Fouling Mechanisms in the Clarification of 1,3-Propanediol Fermentation Broths by Membrane Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Fermentation Broth
2.2. Membrane Separation Setup and Process Parameters
2.3. Evaluation of Membrane Filtration Performance
2.3.1. Permeate Flux
2.3.2. Filtration Efficiency
2.4. Models for Analysis of Complex Process Behavior
2.4.1. Resistance-in-Series Model
2.4.2. Fouling Propensity Model
2.4.3. Hermans and Bredee Model
2.5. Rheological Measurements
2.6. Determination of Basic Properties
2.7. Determination of 1,3-PD and Glycerol Contents
2.8. Determination of Membrane Properties
2.9. Data Processing
3. Results and Discussion
3.1. Evaluation of Membrane Filtration Performance
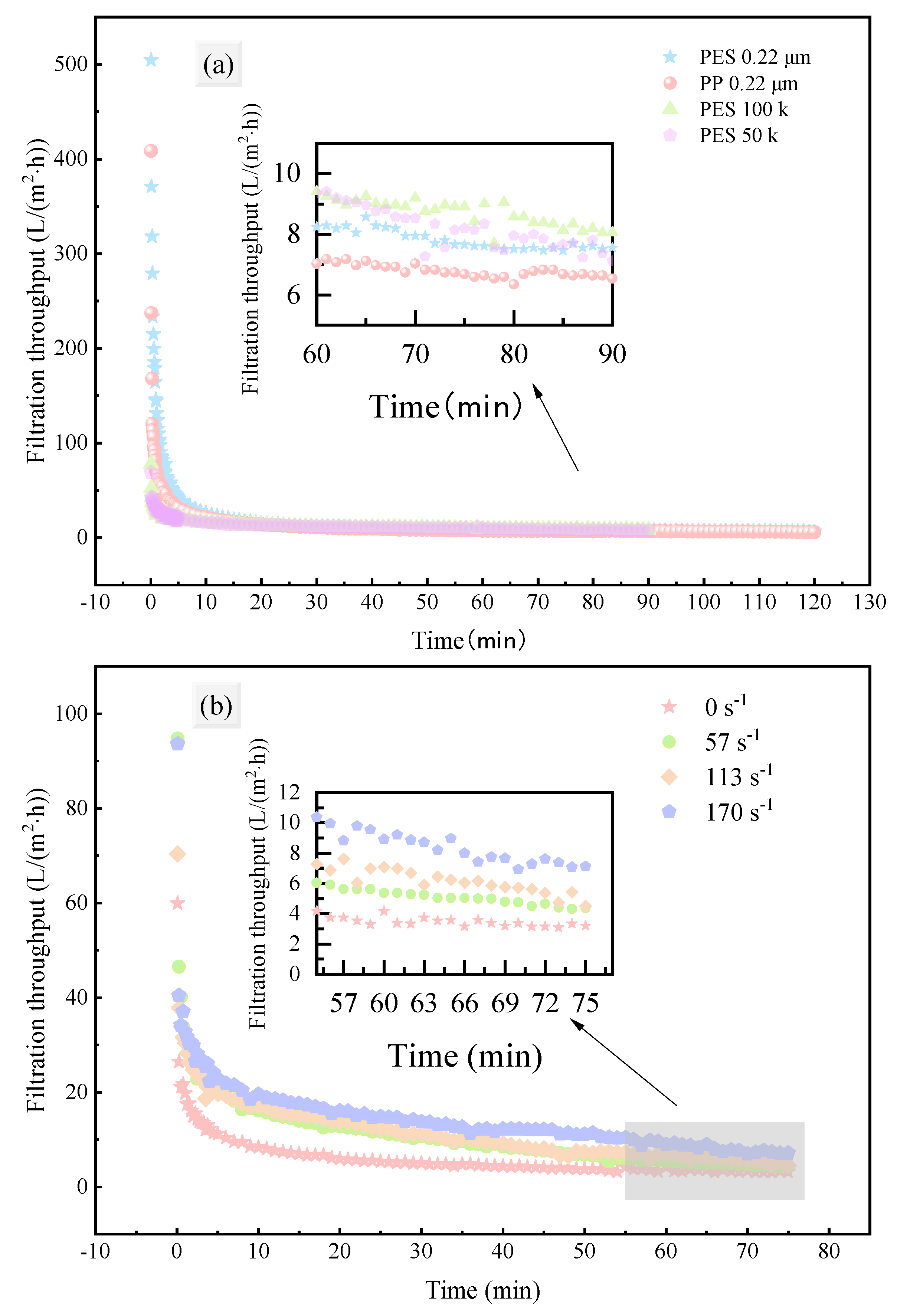
3.1.1. Evolution of Permeate Flux
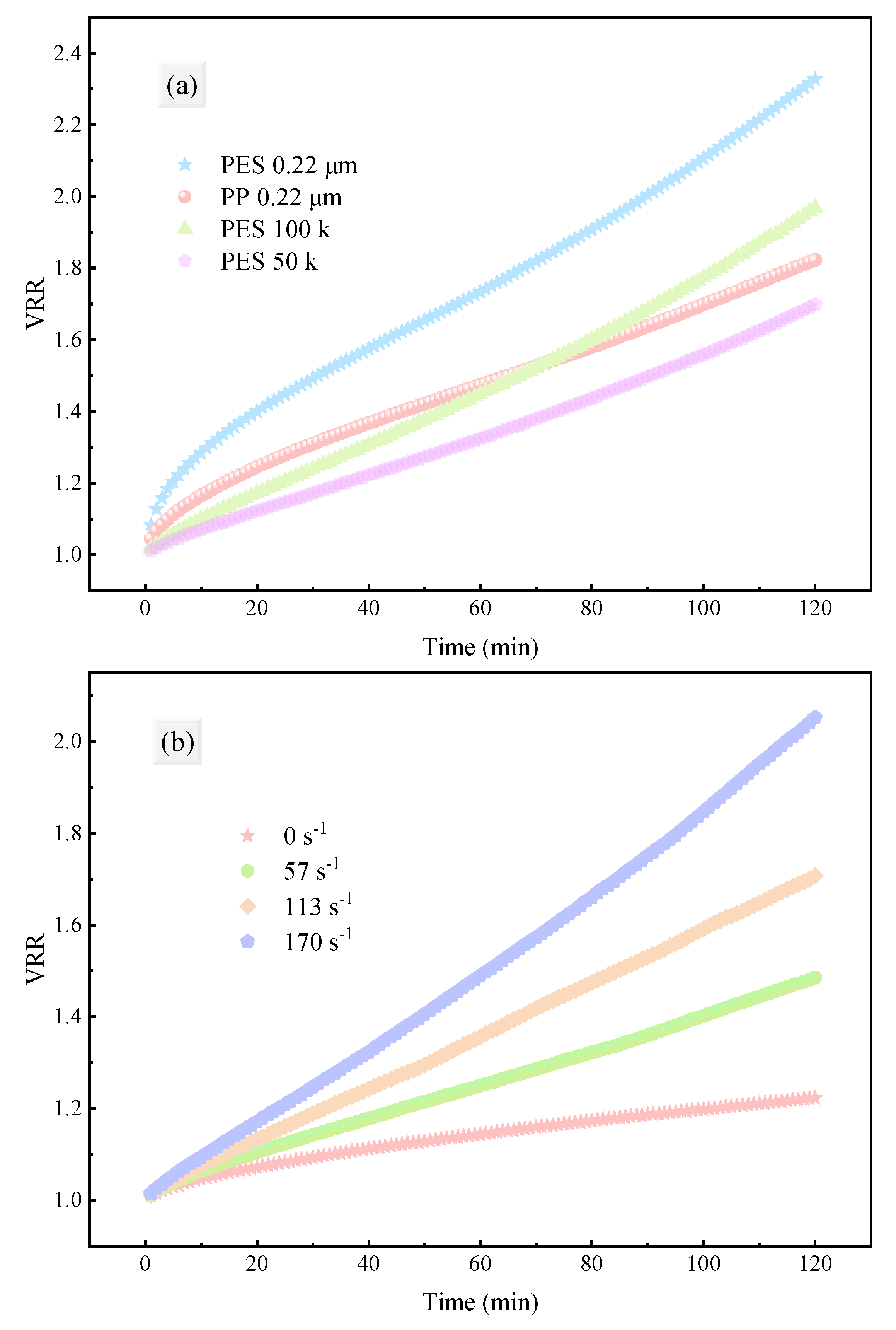
3.1.2. Filtration Efficiency Evaluation
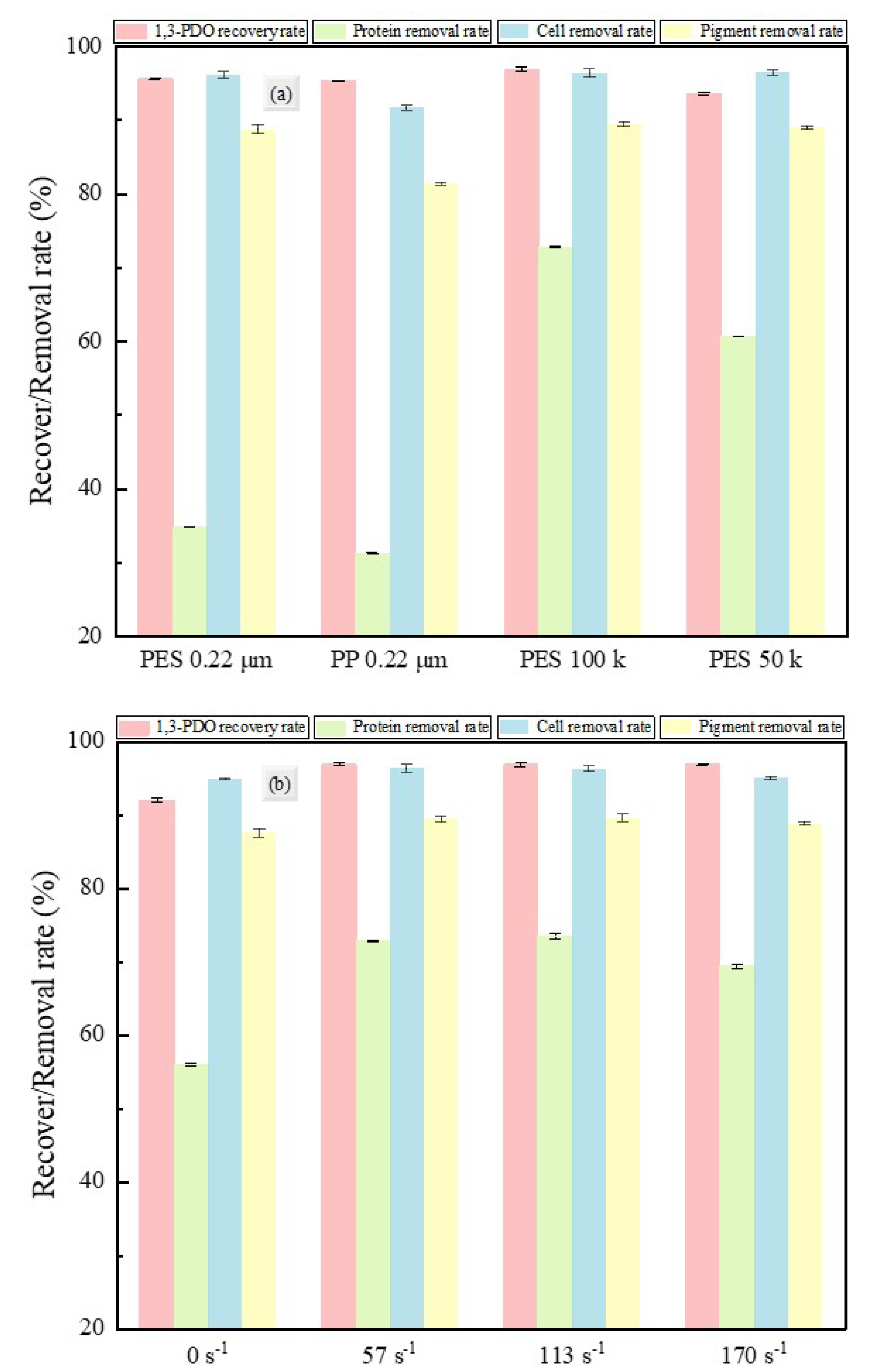
3.1.3. 1,3-PD Recovery and Impurity Removal Rates
3.2. Intrinsic Properties of Fermentation Broth
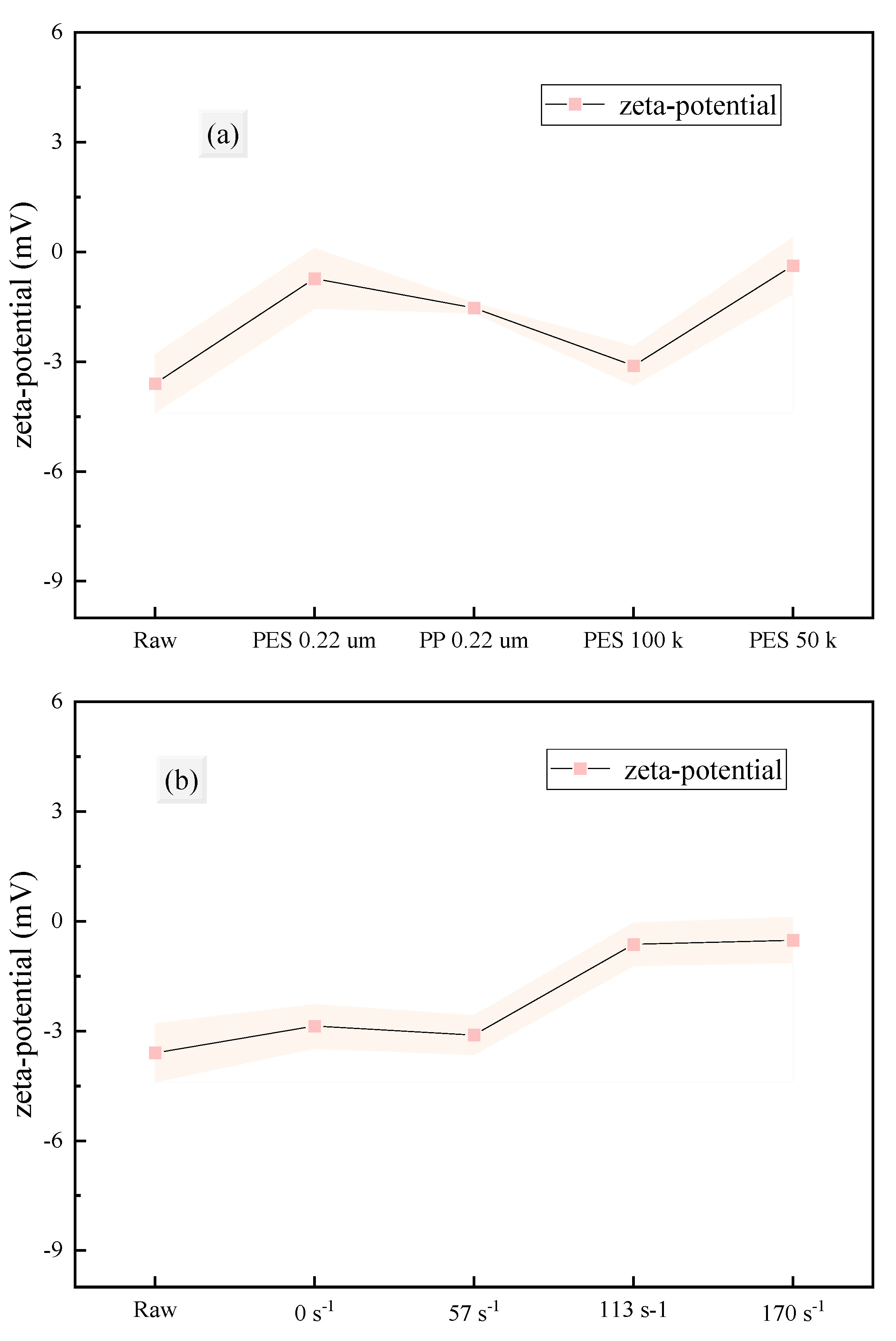
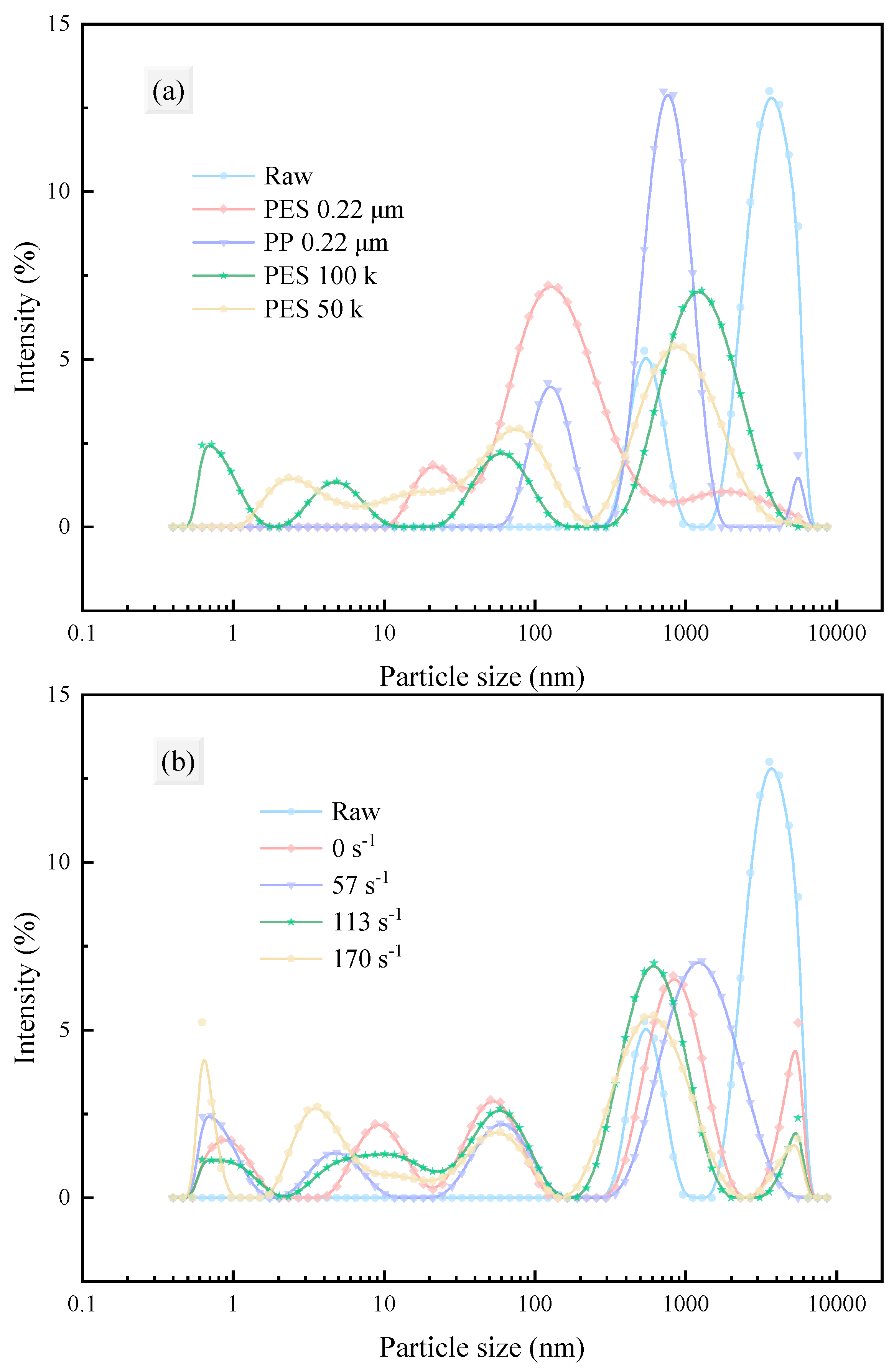
3.2.1. Particle Properties
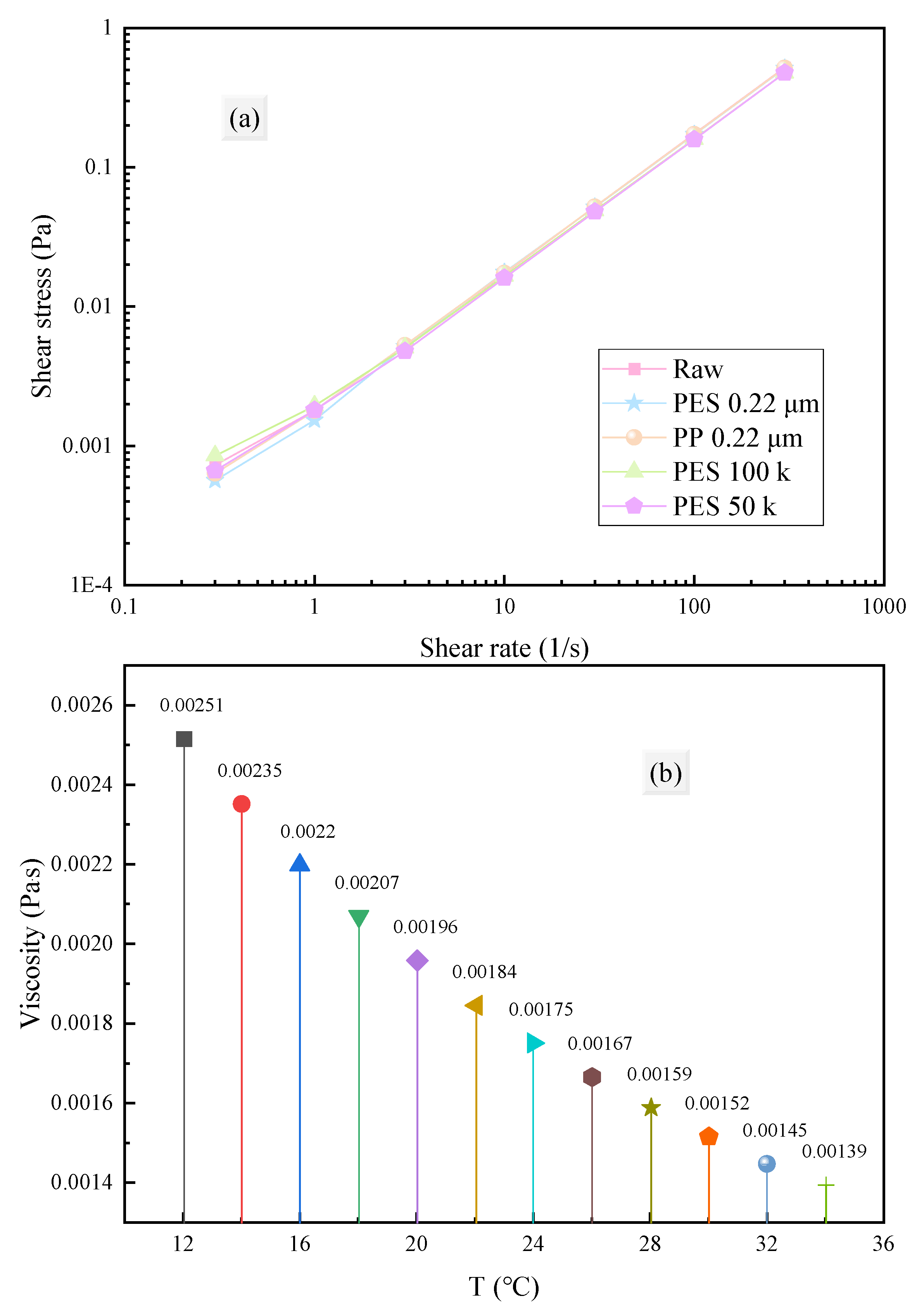
3.2.2. Rheological Properties
3.3. Membrane Fouling Mechanism
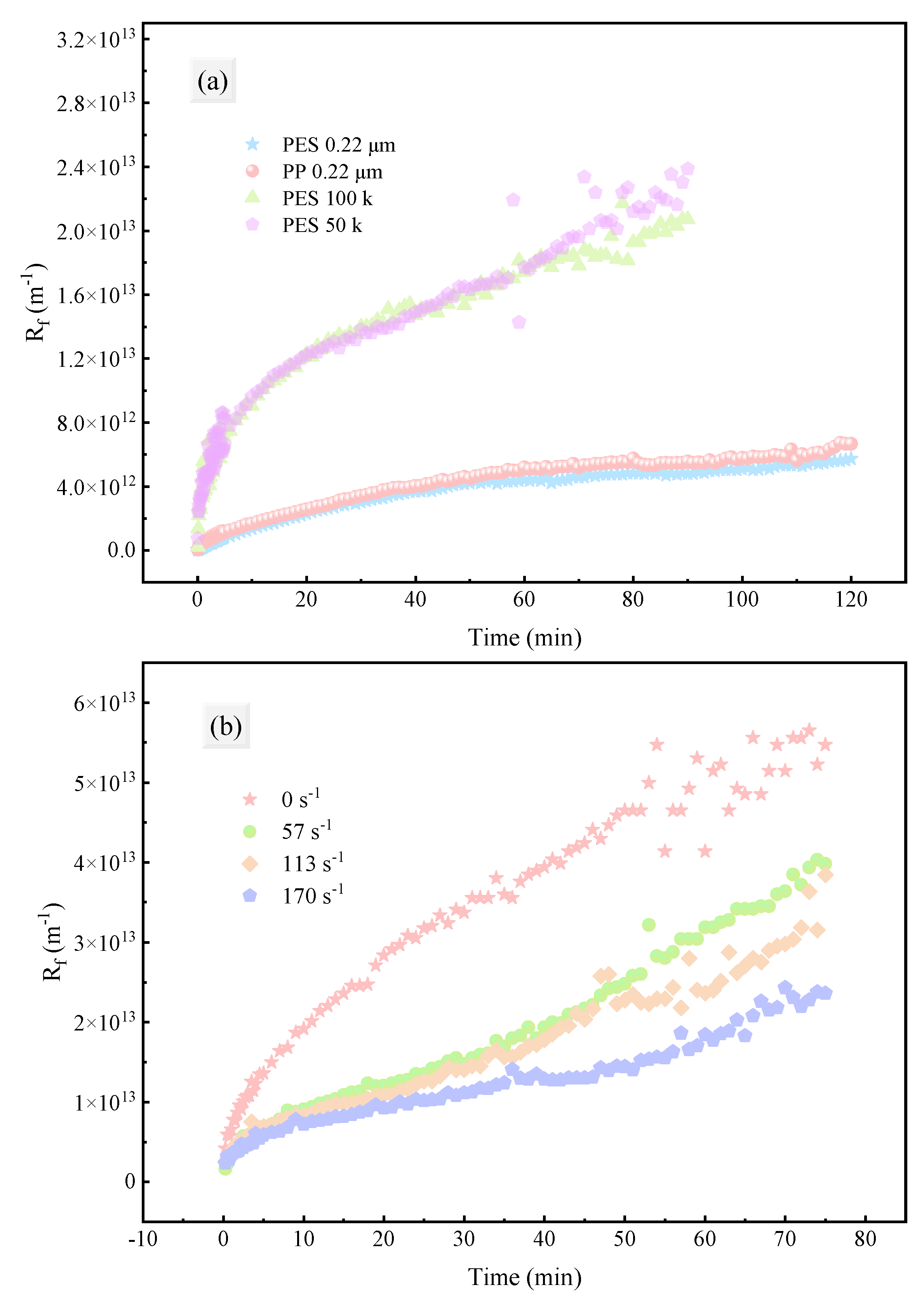
3.3.1. Resistance Distribution
3.3.2. Fouling Propensity
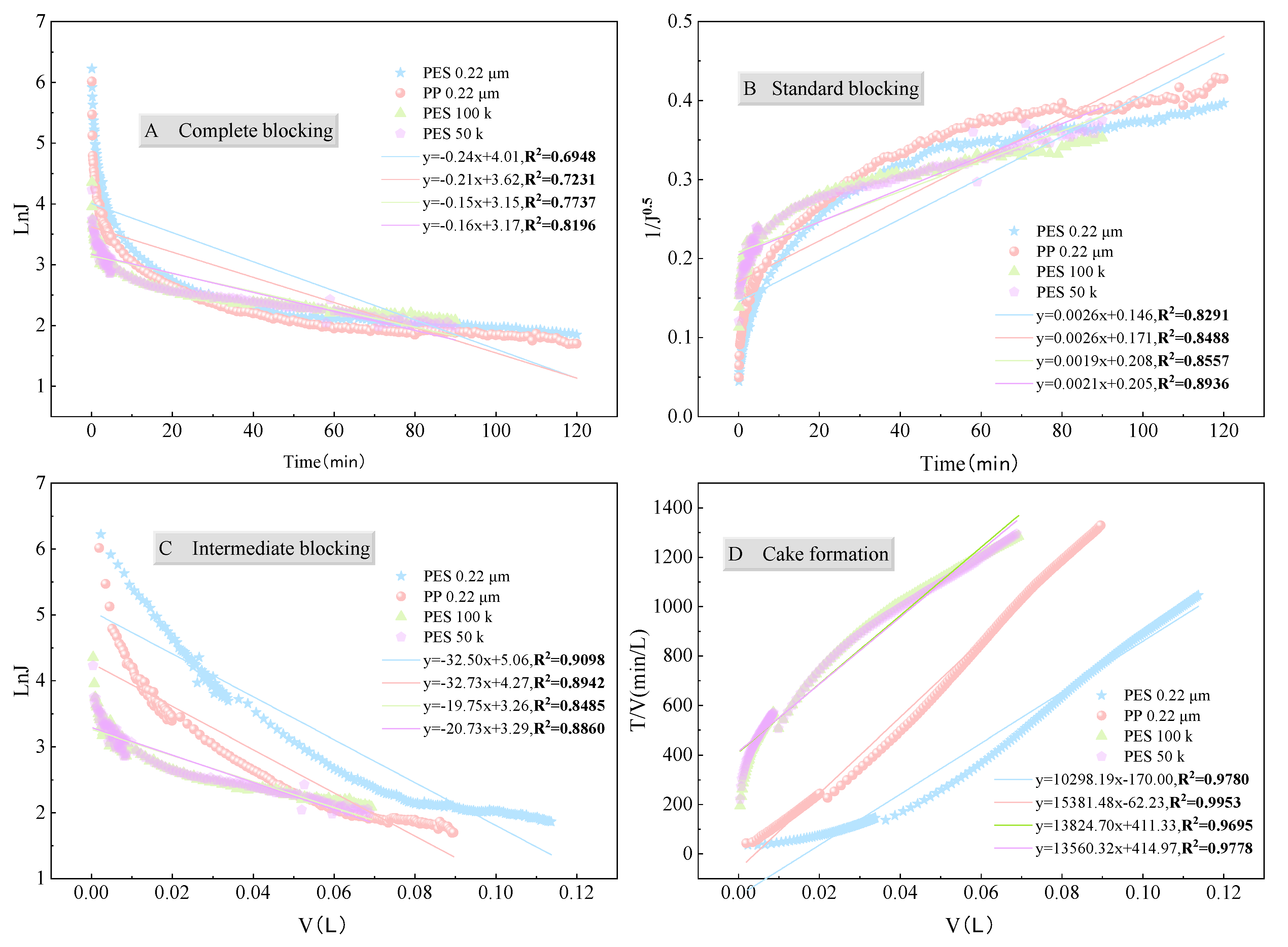
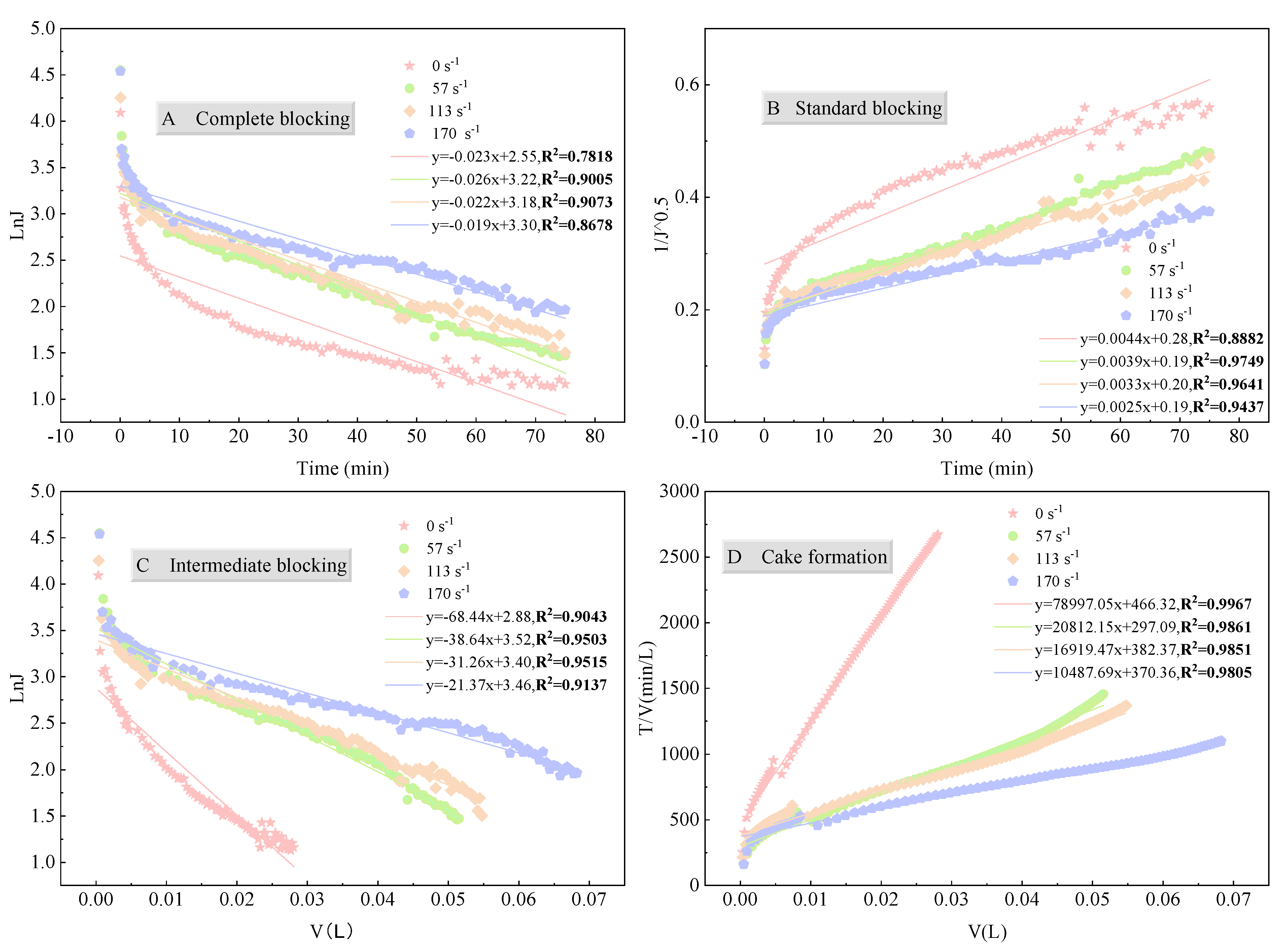
3.3.3. Membrane Pore Blocking Mechanism
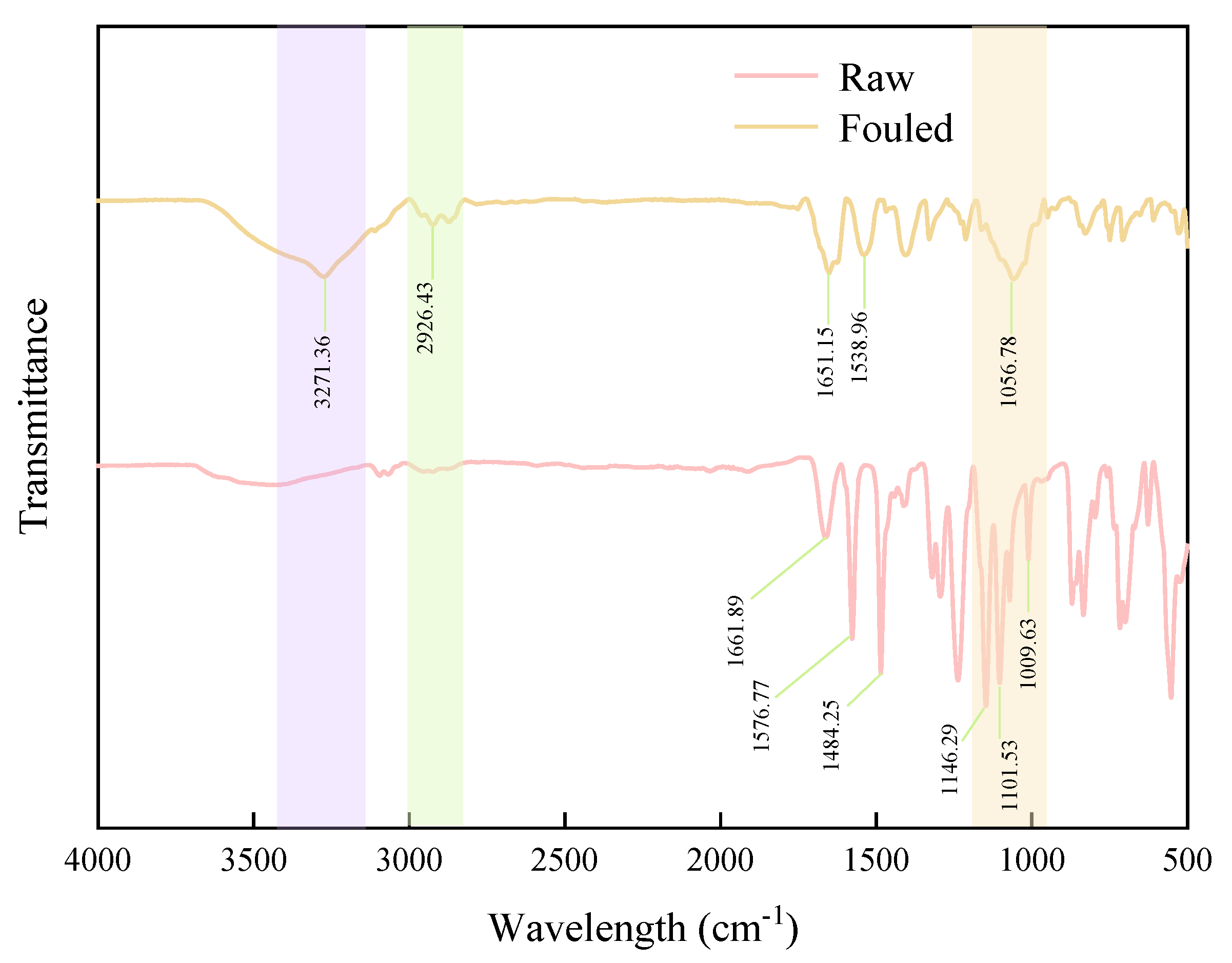
3.4. Foulants Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Chandure, A.; Umare, S.; Pandey, R. Synthesis and biodegradation studies of 1,3-propanediol based aliphatic poly(ester carbonate)s. Eur. Polym. J. 2008, 44, 2068–2086. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Shen, J.-T.; Yan, L.; Zhou, J.-J.; Jiang, L.-L.; Chen, Y.; Yuan, J.-L.; Feng, E.; Xiu, Z.-L. Advances in bioconversion of glycerol to 1,3-propanediol: Prospects and challenges. Process. Biochem. 2018, 71, 134–146. [Google Scholar] [CrossRef]
- Saxena, R.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.-L.; Zeng, A.-P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926. [Google Scholar] [CrossRef]
- Hao, J.; Xu, F.; Liu, H.; Liu, D. Downstream processing of 1,3-propanediol fermentation broth. J. Chem. Technol. Biotechnol. 2005, 81, 102–108. [Google Scholar] [CrossRef]
- Johnson, E.E.; Rehmann, L. The role of 1,3-propanediol production in fermentation of glycerol by Clostridium pasteurianum. Bioresour. Technol. 2016, 209, 1–7. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, B.; Zhang, D.; Xiu, Z. Aqueous two-phase extraction of 1,3-propanediol from glycerol-based fermentation broths. Sep. Purif. Technol. 2009, 66, 472–478. [Google Scholar] [CrossRef]
- Sui, W.-B.; Huang, L.-S.; Wang, X.-L.; Zhou, X.; Sun, Y.-Q.; Xiu, Z.-L. Extractive adsorption of 1,3-propanediol on a novel polystyrene macroporous resin enclosing medium and long-chain alcohols as extractant. Bioresour. Bioprocess. 2023, 10, 28. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Lu, Y.; Zhang, C.; Lu, Z. An efficient method for separation of surfactin from Bacillus amyloliquefaciens fmb50 broth by flocculation. Process. Biochem. 2014, 49, 1182–1188. [Google Scholar] [CrossRef]
- Anand, P.; Saxena, R.K.; Marwah, R.G. A novel downstream process for 1,3-propanediol from glycerol-based fermentation. Appl. Microbiol. Biotechnol. 2011, 90, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sharma, S.; Wang, W.; Zeng, A. A novel downstream process for highly pure 1,3-propanediol from an efficient fed-batch fermentation of raw glycerol by Clostridium pasteurianum. Eng. Life Sci. 2021, 21, 351–363. [Google Scholar] [CrossRef]
- Li, Z.; Yan, L.; Zhou, J.; Wang, X.; Sun, Y.; Xiu, Z.-L. Two-step salting-out extraction of 1,3-propanediol, butyric acid and acetic acid from fermentation broths. Sep. Purif. Technol. 2019, 209, 246–253. [Google Scholar] [CrossRef]
- Sui, W.-B.; Sun, Y.-Q.; Wang, X.-L.; Xiu, Z.-L. Synergistic Extraction of 1,3-Propanediol from Fermentation Broths Using Multialcohol Extractants. ACS Sustain. Chem. Eng. 2022, 10, 11891–11901. [Google Scholar] [CrossRef]
- Bastrzyk, J.; Gryta, M. Separation of post-fermentation glycerol solution by nanofiltration membrane distillation system. Desalination Water Treat. 2015, 53, 319–329. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef]
- Li, S.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Effects of Zeolite Membrane Structure on the Separation of 1,3-Propanediol from Glycerol and Glucose by Pervaporation. Chem. Mater. 2001, 13, 1865–1873. [Google Scholar] [CrossRef]
- Li, S. Separation of 1,3-propanediol from glycerol and glucose using a ZSM-5 zeolite membrane. J. Membr. Sci. 2001, 191, 53–59. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, D.; Sun, Y.; Xiu, Z. Separation of 1,3-propanediol from glycerol-based fermentations of Klebsiella pneumoniae by alcohol precipitation and dilution crystallization. Front. Chem. Eng. China 2007, 1, 202–207. [Google Scholar] [CrossRef]
- Tomczak, W.; Grubecki, I.; Gryta, M. The Use of NaOH Solutions for Fouling Control in a Membrane Bioreactor: A Feasibility Study. Membranes 2021, 11, 887. [Google Scholar] [CrossRef]
- Tomczak, W. The Application of the Nanofiltration Membrane NF270 for Separation of Fermentation Broths. Membranes 2022, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study. Int. J. Mol. Sci. 2022, 23, 11738. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M.; Żak, S.; Daniluk, M. Application of Industrial NF and RO Membranes in Separation of Post-Fermentation Solutions: Preliminary Study. Materials 2025, 18, 2779. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Van Tran, T.T.; Wu, Z.-H.; Juang, R.-S. Comparison of strategies for enhanced production of 1,3-propanediol from fermentation broths of Klebsiella pneumoniae. J. Taiwan Inst. Chem. Eng. 2025, 168, 105944. [Google Scholar] [CrossRef]
- Peng, B.; Li, Z.; Xiong, Q.; Wu, C.; Huang, J.; Zhou, R.; Jin, Y. Casein-dextran complexes subjected to microfiltration: Colloidal properties and their corresponding processing behaviors. J. Food Eng. 2022, 320, 110913. [Google Scholar] [CrossRef]
- Wen, S.; Huang, J.; Zhou, R.; Wu, C.; Hengl, N.; Pignon, F.; Jin, Y. Molecular mechanism of casein-chitosan fouling during microfiltration. Sep. Purif. Technol. 2023, 325, 124659. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Huang, J.; Zhou, R.; Wu, C.; Jin, Y. Microfiltration of soy sauce: Efficiency, resistance and fouling mechanism at different operating stages. Sep. Purif. Technol. 2020, 240, 116656. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, J.; Ding, L.; Bals, O.; Jaffrin, M.Y.; Vorobiev, E. Chicory juice clarification by membrane filtration using rotating disk module. J. Food Eng. 2013, 115, 264–271. [Google Scholar] [CrossRef]
- Acosta, O.; Vaillant, F.; Pérez, A.M.; Dornier, M. Concentration of Polyphenolic Compounds in Blackberry (Rubus Adenotrichos Schltdl.) Juice by Nanofiltration. J. Food Process. Eng. 2016, 40, e12343. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Z.; Huang, J.; Wu, C.; Zhou, R.; Jin, Y. Tanning Wastewater Treatment by Ultrafiltration: Process Efficiency and Fouling Behavior. Membranes 2021, 11, 461. [Google Scholar] [CrossRef]
- Xiong, Q.-M.; Liu, J.; Liu, M.; Shen, C.-H.; Yu, X.-C.; Wu, C.-D.; Huang, J.; Zhou, R.-Q.; Jin, Y. Fouling analysis and permeate quality evaluation of mulberry wine in microfiltration process. RSC Adv. 2020, 10, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, J.; Zhou, R.; Wu, C.; Jin, Y. Microfiltration of raw soy sauce: Membrane fouling mechanisms and characterization of physicochemical, aroma and shelf-life properties. RSC Adv. 2019, 9, 2928–2940. [Google Scholar] [CrossRef] [PubMed]
- de la Garza, F.; Boulton, R. The Modeling of Wine Filtrations. Am. J. Enol. Vitic. 1984, 35, 189–195. [Google Scholar] [CrossRef]
- Gökmen, V.; Çetinkaya, Ö. Effect of pretreatment with gelatin and bentonite on permeate flux and fouling layer resistance during apple juice ultrafiltration. J. Food Eng. 2007, 80, 300–305. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, X.; Yin, F.; Li, S.; He, J. Clarification of Jerusalem Artichoke Extract Using Ultra-filtration: Effect of Membrane Pore Size and Operation Conditions. Food Bioprocess Technol. 2018, 11, 864–873. [Google Scholar] [CrossRef]
- Hermans, P.H.; Bredée, H.L. Principles of the Mathematical Treatment of Constant-Pressure Filtration. J. Soc. Chem. Ind. 1936, 55, 1–4. [Google Scholar]
- Zheng, Y.; Zhang, W.; Tang, B.; Ding, J.; Zheng, Y.; Zhang, Z. Membrane fouling mechanism of biofilm-membrane bioreactor (BF-MBR): Pore blocking model and membrane cleaning. Bioresour. Technol. 2018, 250, 398–405. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.-J.; Álvarez-Blanco, S.; Vincent-Vela, M.-C. Fouling mechanisms of ultrafiltration membranes fouled with whey model solutions. Desalination 2015, 360, 87–96. [Google Scholar] [CrossRef]
- Moller, P.; Fall, A.; Chikkadi, V.; Derks, D.; Bonn, D. An attempt to categorize yield stress fluid behaviour. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 5139–5155. [Google Scholar] [CrossRef]
- Jin, Y.; Hengl, N.; Baup, S.; Pignon, F.; Gondrexon, N.; Sztucki, M.; Gésan-Guiziou, G.; Magnin, A.; Abyan, M.; Karrouch, M.; et al. Effects of ultrasound on cross-flow ultrafiltration of skim milk: Characterization from macro-scale to nano-scale. J. Membr. Sci. 2014, 470, 205–218. [Google Scholar] [CrossRef]
- Pierce, J.; Suelter, C. An evaluation of the Coomassie brilliant blue G-250 dye-binding method for quantitative protein determination. Anal. Biochem. 1977, 81, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, X.; Wei, Y.; Ledesma-Amaro, R.; Ji, X.-J. Reprogramming the metabolism of Klebsiella pneumoniae for efficient 1,3-propanediol production. Chem. Eng. Sci. 2021, 236, 116539. [Google Scholar] [CrossRef]
- Toräng, A.; Jönsson, A.-S.; Zacchi, G. Separation of Cells and Proteins from Fermentation Broth in a Shear-Enhanced Cross-Flow Ultrafiltration Module as the First Step in the Refinement of Lactic Acid. Appl. Biochem. Biotechnol. 1999, 76, 143–158. [Google Scholar] [CrossRef]
- Parsin, S.; Kaltschmitt, M. Processing of hemicellulose in wheat straw by steaming and ultrafiltration—A novel approach. Bioresour. Technol. 2023, 393, 130071. [Google Scholar] [CrossRef]
- Marke, H.S.; Breil, M.P.; Hansen, E.B.; Pinelo, M.; Krühne, U. Investigation of the velocity factor in a rotational dynamic microfiltration system. Sep. Purif. Technol. 2019, 220, 69–77. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Borda, D.; Aprodu, I. Alternative Processing Options for Improving the Proteins Functionality by Maillard Conjugation. Foods 2023, 12, 3588. [Google Scholar] [CrossRef]
- Youravong, W.; Grandison, A.S.; Lewis, M.J. Effect of hydrodynamic and physicochemical changes on critical flux of milk protein suspensions. J. Dairy Res. 2002, 69, 443–455. [Google Scholar] [CrossRef]
- Susanto, H.; Franzka, S.; Ulbricht, M. Dextran fouling of polyethersulfone ultrafiltration membranes—Causes, extent and consequences. J. Membr. Sci. 2007, 296, 147–155. [Google Scholar] [CrossRef]
- Šegota, S.; Heimer, S.; Težak, Đ. New catanionic mixtures of dodecyldimethylammonium bromide/sodium dodecylbenzenesulphonate/water. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 274, 91–99. [Google Scholar] [CrossRef]
- Bouzid, H.; Rabiller-Baudry, M.; Paugam, L.; Rousseau, F.; Derriche, Z.; Bettahar, N.E. Impact of zeta potential and size of caseins as precursors of fouling deposit on limiting and critical fluxes in spiral ultrafiltration of modified skim milks. J. Membr. Sci. 2008, 314, 67–75. [Google Scholar] [CrossRef]
- Moayedi, H.; Kazemian, S. Zeta Potentials of Suspended Humus in Multivalent Cationic Saline Solution and Its Effect on Electro-Osomosis Behavior. J. Dispers. Sci. Technol. 2013, 34, 283–294. [Google Scholar] [CrossRef]
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Characteristic properties of crude pineapple waste extract for bromelain purification by membrane processing. J. Food Sci. Technol. 2015, 52, 7103–7112. [Google Scholar] [CrossRef]
- Hongvaleerat, C.; Cabral, L.M.; Dornier, M.; Reynes, M.; Ningsanond, S. Concentration of pineapple juice by osmotic evaporation. J. Food Eng. 2008, 88, 548–552. [Google Scholar] [CrossRef]
- Pradhan, M.; Johir, A.H.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Effects of Viscosity on Submerged Membrane Microfiltration Systems. Membranes 2022, 12, 780. [Google Scholar] [CrossRef]
- Poorasgari, E.; Bugge, T.V.; Christensen, M.L.; Jørgensen, M.K. Compressibility of fouling layers in membrane bioreactors. J. Membr. Sci. 2015, 475, 65–70. [Google Scholar] [CrossRef]
- Meng, F.G.; Zhang, H.M.; Li, Y.S.; Zhang, X.W.; Yang, F.L.; Xiao, J.N. Cake layer morphology in microfiltration of activated sludge wastewater based on fractal analysis. Sep. Purif. Technol. 2005, 44, 250–257. [Google Scholar] [CrossRef]
- Aoustin, E. Ultrafiltration of natural organic matter. Sep. Purif. Technol. 2001, 22-23, 63–78. [Google Scholar] [CrossRef]
- Wu, S.; Hua, X.; Ma, B.; Fan, H.; Miao, R.; Ulbricht, M.; Hu, C.; Qu, J. Three-Dimensional Analysis of the Natural-Organic-Matter Distribution in the Cake Layer to Precisely Reveal Ultrafiltration Fouling Mechanisms. Environ. Sci. Technol. 2021, 55, 5442–5452. [Google Scholar] [CrossRef]
- Saha, N.; Balakrishnan, M.; Ulbricht, M. Sugarcane juice ultrafiltration: FTIR and SEM analysis of polysaccharide fouling. J. Membr. Sci. 2007, 306, 287–297. [Google Scholar] [CrossRef]




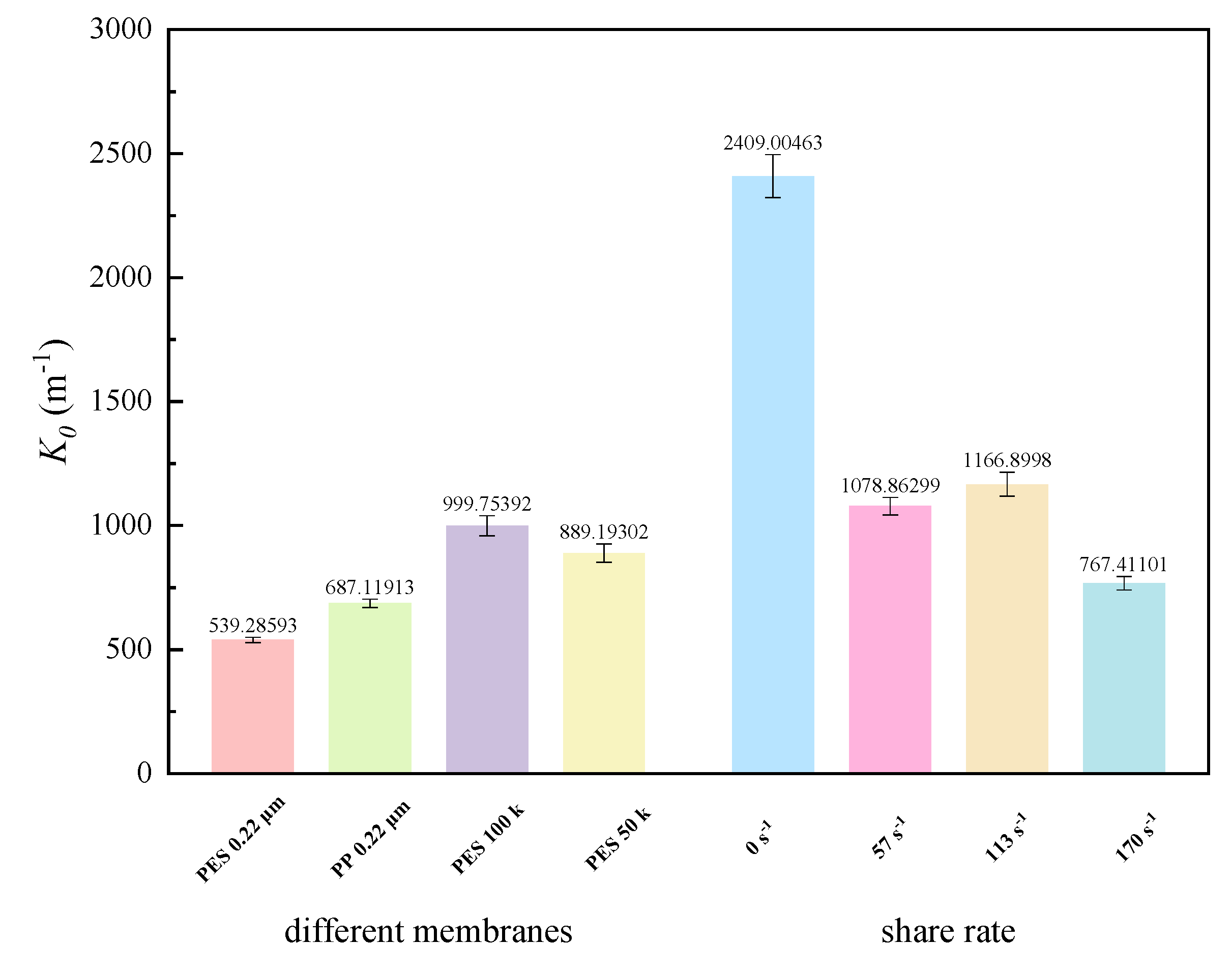
| Reference | Membrane Separation Technology | Research Content (Membrane-Related) | Research on Membrane Fouling Mechanism |
|---|---|---|---|
| [15] | NF and MD | Separation efficiency and process optimization | Mentioned but not thoroughly investigated |
| [9,12,13,14] | UF | Membrane filtration as a pre-treatment step in downstream processing | None |
| [11] | MF | ||
| [16] | MF | Macroscopic performance (flux, turbidity) and cleaning methods | Qualitative description via SEM |
| [20] | UF | Optimization of operational parameters (TMP, feed flow rate Q, feed pH) | Resistance-in-series analysis |
| [22] | UF | Integration of bioreactor with ceramic membrane to product 1,3-PD | None |
| [24] | RO | Exploration on rejection characteristics and antifouling performance of RO for 1,3-PD fermentation broth | None |
| [25] | NF and RO | Investigation of applicability of NF and RO to separate 1,3-PD fermentation broth | None |
| [26] | MF | Combination of ceramic membrane filtration with membrane extraction to improve production of 1,3-PD | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yang, F.; Wang, Q.; Zheng, T.; Zhou, R.; Wu, C.; Jin, Y. Fouling Mechanisms in the Clarification of 1,3-Propanediol Fermentation Broths by Membrane Processes. Membranes 2025, 15, 276. https://doi.org/10.3390/membranes15090276
Chen H, Yang F, Wang Q, Zheng T, Zhou R, Wu C, Jin Y. Fouling Mechanisms in the Clarification of 1,3-Propanediol Fermentation Broths by Membrane Processes. Membranes. 2025; 15(9):276. https://doi.org/10.3390/membranes15090276
Chicago/Turabian StyleChen, Hong, Fu Yang, Qianyu Wang, Tianyu Zheng, Rongqing Zhou, Chongde Wu, and Yao Jin. 2025. "Fouling Mechanisms in the Clarification of 1,3-Propanediol Fermentation Broths by Membrane Processes" Membranes 15, no. 9: 276. https://doi.org/10.3390/membranes15090276
APA StyleChen, H., Yang, F., Wang, Q., Zheng, T., Zhou, R., Wu, C., & Jin, Y. (2025). Fouling Mechanisms in the Clarification of 1,3-Propanediol Fermentation Broths by Membrane Processes. Membranes, 15(9), 276. https://doi.org/10.3390/membranes15090276








