 0.7 | 6 |
| TA/ZIF-8-UF | - | 4 | 0.015 | 0.7 | 6 |
| DA/TA/ZIF-8-UF | 1 | 4 | 0.015 | 0.7 | 6 |
| Membrane | C | N | O | F | Zn |
|---|---|---|---|---|---|
| PVDF substrate | 58.23 | 0.97 | 6.87 | 33.94 | - |
| DA/TA-UF | 67.98 | 3.93 | 27.44 | 0.66 | - |
| DA/TA/ZIF-8-UF | 69.44 | 17.75 | 6.12 | 0.55 | 6.15 |
| Materials | γAB | γTOT | |||
| Commodity UF | 33.65 | 0.11 | 2.33 | 1.01 | 34.66 |
| DA/TA/ZIF-8-UF | 38.18 | 0.42 | 64.97 | 10.45 | 48.63 |
| BSA | 43.22 | 1.065 | 47.68 | 14.25 | 57.47 |
| OVA | 35.57 | 0.41 | 50.76 | 9.12 | 44.69 |
| LYZ | 26.71 | 0.93 | 28.31 | 10.26 | 36.97 |
| Commercial UF-BSA | −4.31 | 24.94 | −0.00080 | 20.63 | - |
| DA/TA/ZIF-8-UF-BSA | −5.75 | 40.51 | −0.00080 | 34.76 | - |
| BSA-BSA | −2.15 | 39.77 | - | - | 37.62 |
| Commercial UF-OVA | −2.93 | 27.12 | −0.00053 | 24.19 | - |
| DA/TA/ZIF-8-UF-OVA | −3.91 | 44.82 | −0.00053 | 40.90 | - |
| OVA-OVA | −1.83 | 52.36 | - | - | 50.53 |
| Commercial UF-LYZ | −1.13 | −36.77 | −0.00137 | −37.90 | - |
| DA/TA/ZIF-8-UF-LYZ | −1.51 | −26.98 | −0.00137 | −28.49 | - |
| LYZ-LYZ | −3.74 | −6.52 | - | - | −10.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Cui, Q.; Wang, Z.; Zhang, X.; Ma, J.; Zhang, W.; Liu, C. Zn2+-Mediated Co-Deposition of Dopamine/Tannic Acid/ZIF-8 on PVDF Hollow Fiber Membranes for Enhanced Antifouling Performance and Protein Separation. Membranes 2025, 15, 277. https://doi.org/10.3390/membranes15090277
Ni L, Cui Q, Wang Z, Zhang X, Ma J, Zhang W, Liu C. Zn2+-Mediated Co-Deposition of Dopamine/Tannic Acid/ZIF-8 on PVDF Hollow Fiber Membranes for Enhanced Antifouling Performance and Protein Separation. Membranes. 2025; 15(9):277. https://doi.org/10.3390/membranes15090277
Chicago/Turabian StyleNi, Lei, Qiancheng Cui, Zhe Wang, Xueting Zhang, Jun Ma, Wenjuan Zhang, and Caihong Liu. 2025. "Zn2+-Mediated Co-Deposition of Dopamine/Tannic Acid/ZIF-8 on PVDF Hollow Fiber Membranes for Enhanced Antifouling Performance and Protein Separation" Membranes 15, no. 9: 277. https://doi.org/10.3390/membranes15090277
APA StyleNi, L., Cui, Q., Wang, Z., Zhang, X., Ma, J., Zhang, W., & Liu, C. (2025). Zn2+-Mediated Co-Deposition of Dopamine/Tannic Acid/ZIF-8 on PVDF Hollow Fiber Membranes for Enhanced Antifouling Performance and Protein Separation. Membranes, 15(9), 277. https://doi.org/10.3390/membranes15090277






