Interpenetrating Nanofibrous Composite Membranes for Removal and Reutilization of P (V) Ions from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
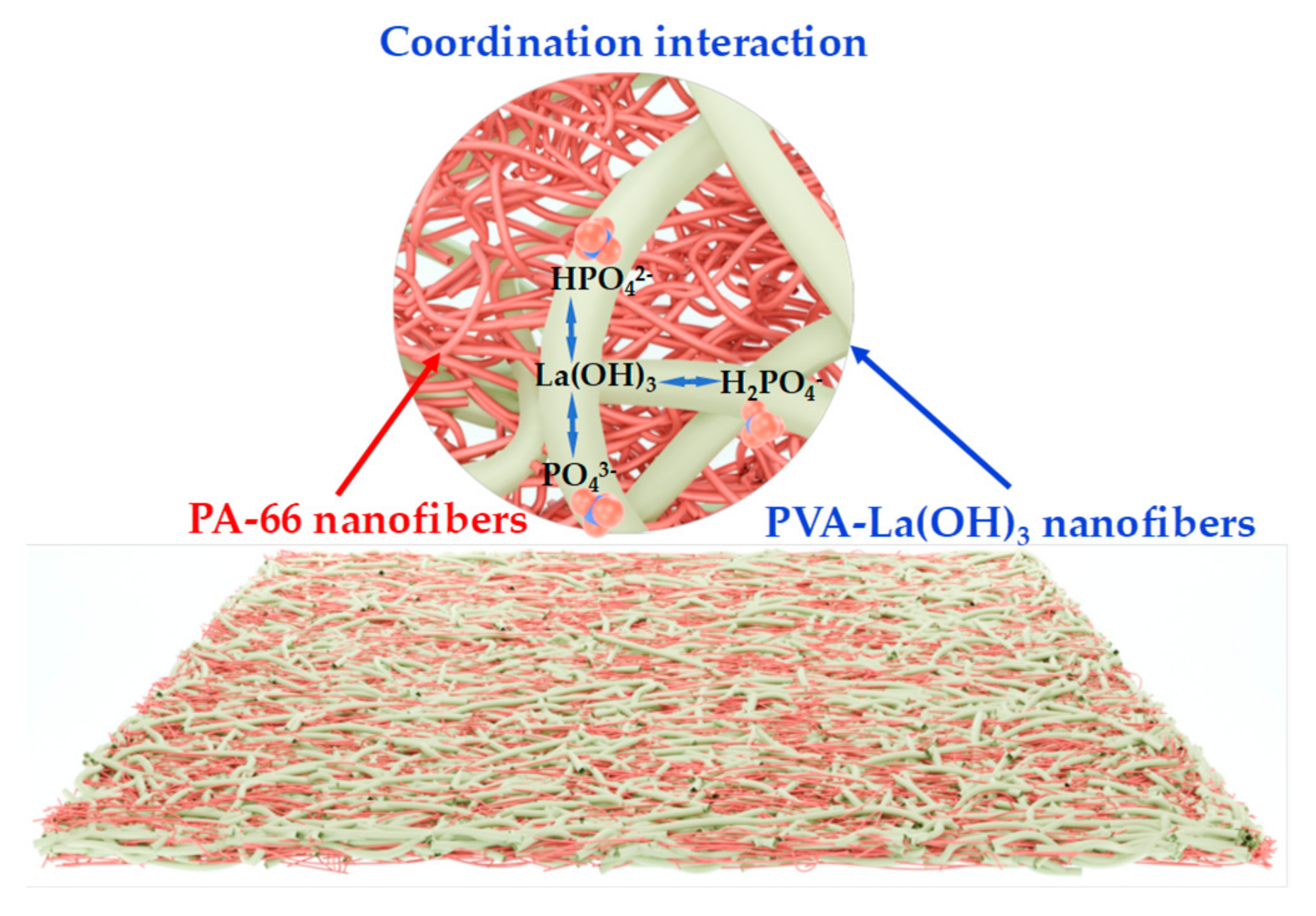
2.2. Membrane Fabrication
2.3. Adsorption Experiments
2.3.1. Static Adsorption of the Nanofibrous Composite Membranes
2.3.2. Dynamic Adsorption of the Nanofibrous Composite Membranes
2.3.3. Phosphorus Release of the Phosphate-Loaded Nanofibrous Nanofiber Membranes
2.3.4. Desorption and Regeneration Experiments
2.4. Characterizations
2.4.1. Surface Morphologies and Pore Size
2.4.2. Mechanical Property Tests
2.4.3. Thermogravimetric Analysis
2.4.4. Fourier Transform Infrared Spectroscopy
2.4.5. X-Ray Photoelectron Spectroscopy Measurements
3. Results and Discussion
3.1. Characterization of Composite Nanofiber Membranes
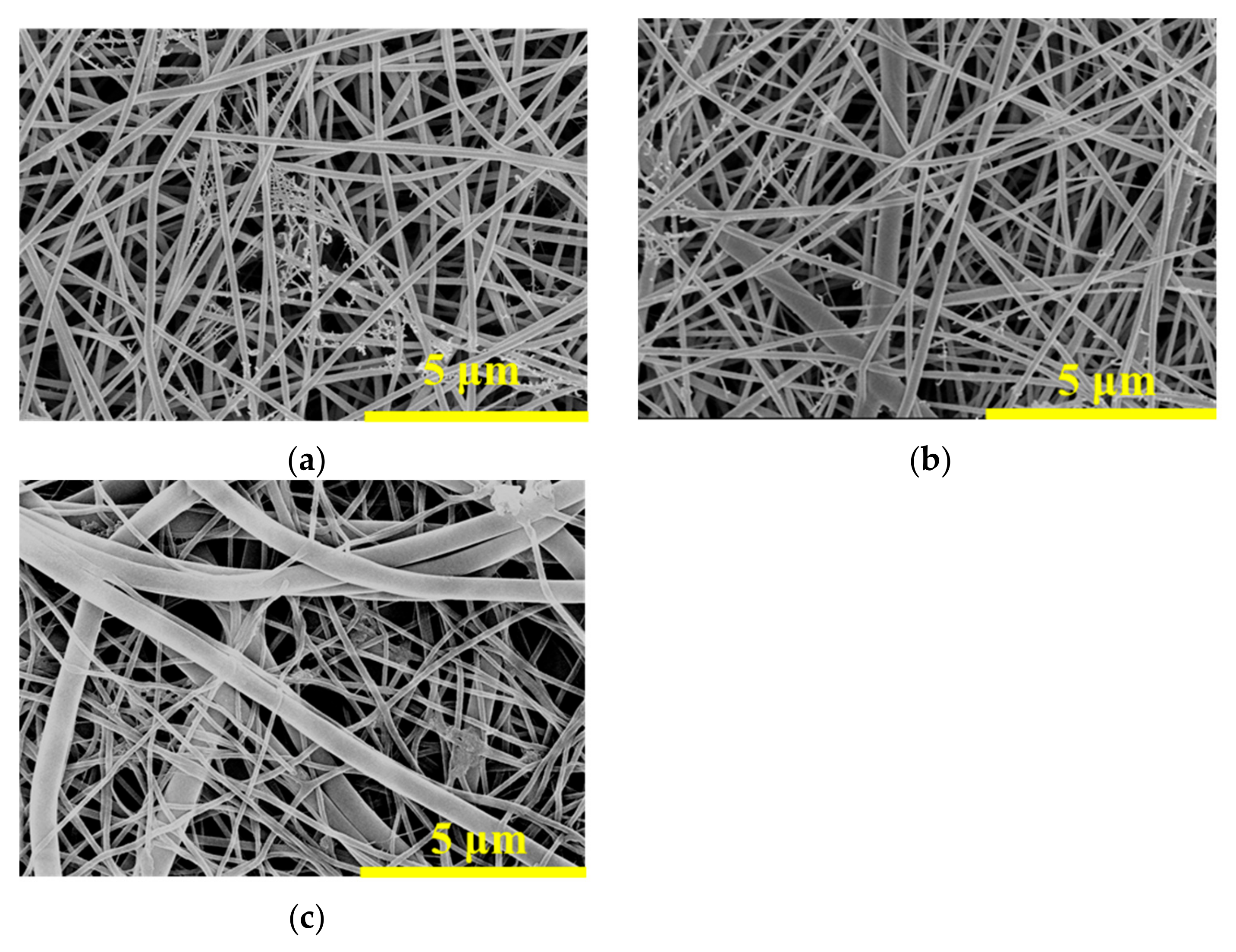
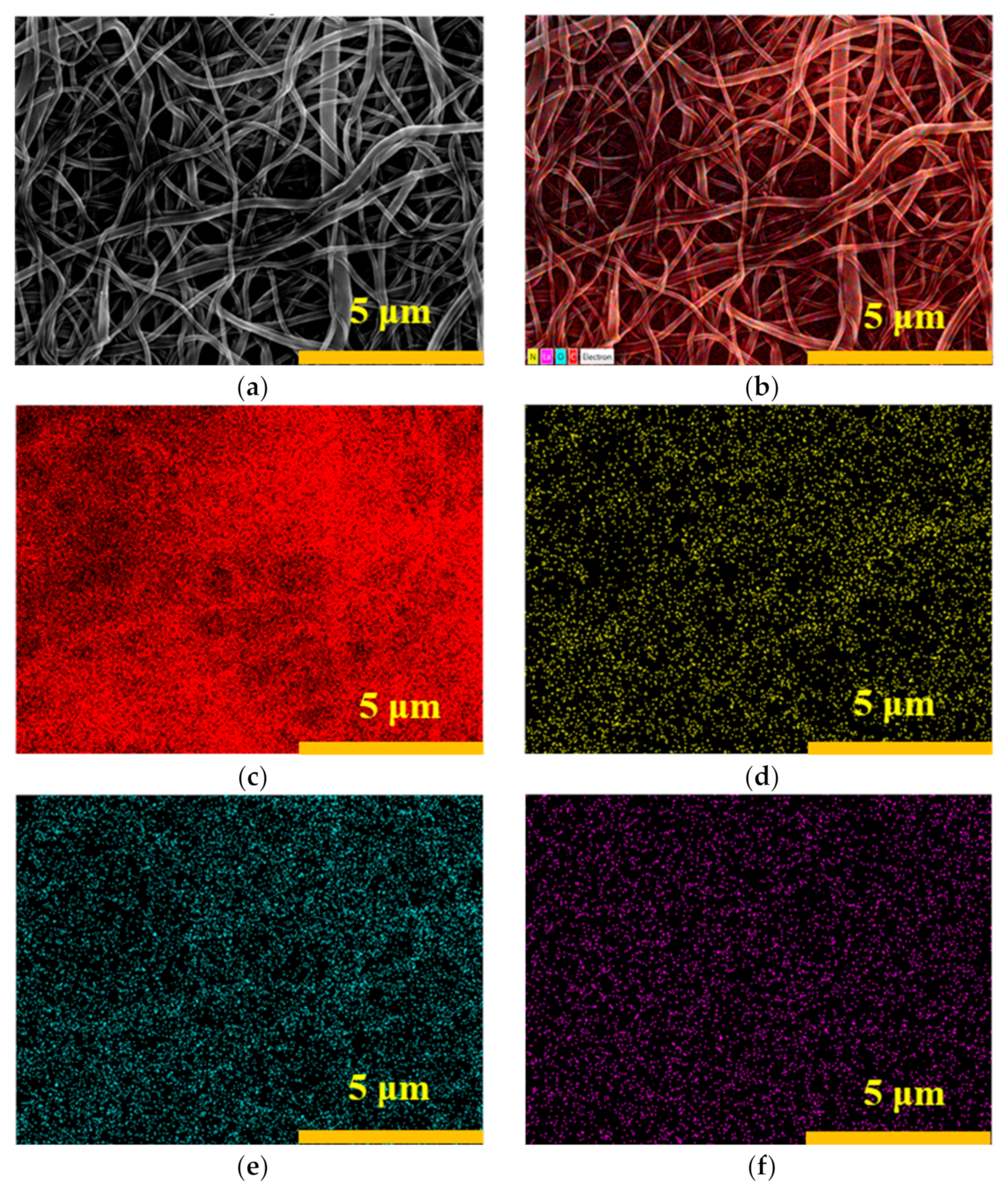
3.1.1. Surface Topography of the Nanofibrous Composite Membranes
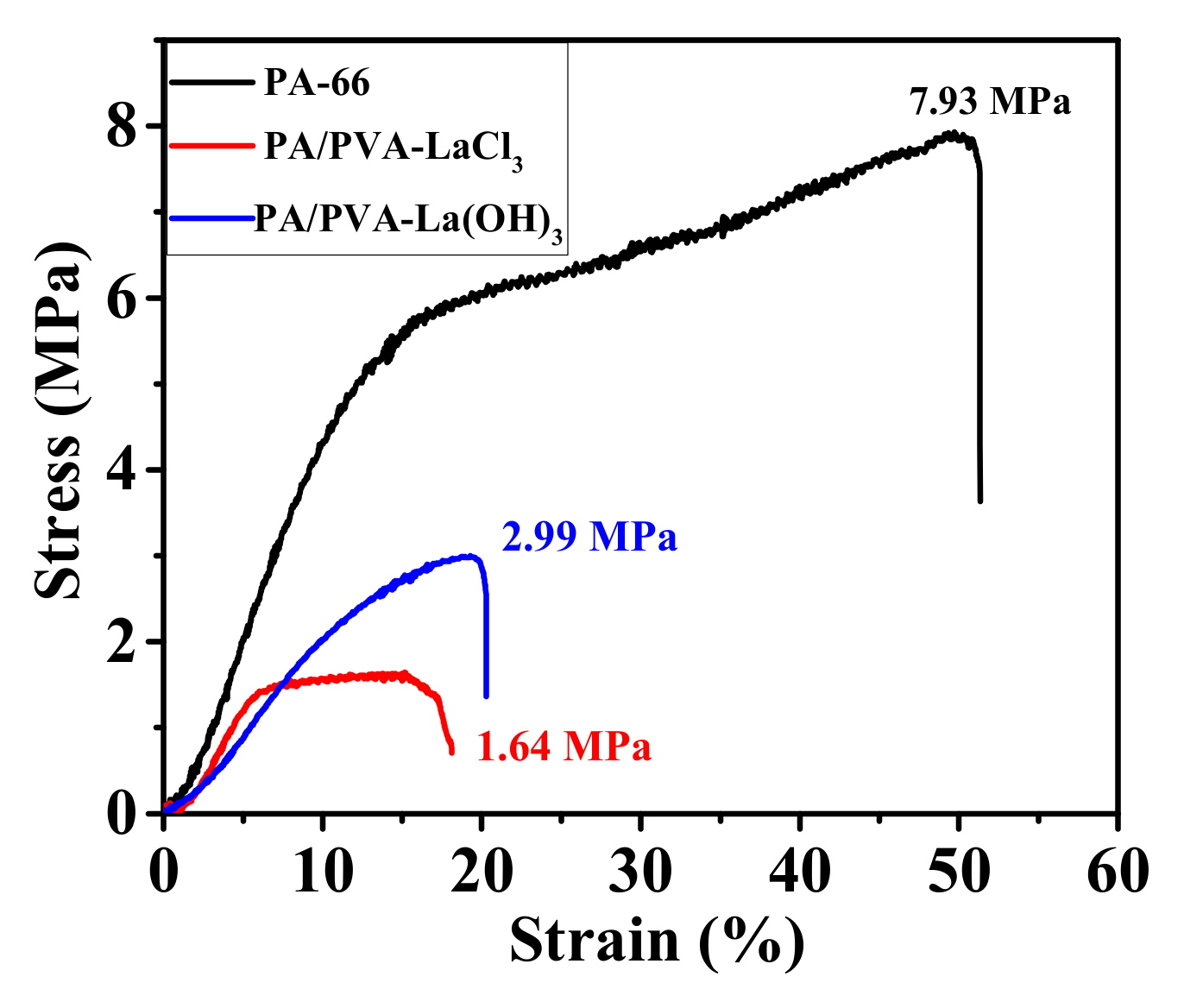
3.1.2. Mechanical Properties
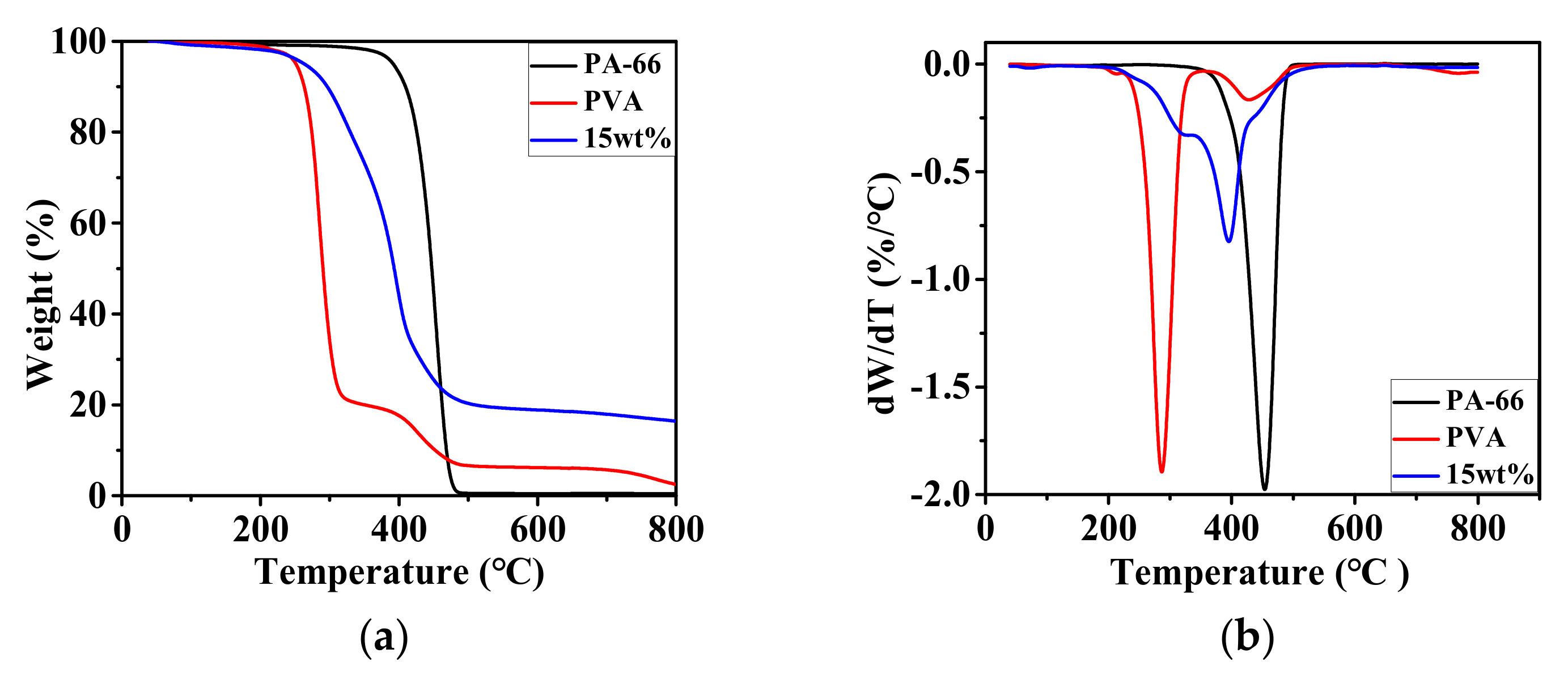
3.1.3. Thermal Behavior
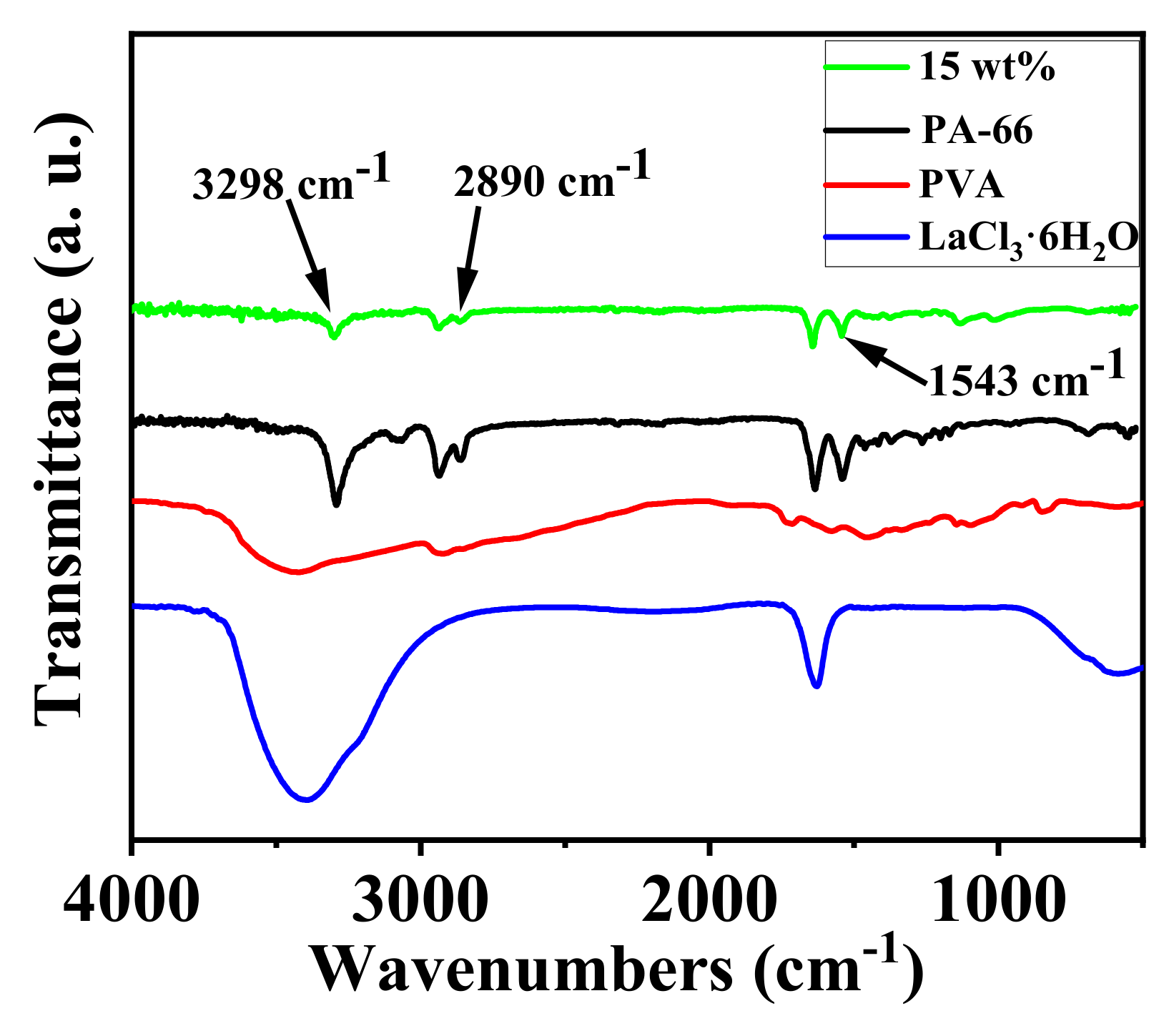
3.1.4. ATR-FTIR Measurements
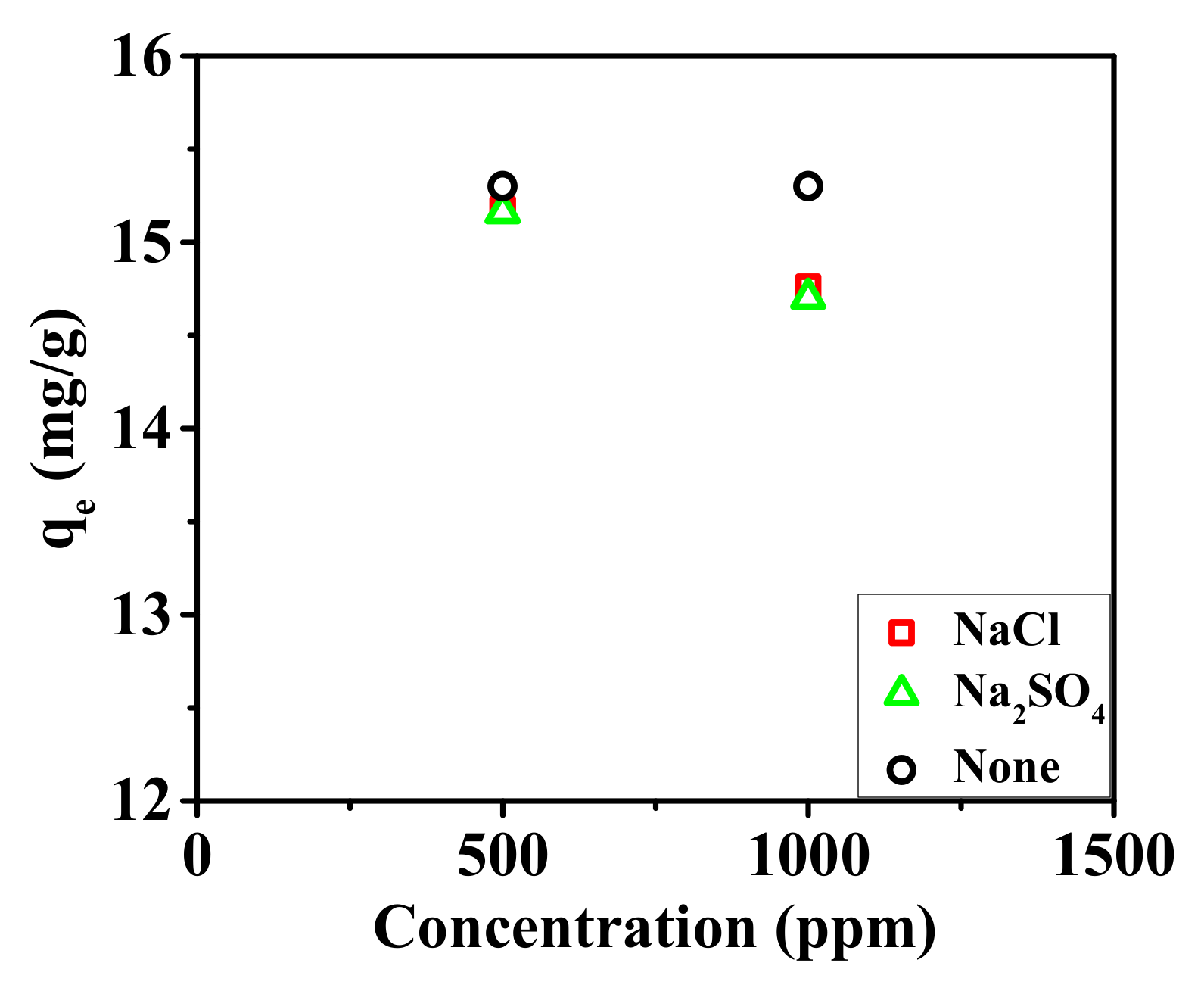
3.2. Adsorption Experiments of the Nanofibrous Composite Membrane
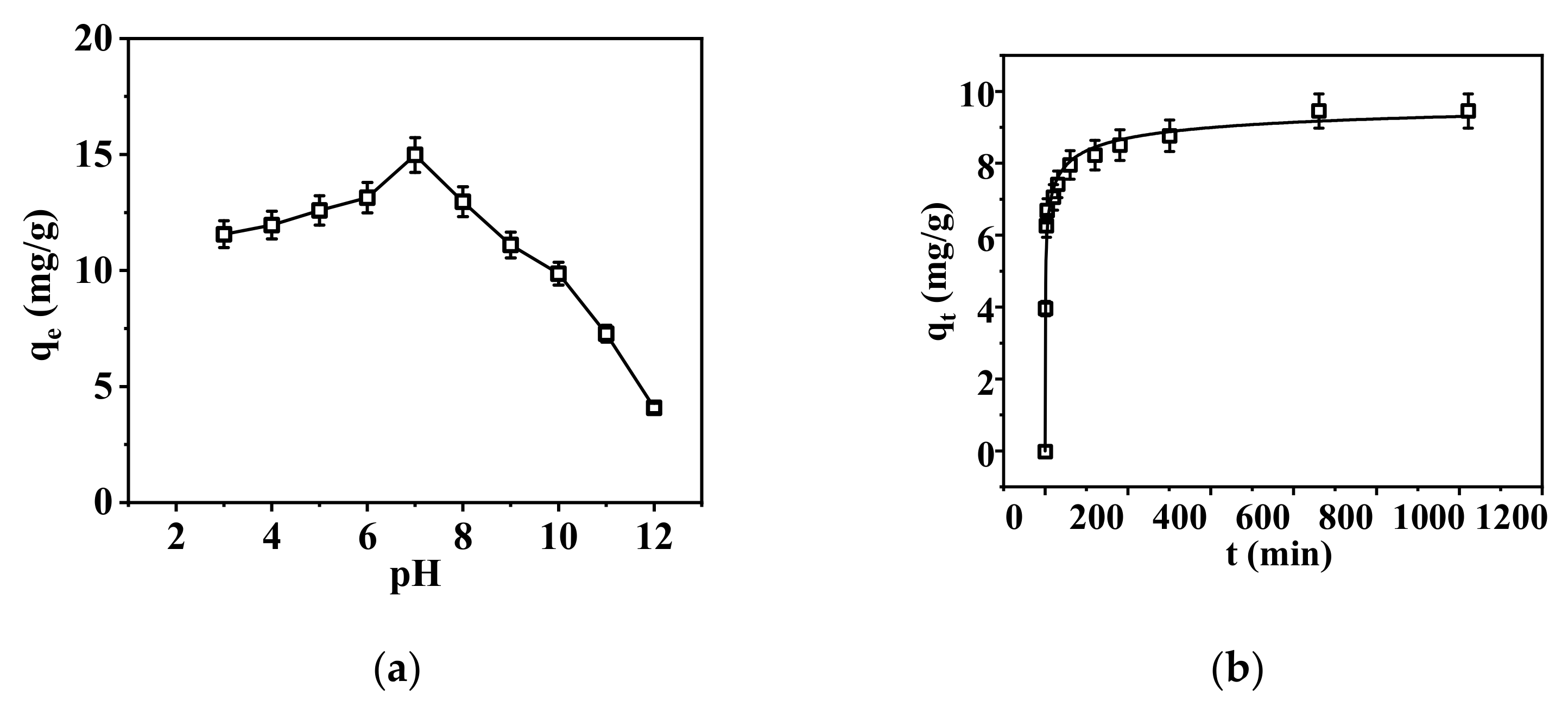
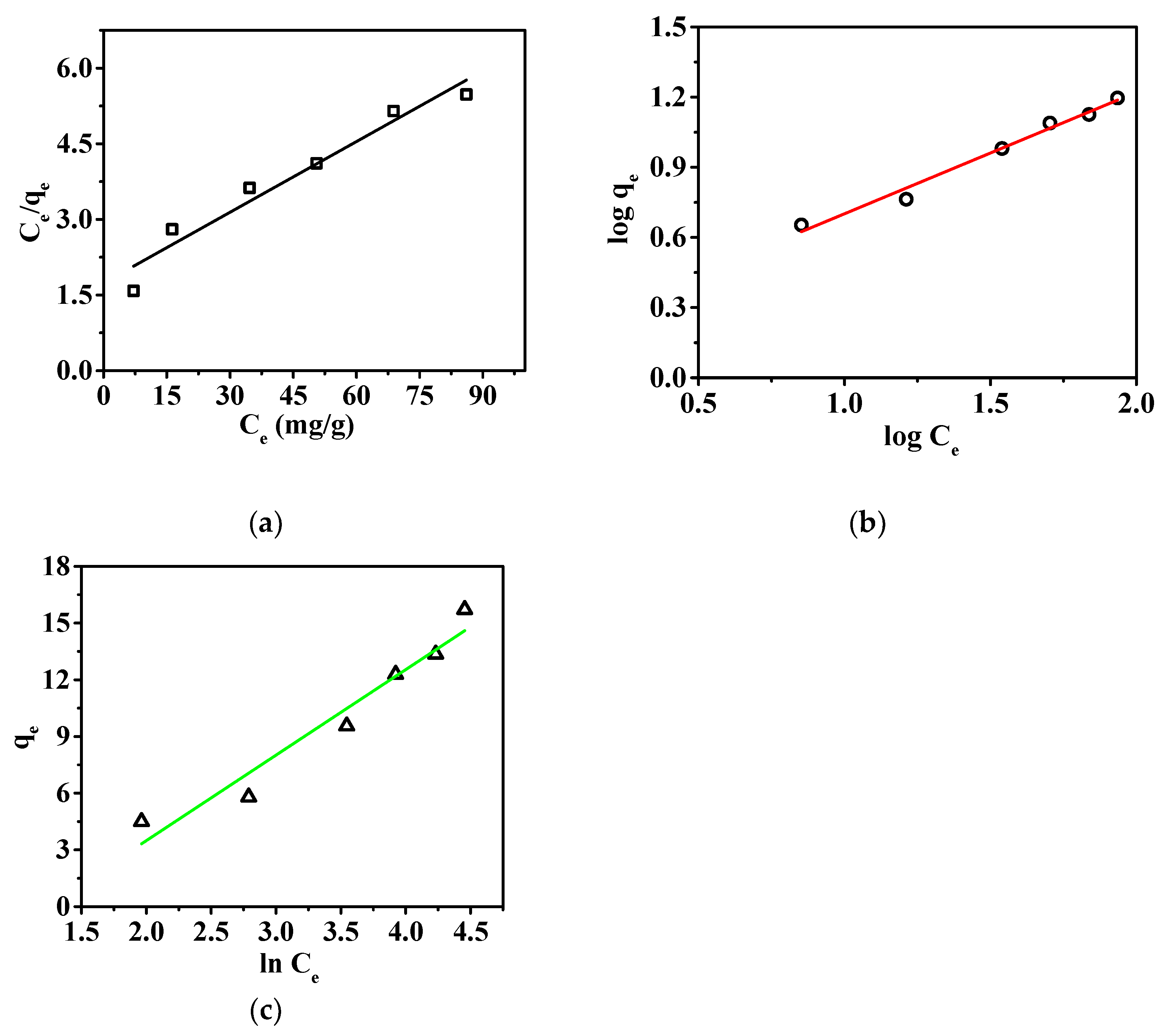
3.2.1. Static Adsorption of the Nanofibrous Composite Membrane
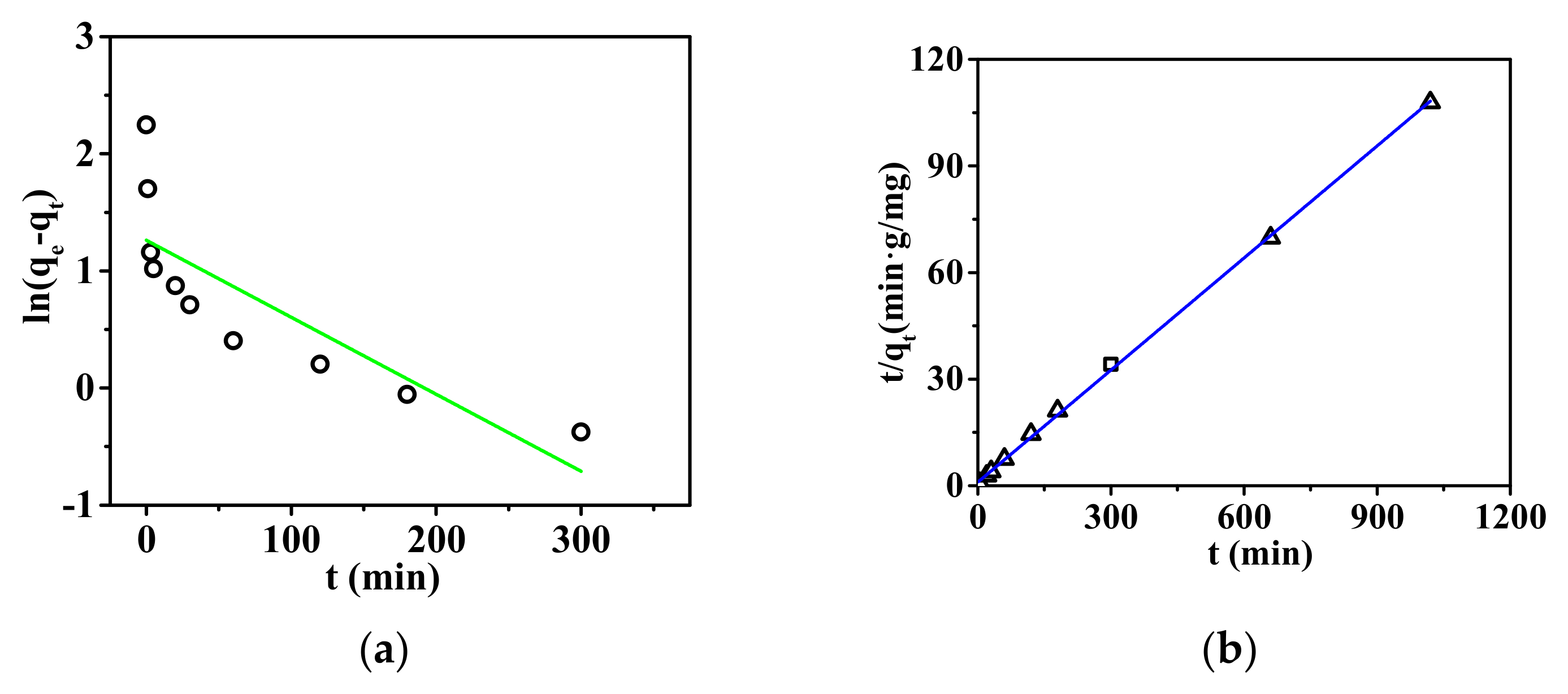
3.2.2. Dynamic Adsorption of the Nanofibrous Composite Membrane
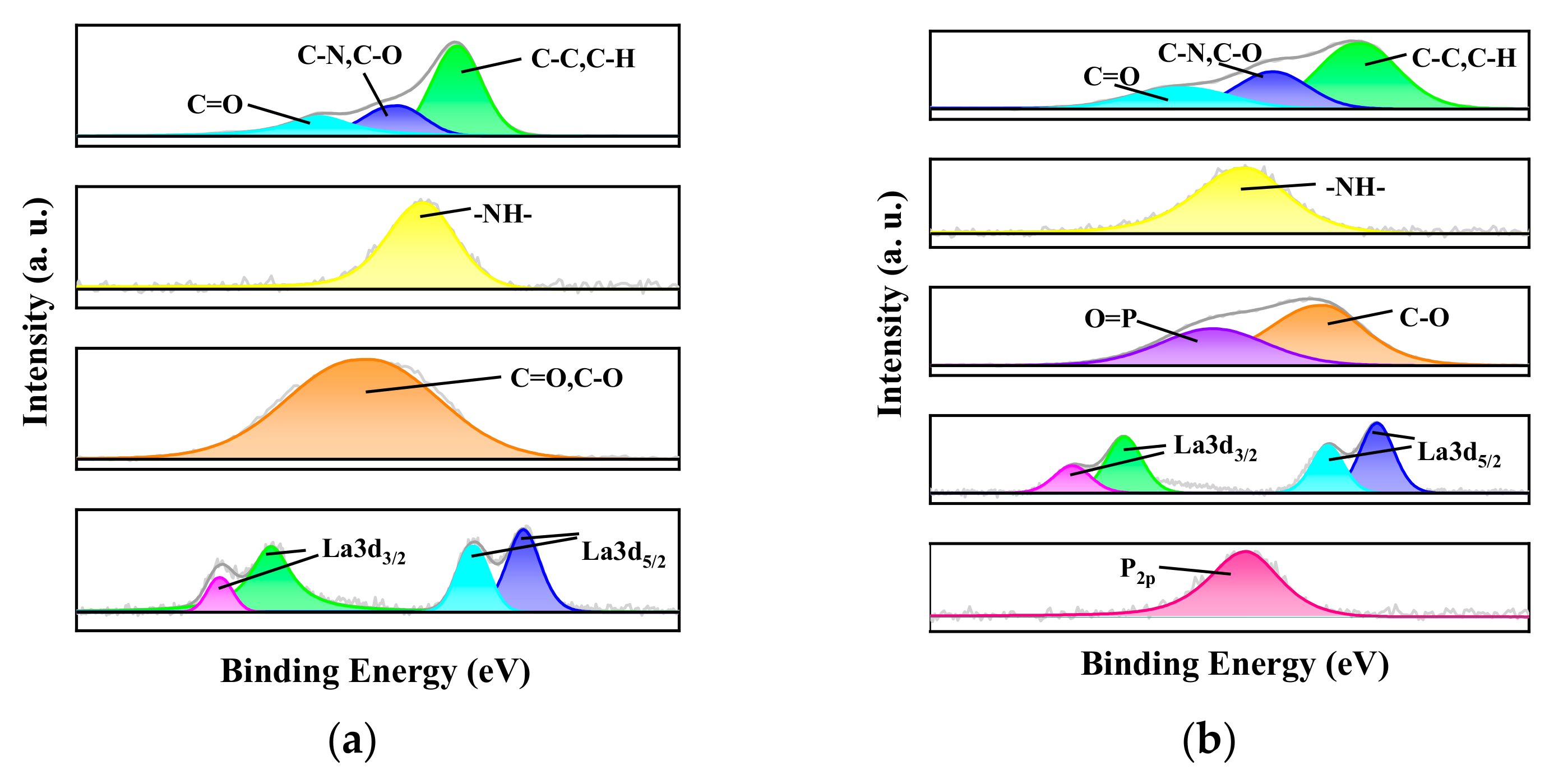
3.3. Adsorption Mechanism
3.4. Phosphorus-Release of the Phosphate-Loaded Nanofibrous Composite Membrane
3.5. Desorption and Regeneration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boretti, A. Covid19 Pandemic as a Further Driver of Water Scarcity in Africa. GeoJournal 2022, 87, 787–814. [Google Scholar] [CrossRef]
- Gräslund, S.; Bengtsson, B.-E. Chemicals and Biological Products Used in South-East Asian Shrimp Farming, and Their Potential Impact on the Environment—A Review. Sci. Total Environ. 2001, 280, 93–131. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Guzman, L.; Gunawan, G.; Viswanathan, T. A Comprehensive Review of Phosphorus Removal Technologies and Processes. J. Macromol. Sci. Part A 2014, 51, 538–545. [Google Scholar] [CrossRef]
- Mulkerrins, D.; Dobson, A.D.W.; Colleran, E. Parameters Affecting Biological Phosphate Removal from Wastewaters. Environ. Int. 2004, 30, 249–259. [Google Scholar] [CrossRef]
- Thistleton, J.; Berry, T.-A.; Pearce, P.; Parsons, S.A. Mechanisms of Chemical Phosphorus Removal II. Process Saf. Environ. Prot. 2002, 80, 265–269. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van Der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Faccini, M.; Borja, G.; Boerrigter, M.; Morillo Martín, D.; Martìnez Crespiera, S.; Vázquez-Campos, S.; Aubouy, L.; Amantia, D. Electrospun Carbon Nanofiber Membranes for Filtration of Nanoparticles from Water. J. Nanomater. 2015, 2015, 247471. [Google Scholar] [CrossRef]
- Ahmed, A.; Zhang, M.; Li, S.; Xu, L. Batch Preparation and Characterization of Anthocyanin/CS/PEO Nanofiber Membranes for Food Packages. Fibers Polym. 2023, 24, 3075–3084. [Google Scholar] [CrossRef]
- Yin, J.; Li, J.; Ramakrishna, S.; Xu, L. Hybrid-Structured Electrospun Nanofiber Membranes as Triboelectric Nanogenerators for Self-Powered Wearable Electronics. ACS Sustain. Chem. Eng. 2023, 11, 14020–14030. [Google Scholar] [CrossRef]
- Chee, T.Y.; Mohd Yusoff, A.R.; Abdullah, F.; Asyraf Wan Mahmood, W.M.; Fathi Jasni, M.J.; Nizam Nik Malek, N.A.; Buang, N.A.; Govarthanan, M. Fabrication, Characterization and Application of Electrospun Polysulfone Membrane for Phosphate Ion Removal in Real Samples. Chemosphere 2022, 303, 135228. [Google Scholar] [CrossRef]
- Ko, Y.G.; Do, T.; Chun, Y.; Kim, C.H.; Choi, U.S.; Kim, J.-Y. CeO2-Covered Nanofiber for Highly Efficient Removal of Phosphorus from Aqueous Solution. J. Hazard. Mater. 2016, 307, 91–98. [Google Scholar] [CrossRef]
- Wang, C.; Yu, S.; Cwiertny, D.M.; Yin, Y.; Myung, N.V. Phosphate Removal Using Surface Enriched Hematite and Tetra-n-Butylammonium Bromide Incorporated Polyacrylonitrile Composite Nanofibers. Sci. Total Environ. 2021, 770, 145364. [Google Scholar] [CrossRef]
- Abu-Obaid, S.; Aktij, S.A.; Tabe, S.; Sadrzadeh, M.; Farnood, R.R. Surfactant-Modified Adsorptive Electrospun Nanofiber Membrane Impregnated with Akageneite for Phosphorus Recovery from Wastewater. J. Environ. Chem. Eng. 2022, 10, 108786. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Tang, A.; Yu, Y.; Qu, X.; Chen, Z.; Wen, Q.; Li, A. Insight into the Synthesis and Adsorption Mechanism of Adsorbents for Efficient Phosphate Removal: Exploration from Synthesis to Modification. Chem. Eng. J. 2022, 442, 136147. [Google Scholar] [CrossRef]
- Jia, X.; Wang, H.; Li, Y.; Xu, J.; Cheng, H.; Li, M.; Zhang, S.; Zhang, H.; Hu, G. Separable Lanthanum-Based Porous PAN Nanofiber Membrane for Effective Aqueous Phosphate Removal. Chem. Eng. J. 2022, 433, 133538. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Sun, F.; Shi, W.; Qi, D.; Wang, K.; Shi, R.; Cui, F.; Wang, C.; Chen, X. Highly Efficient Phosphate Scavenger Based on Well-Dispersed La(OH)3 Nanorods in Polyacrylonitrile Nanofibers for Nutrient-Starvation Antibacteria. ACS Nano 2015, 9, 9292–9302. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, X.; Liu, J.; Lu, L.; Peng, K.; Bhattarai, R. PVA/PEI Crosslinked Electrospun Nanofibers with Embedded La(OH)3 Nanorod for Selective Adsorption of High Flux Low Concentration Phosphorus. J. Hazard. Mater. 2020, 384, 121457. [Google Scholar] [CrossRef] [PubMed]
- Amini, G.; Gharehaghaji, A.A. Improving Adhesion of Electrospun Nanofiber Mats to Supporting Substrate by Using Adhesive Bonding. Int. J. Adhes. Adhes. 2018, 86, 40–44. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.; Yin, X.; Ma, H.; Hsiao, B.S. Highly Permeable Nanofibrous Composite Microfiltration Membranes for Removal of Nanoparticles and Heavy Metal Ions. Sep. Purif. Technol. 2020, 233, 115976. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Hsiao, B.S. Interpenetrating Nanofibrous Composite Membranes for Water Purification. ACS Appl. Nano Mater. 2019, 2, 3606–3614. [Google Scholar] [CrossRef]
- Sjösten, A.; Blomqvist, S. Influence of Phosphate Concentration and Reaction Temperature When Using the Molybdenum Blue Method for Determination of Phosphate in Water. Water Res. 1997, 31, 1818–1823. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhu, Y.; Yang, S.; Dong, Z.; Zhang, X.; Wang, R. Crystallization Regulation Determined Spinnability and Mechanical Properties toward PA66/Calcium Chloride and Its Fibers. ACS Omega 2025, 10, 19444–19452. [Google Scholar] [CrossRef] [PubMed]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat Polymer Ribbons and Other Shapes by Electrospinning. J. Polym. Sci. B Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Duemichen, E.; Braun, U.; Kraemer, R.; Deglmann, P.; Senz, R. Thermal Extraction Combined with Thermal Desorption: A Powerful Tool to Investigate the Thermo-Oxidative Degradation of Polyamide 66 Materials. J. Anal. Appl. Pyrolysis 2015, 115, 288–298. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Ozturk, M.K.; Nergis, B.; Candan, C. Thermal Analysis of PVA Nanofibrous Membranes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012048. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Mishra, R.; Singh, H.; Krishnamurthy, N. Determination of Thermodynamic Stability of Lanthanum Chloride Hydrates (LaCl3⋅xH2O) by Dynamic Transpiration Method. J. Alloys Compd. 2014, 588, 578–584. [Google Scholar] [CrossRef]
- Smirnov, L.V.; Kulikova, N.P.; Platonova, N.V. Infrared Spectra of Polyvinylalcohol. Polym. Sci. USSR 1967, 9, 2849–2856. [Google Scholar] [CrossRef]
- Guerrini, L.M.; Branciforti, M.C.; Canova, T.; Bretas, R.E.S. Electrospinning and Characterization of Polyamide 66 Nanofibers with Different Molecular Weights. Mat. Res. 2009, 12, 181–190. [Google Scholar] [CrossRef]
- Manoilova, O.V.; Podkolzin, S.G.; Tope, B.; Lercher, J.; Stangland, E.E.; Goupil, J.-M.; Weckhuysen, B.M. Surface Acidity and Basicity of La2O3, LaOCl, and LaCl3 Characterized by IR Spectroscopy, TPD, and DFT Calculations. J. Phys. Chem. B 2004, 108, 15770–15781. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(Vinyl Alcohol) Films Crosslinked by Glutaraldehyde under Mild Conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Hu, P.; Huang, R. Adsorption of Phosphate from Aqueous Solution by Zr(IV)-crosslinked Quaternized Chitosan/Bentonite Composite. Environ. Prog. Sustain. Energy 2018, 37, 267–275. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, H.; Shen, T.; Wang, N.; Wang, P. Critical Review of La(III)-Based Absorbents toward Phosphate Adsorption from Aqueous Solutions: Mechanisms, Adsorbent Design, and Prospects. J. Environ. Chem. Eng. 2024, 12, 112571. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of Phosphate from Aqueous Solutions by Adsorption onto Ca(OH)2 Treated Natural Clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From Theory to Practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Mokhtari-Shourijeh, Z.; Montazerghaem, L.; Olya, M.E. Preparation of Porous Nanofibers from Electrospun Polyacrylonitrile/Polyvinylidene Fluoride Composite Nanofibers by Inexpensive Salt Using for Dye Adsorption. J. Polym. Environ. 2018, 26, 3550–3563. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, W.; Sun, J.; Zhou, Q. Efficient Removal of Cr(VI) from Water by the Uniform Fiber Ball Loaded with Polypyrrole: Static Adsorption, Dynamic Adsorption and Mechanism Studies. Chemosphere 2020, 248, 126102. [Google Scholar] [CrossRef]
- Zavareh, S.; Behrouzi, Z.; Avanes, A. Cu (II) Binded Chitosan/Fe3O4 Nanocomomposite as a New Biosorbent for Efficient and Selective Removal of Phosphate. Int. J. Biol. Macromol. 2017, 101, 40–50. [Google Scholar] [CrossRef]
- Nie, W.; Zhang, X.; Luo, X.; Xie, L.; Zhu, Y.; Tang, A.; Liang, D. Efficient Phosphate Adsorption from Real Industrial Wastewater Using Fe/La-Impregnated Biochar. J. Environ. Chem. Eng. 2025, 13, 117967. [Google Scholar] [CrossRef]
- Lei, X.; Dai, X.; Long, S.; Cai, N.; Ma, Z.; Luo, X. Facile Design of Green Engineered Cellulose/Metal Hybrid Macrogels for Efficient Trace Phosphate Removal. Ind. Eng. Chem. Res. 2017, 56, 7525–7533. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced Phosphate Removal by Zeolite Loaded with Mg–Al–La Ternary (Hydr)Oxides from Aqueous Solutions: Performance and Mechanism. Chem. Eng. J. 2019, 357, 33–44. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Li, D.; Liu, Z.-Q.; Tao, Q.; Zhu, Y.; Yang, J.; Zhang, Y.-M. Kinetics, Isotherm, Thermodynamic, and Adsorption Mechanism Studies of La(OH)3-Modified Exfoliated Vermiculites as Highly Efficient Phosphate Adsorbents. Chem. Eng. J. 2014, 236, 191–201. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Zhao, Y.; Zhou, X.; Zheng, H. La3+/La(OH)3 Loaded Magnetic Cationic Hydrogel Composites for Phosphate Removal: Effect of Lanthanum Species and Mechanistic Study. Water Res. 2017, 126, 433–441. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate Adsorption on Lanthanum Loaded Biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Wang, L. Preferable Adsorption of Phosphate Using Lanthanum-Incorporated Porous Zeolite: Characteristics and Mechanism. Appl. Surf. Sci. 2017, 426, 995–1004. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of Phosphate from Water by Lanthanum-Modified Zeolites Obtained from Fly Ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef]
- Yahya, R.; Elshaarawy, R.F.M. Cross-Linked Quaternized Polyethersulfone-Amino Crystalline Nanocellulose Composite Membrane for Enhanced Phosphate Removal from Wastewater. Int. J. Biol. Macromol. 2023, 236, 123995. [Google Scholar] [CrossRef]
- Loffreda, D. Theoretical Insight of Adsorption Thermodynamics of Multifunctional Molecules on Metal Surfaces. Surf. Sci. 2006, 600, 2103–2112. [Google Scholar] [CrossRef]
- Antelo, J.; Avena, M.; Fiol, S.; López, R.; Arce, F. Effects of pH and Ionic Strength on the Adsorption of Phosphate and Arsenate at the Goethite–Water Interface. J. Colloid Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Omitola, O.B.; Abonyi, M.N.; Akpomie, K.G.; Dawodu, F.A. Adams-Bohart, Yoon-Nelson, and Thomas Modeling of the Fix-Bed Continuous Column Adsorption of Amoxicillin onto Silver Nanoparticle-Maize Leaf Composite. Appl. Water Sci. 2022, 12, 94. [Google Scholar] [CrossRef]
- Belat, B.; Veli, S.; Isgoren, M. Modeling of Linear Alkyl Benzene Sulphonic Acid Removal from Aqueous Solution with Fixed Bed Adsorption Column: Thomas and Yoon–Nelson Methods. J. Chem. Tech. Biotech. 2022, 97, 1771–1780. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, J.; Ma, Q.; Baihetiyaer, B.; Sun, J.; Zhang, Y.; Jiang, Y.; Wang, J.; Yin, X. Montmorillonite-Reduced Graphene Oxide Composite Aerogel (M−rGO): A Green Adsorbent for the Dynamic Removal of Cadmium and Methylene Blue from Wastewater. Sep. Purif. Technol. 2022, 296, 121416. [Google Scholar] [CrossRef]
- Fang, L.; Shi, Q.; Nguyen, J.; Wu, B.; Wang, Z.; Lo, I.M.C. Removal Mechanisms of Phosphate by Lanthanum Hydroxide Nanorods: Investigations Using EXAFS, ATR-FTIR, DFT, and Surface Complexation Modeling Approaches. Environ. Sci. Technol. 2017, 51, 12377–12384. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate Adsorption on Lanthanum Hydroxide-Doped Activated Carbon Fiber. Chem. Eng. J. 2012, 185–186, 160–167. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Lu, Y.; Tariq, M.; Yuan, L.; Yang, J.; Li, Z.; Lyu, S. Development of Lanthanum Hydroxide Loaded on Molecular Sieve Adsorbent and Mechanistic Study for Phosphate Removal. J. Alloys Compd. 2018, 768, 953–961. [Google Scholar] [CrossRef]
- Wilharm, R.K.; Huang, S.-Y.; Gugger, I.J.; Pierre, V.C. A Walk Across the Lanthanide Series: Trend in Affinity for Phosphate and Stability of Lanthanide Receptors from La(III) to Lu(III). Inorg. Chem. 2021, 60, 15808–15817. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Zhang, H.; Wang, H.; Li, W.; Li, Y.; Xu, J.; Wang, B.; Hu, G. Selective Adsorption Behavior and Mechanism of Phosphate in Water by Different Lanthanum Modified Biochar. J. Environ. Chem. Eng. 2022, 10, 107476. [Google Scholar] [CrossRef]
- Lv, L.; Deng, T.; Wang, L.; Peng, H.; Chen, H.; Li, X.; Chi, F. Hydrophilic Modification and Synergistic Interaction of Phosphate-Amidoxime Adsorbent for Enhanced Uranium Extraction from Seawater. Desalination 2025, 600, 118491. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of Surface Oxygen Complexes on Carbon Nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Pang, Y.; Zhou, X.; Vovk, E.I.; Guan, C.; Li, S.; van Bavel, A.P.; Yang, Y. Understanding Lanthanum Oxide Surface Structure by DFT Simulation of Oxygen 1s Calibrated Binding Energy in XPS after in Situ Treatment. Appl. Surf. Sci. 2021, 548, 149214. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Lu, S.; Wu, D.; Zhang, Z.; Kong, H. Removal and Recovery of Phosphate from Water by Lanthanum Hydroxide Materials. Chem. Eng. J. 2014, 254, 163–170. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Pizarro, C. Biofertilizers from Algal Treatment of Dairy and Swine Manure Effluents: Characterization of Algal Biomass as a Slow Release Fertilizer. J. Veg. Sci. 2007, 12, 107–125. [Google Scholar] [CrossRef]
- Strauss, R.; Brümmer, G.w.; Barrow, N.j. Effects of Crystallinity of Goethite: II. Rates of Sorption and Desorption of Phosphate. Eur. J. Soil Sci. 1997, 48, 101–114. [Google Scholar] [CrossRef]





| Constituencies | Group Number | Membrane Weight (mg) | Whether or Not to Add Culture Medium |
|---|---|---|---|
| Experimental group | 1 | 20 | Yes |
| 2 | 10 | Yes | |
| Blank control group | 3 | 0 | No |
| Control group | 4 | 0 | Yes |
| Samples | Fiber Diameter (μm) | Mean Pore Size (μm) | ||
|---|---|---|---|---|
| PA-66 | PVA | Before Crosslinking | After Crosslinking | |
| PA-66 | 0.110 ± 0.006 | -- | 0.28 | -- |
| PA-66/PVA-5%La(OH)3 | 0.110 ± 0.005 | 0.565 ± 0.015 | 0.38 | 0.35 |
| PA-66/PVA-15%La(OH)3 | 0.109 ± 0.006 | 0.605 ± 0.012 | 0.50 | 0.45 |
| Kinetic Equations | Parameter | P |
|---|---|---|
| qe1 | 3.53 | |
| K1 | 0.00657 | |
| R12 | 0.680 | |
| qe2 | 9.51 | |
| K2 R22 | 0.0108 0.999 |
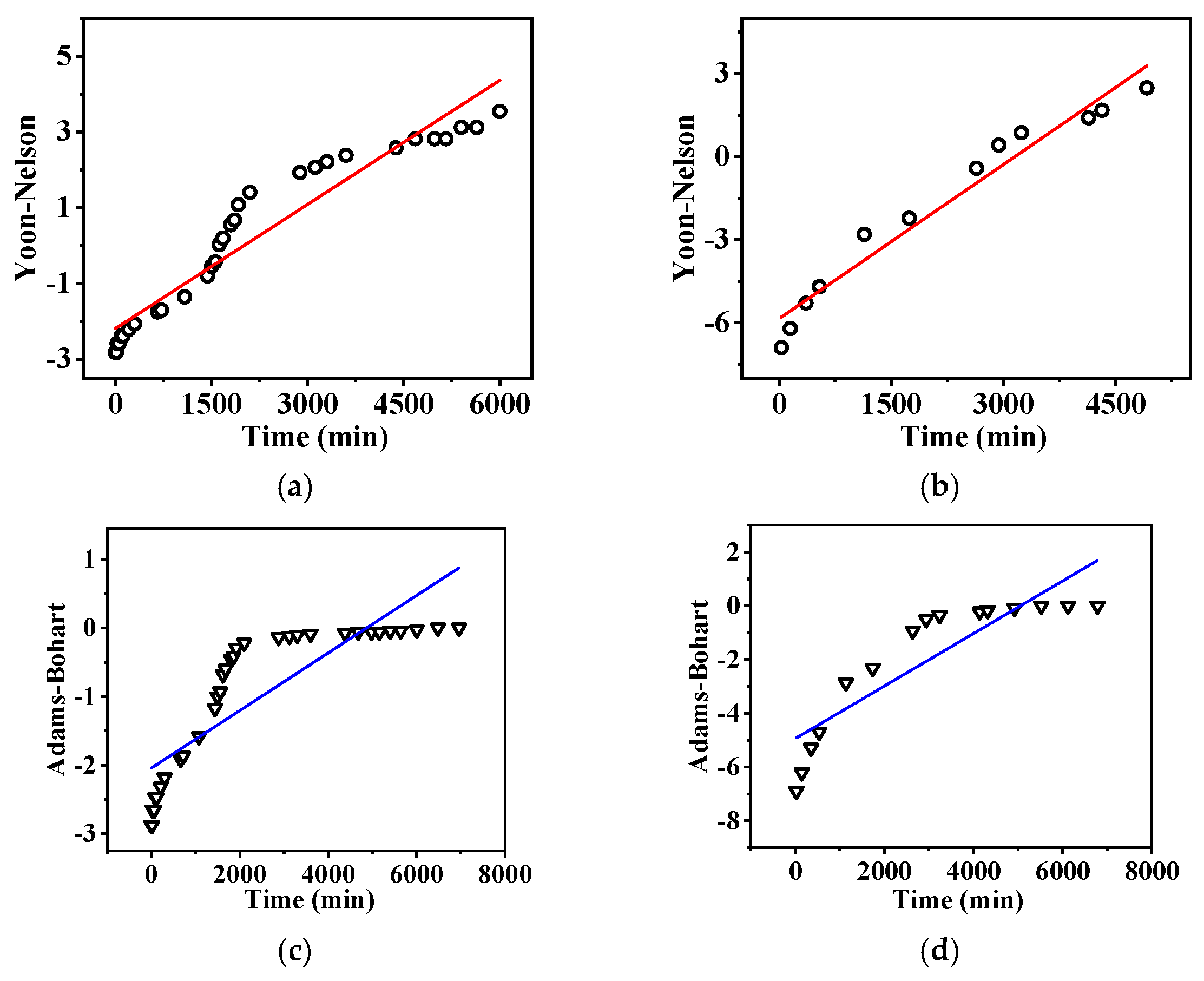
| Models | Parameter | Low Flow Rate | High Flow Rate |
|---|---|---|---|
| Yoon–Nelson | KYN (1/min) | −0.00109 | −0.00185 |
| (min) | 2008.52 | 3161 | |
| R2 | 0.92 | 0.96 | |
| Adams–Bohart | KAB (L/(mg·min) | 0.000298 | 0.00098 |
| N0 (mg/L) | 41,124 | 6900 | |
| R2 | 0.71 | 0.78 |
| Adsorption Isotherms | Isotherm Parameters | P |
|---|---|---|
| Langmuir | KL (L/mg) | 0.027 |
| qm (mg/g) | 21.39 | |
| RL2 | 0.95 | |
| Freundlich | KF (mg/g)/(ppm)1/n | 1.52 |
| 1/n | 0.52 | |
| RF2 | 0.98 | |
| Tempkin | AT | 0.29 |
| bT | 547.82 | |
| RT2 | 0.95 |
| Adsorbents | qm (mg/g) |
|---|---|
| Chitosan [41] | 23.98 |
| Fe/La@BC [42] | 44.12 |
| CHM [43] | 22.25 |
| Mg–Al–La ternary (hydr)oxides [44] | 80.8 |
| La(OH)3-modified exfoliated vermiculites [45] | 79.6 |
| MCH-La(OH)3 [46] | 90.2 |
| La-BC [47] | 46.37 |
| La2O3 [48] | 17.2 |
| La-P1 [49] | 58.2 |
| La-A [49] | 44.0 |
| La-CLP [49] | 24.6 |
| This work | 21.39 |
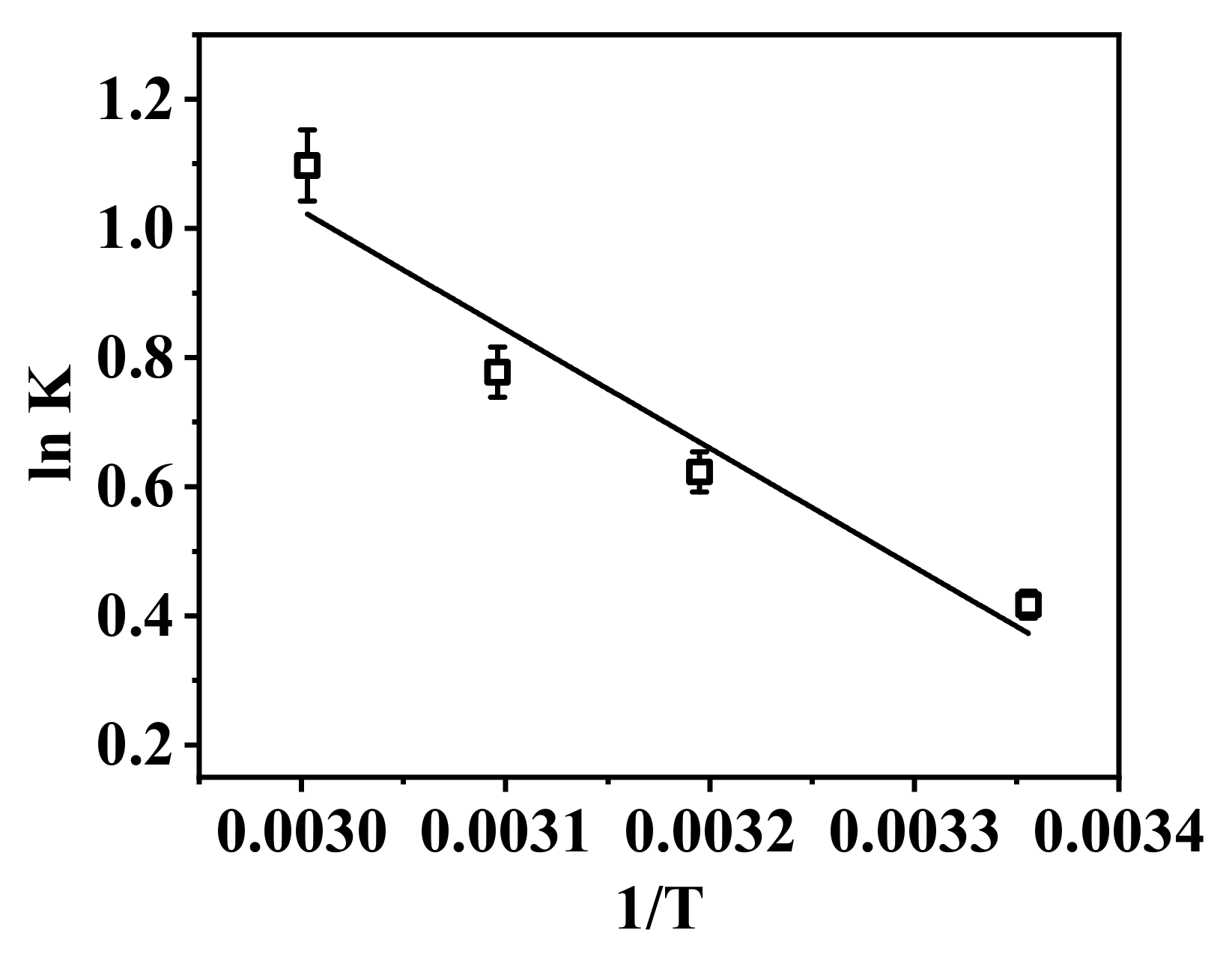
| T (K) | ΔS0 (J/(mol·K)) | ΔH0 (kJ/mol) | ΔG (kJ/mol) |
|---|---|---|---|
| 298 | 54.44 | 15.297 | −0.93 |
| 313 | −1.74 | ||
| 323 | −2.29 | ||
| 333 | −2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, G.; Ma, H.; Hsiao, B.S. Interpenetrating Nanofibrous Composite Membranes for Removal and Reutilization of P (V) Ions from Wastewater. Membranes 2025, 15, 262. https://doi.org/10.3390/membranes15090262
You G, Ma H, Hsiao BS. Interpenetrating Nanofibrous Composite Membranes for Removal and Reutilization of P (V) Ions from Wastewater. Membranes. 2025; 15(9):262. https://doi.org/10.3390/membranes15090262
Chicago/Turabian StyleYou, Guibin, Hongyang Ma, and Benjamin S. Hsiao. 2025. "Interpenetrating Nanofibrous Composite Membranes for Removal and Reutilization of P (V) Ions from Wastewater" Membranes 15, no. 9: 262. https://doi.org/10.3390/membranes15090262
APA StyleYou, G., Ma, H., & Hsiao, B. S. (2025). Interpenetrating Nanofibrous Composite Membranes for Removal and Reutilization of P (V) Ions from Wastewater. Membranes, 15(9), 262. https://doi.org/10.3390/membranes15090262








