Treatment of Industrial Brine Using a Poly (Vinylidene Fluoride) Membrane Modified with Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Functionalization of Pristine-MWCNTs (p-MWCNTs)
2.1.2. Synthesis of Mixed Matrix Membranes with Carbon Nanomaterials
2.2. Membrane Testing
Brine Testing
3. Results and Discussion
3.1. Characterization of Nanomaterials
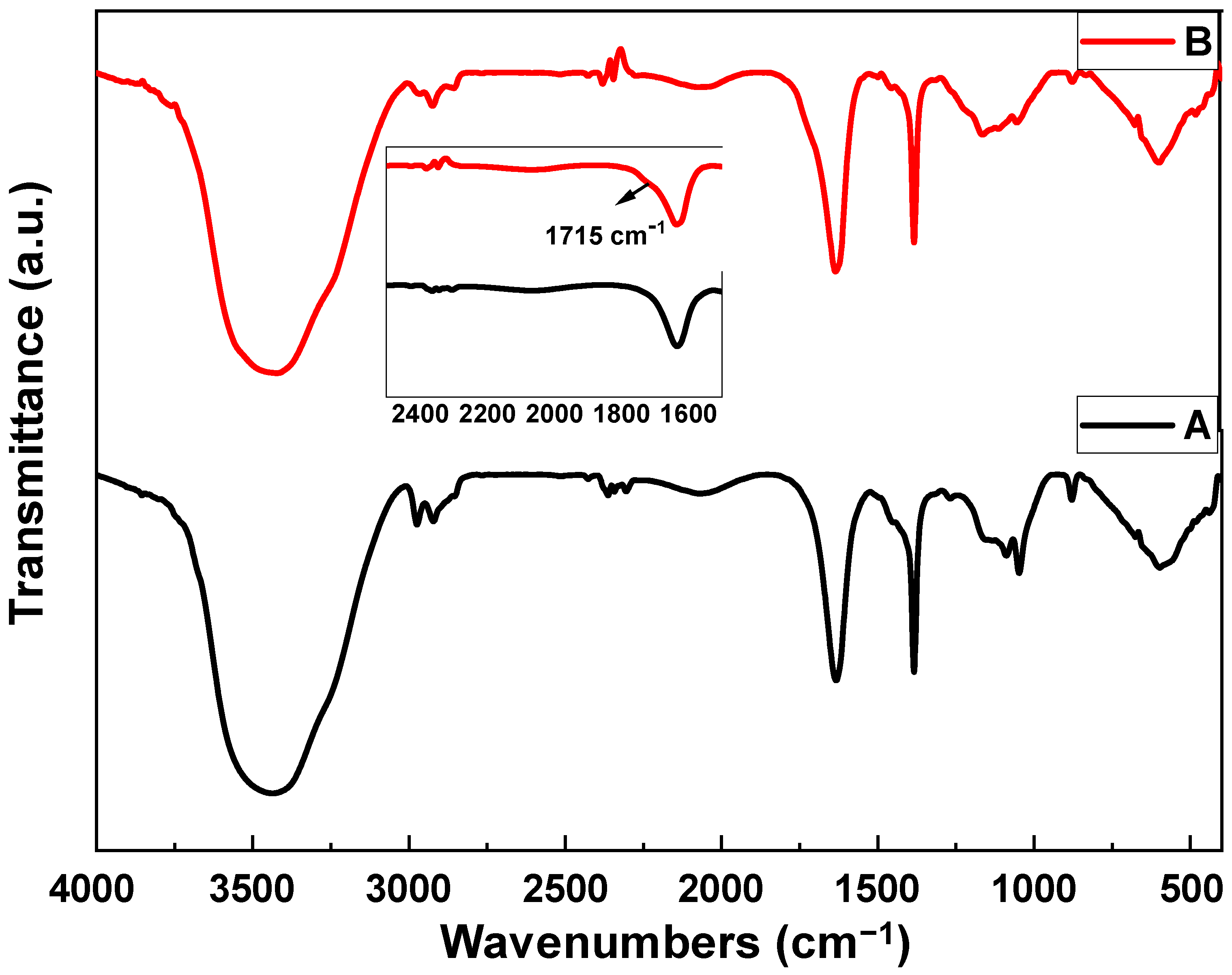
3.1.1. FTIR Analysis
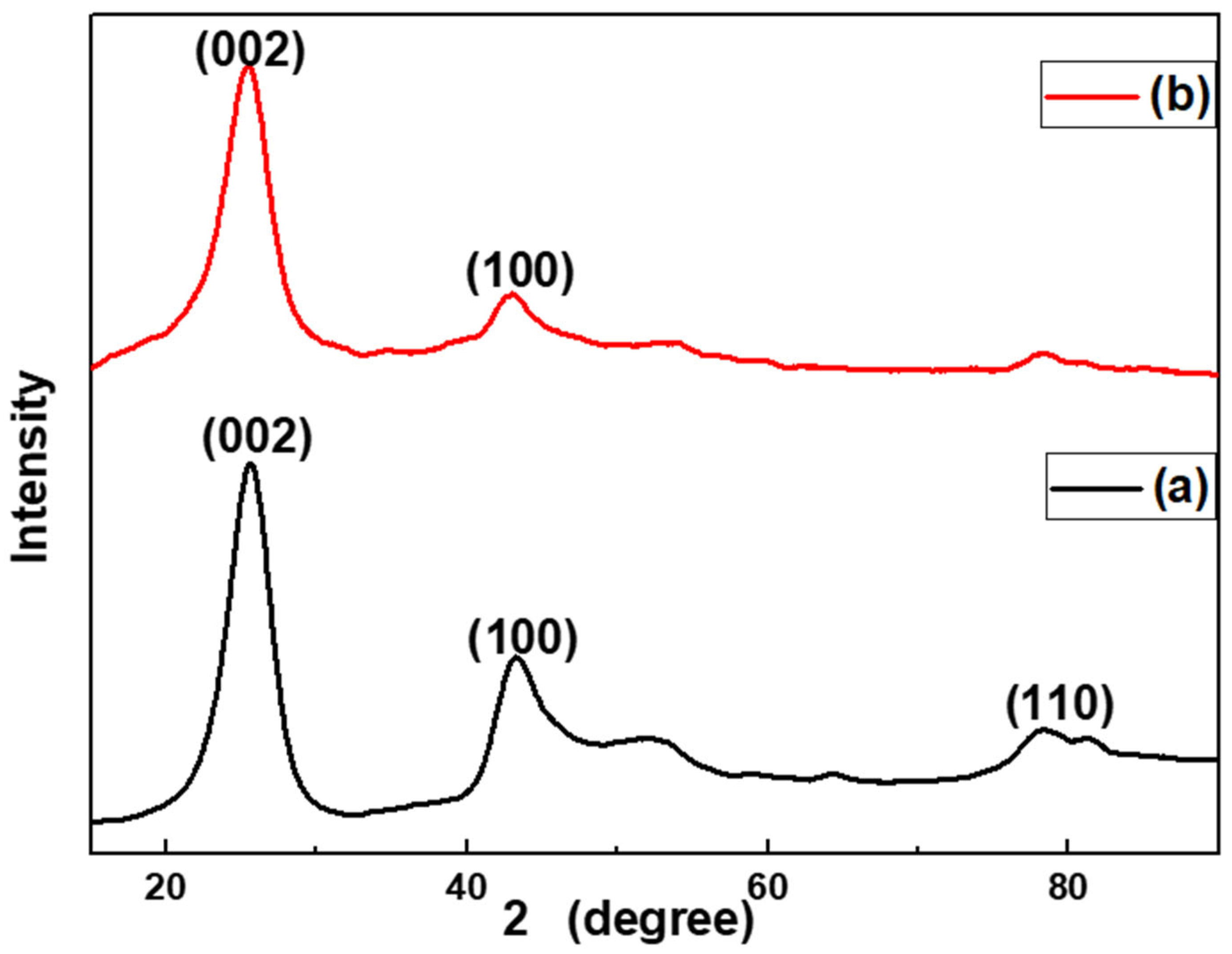
3.1.2. XRD Results
3.1.3. Raman Analysis
3.1.4. Thermal Stability (TGA Analysis)
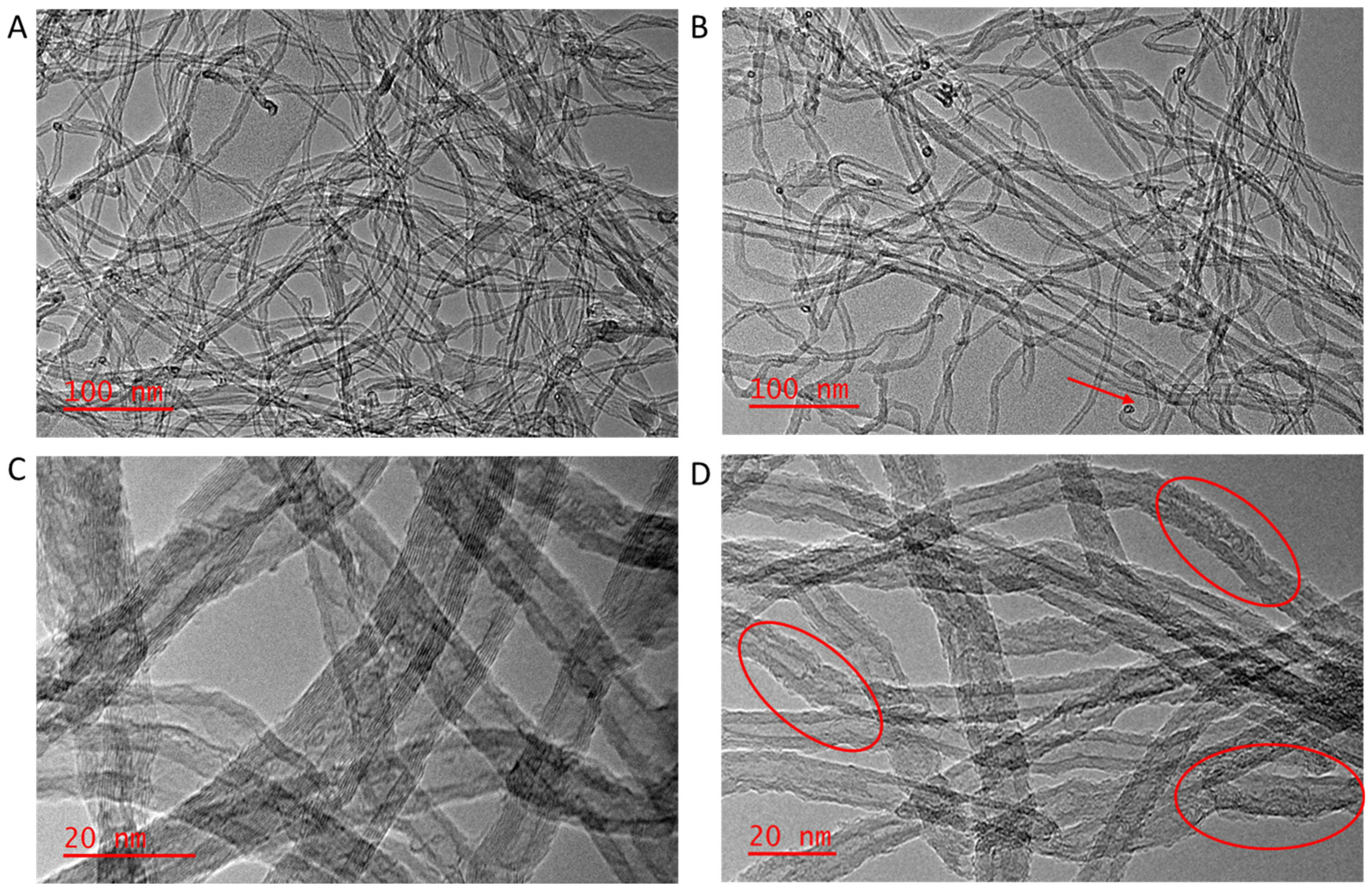
3.1.5. TEM Analysis
3.1.6. Textural Characteristics of the MWCNTs
3.2. Membrane Characterizations
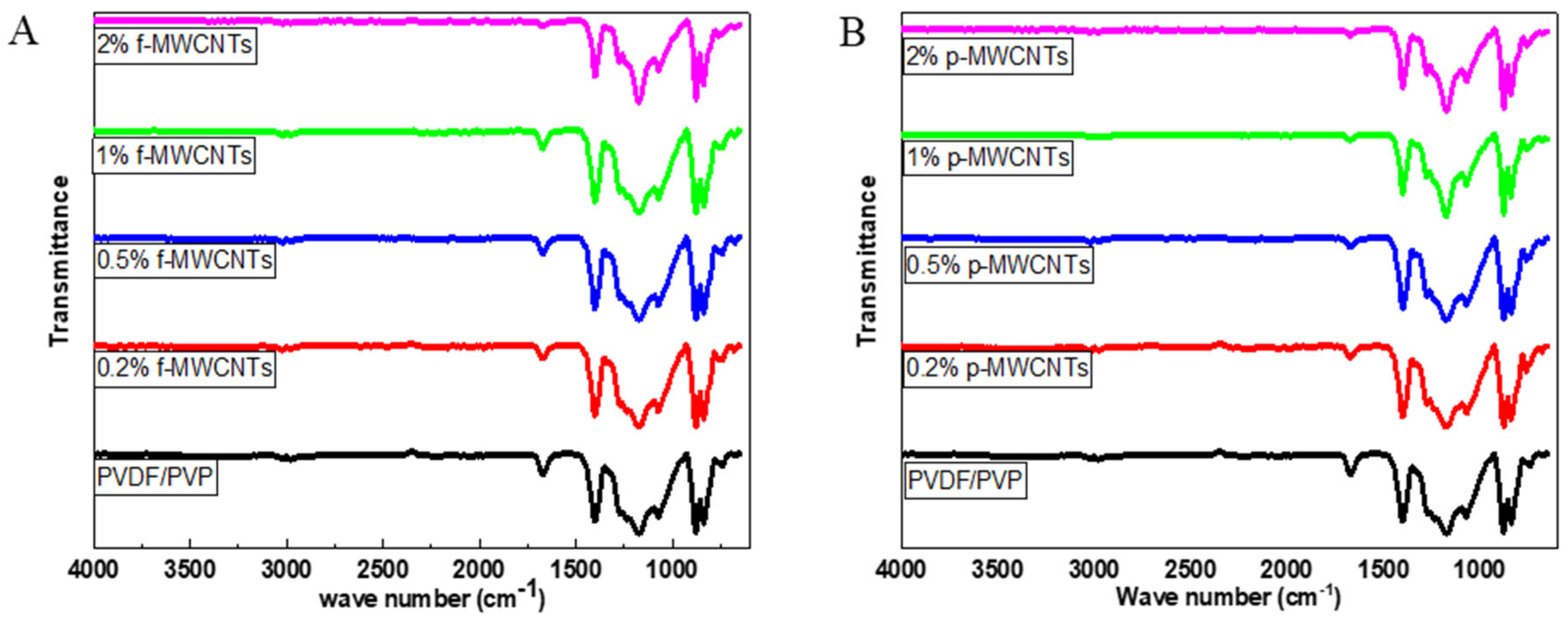
3.2.1. ATR-FTIR
3.2.2. XRD Analysis
3.2.3. SEM (Surface and Cross-Sectional)
3.2.4. Contact Angle and Water Intake Measurements
3.3. Performance Tests on the Membranes
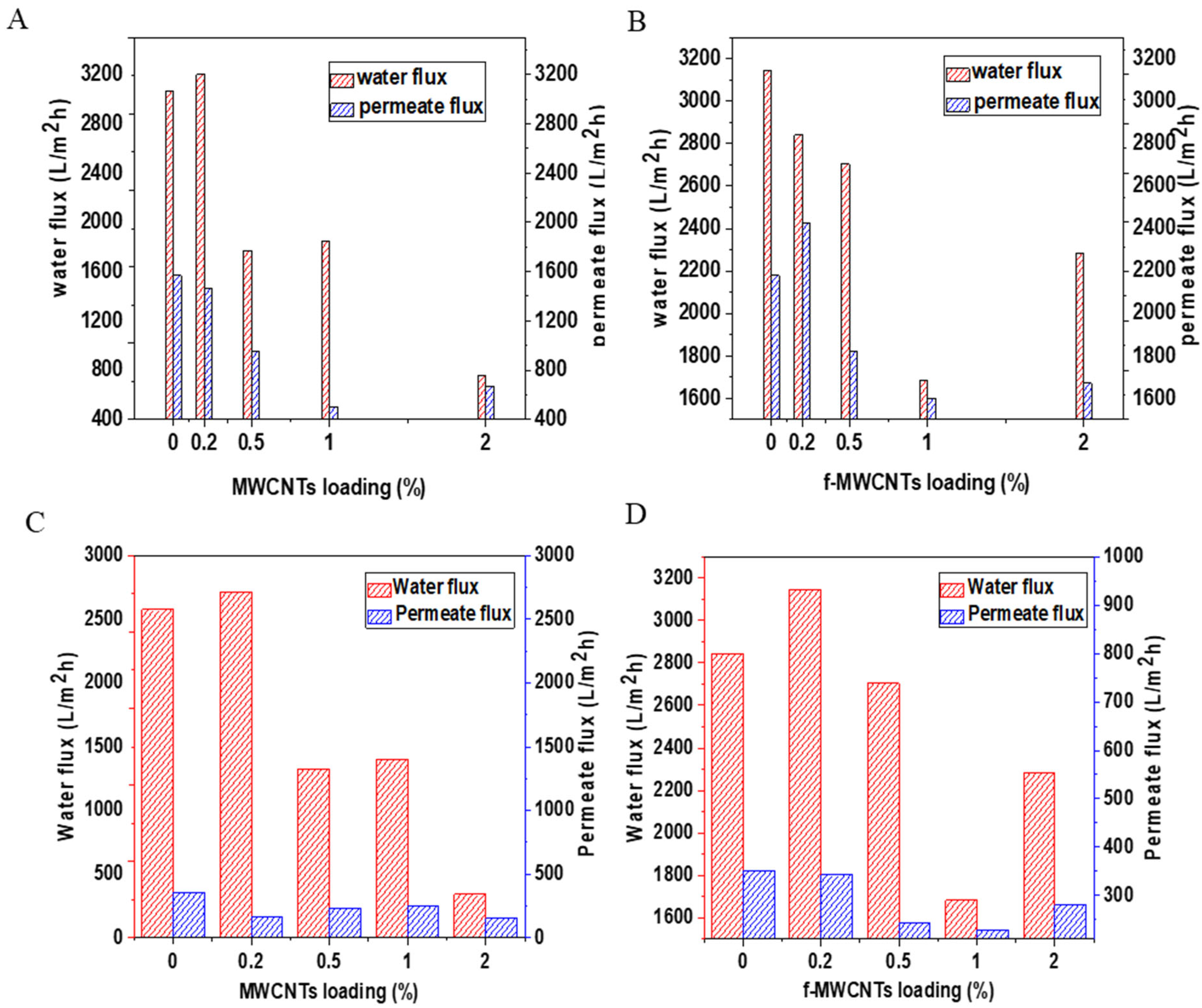
3.3.1. Water Flux and Permeate Flux Tests
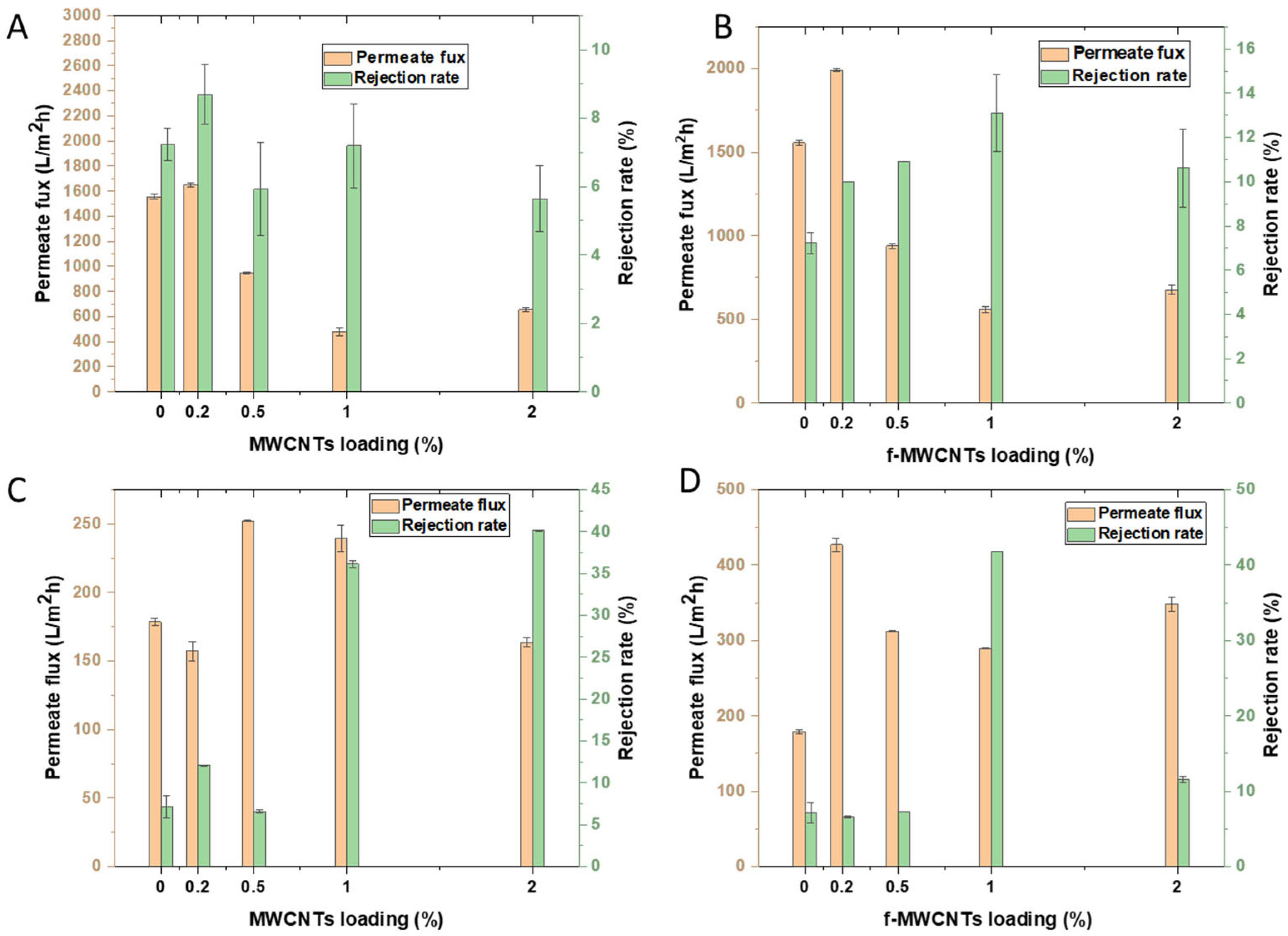
3.3.2. Rejection Tests: Effects of Nanomaterial Loading
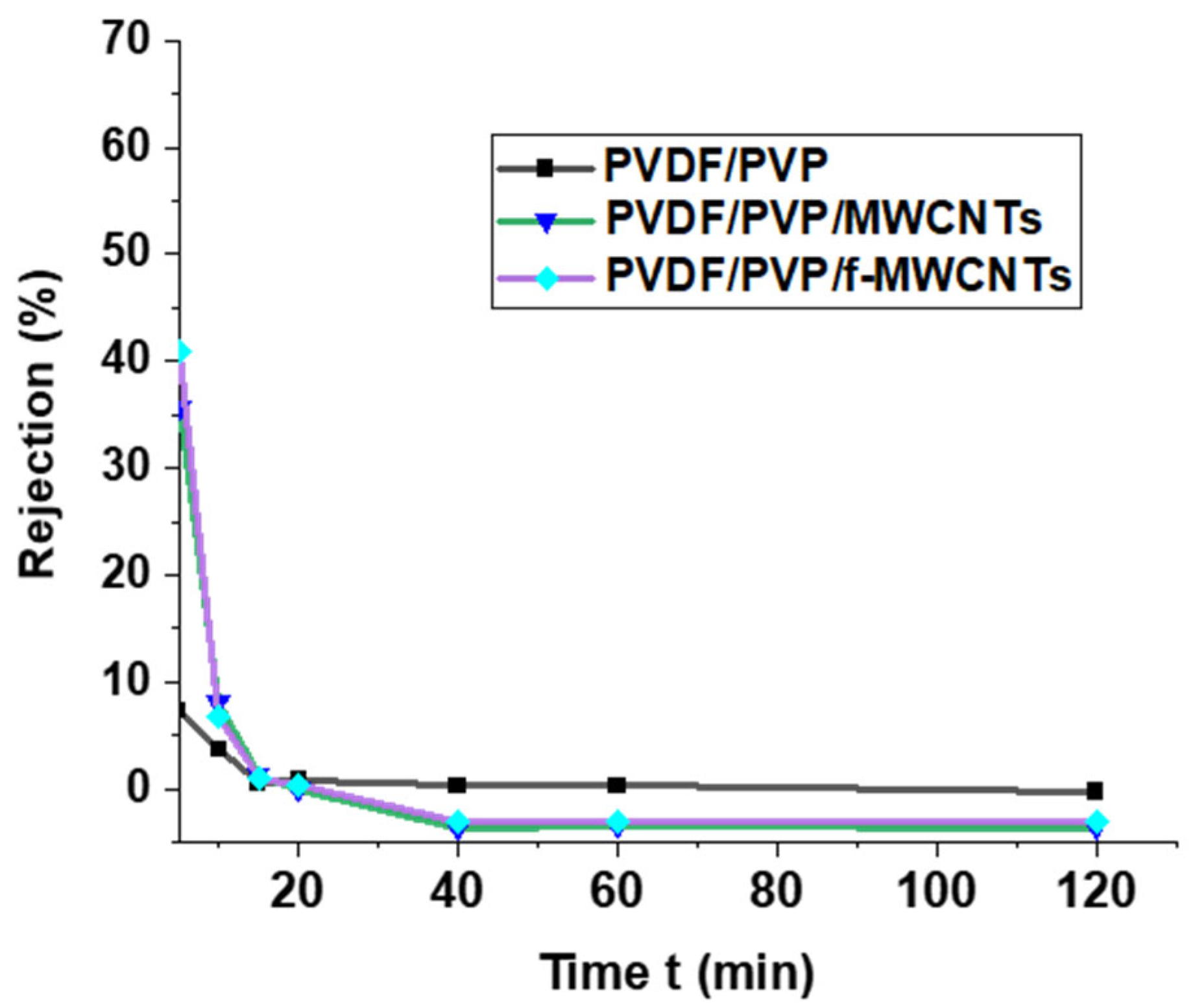
3.3.3. Effects of Contact Time
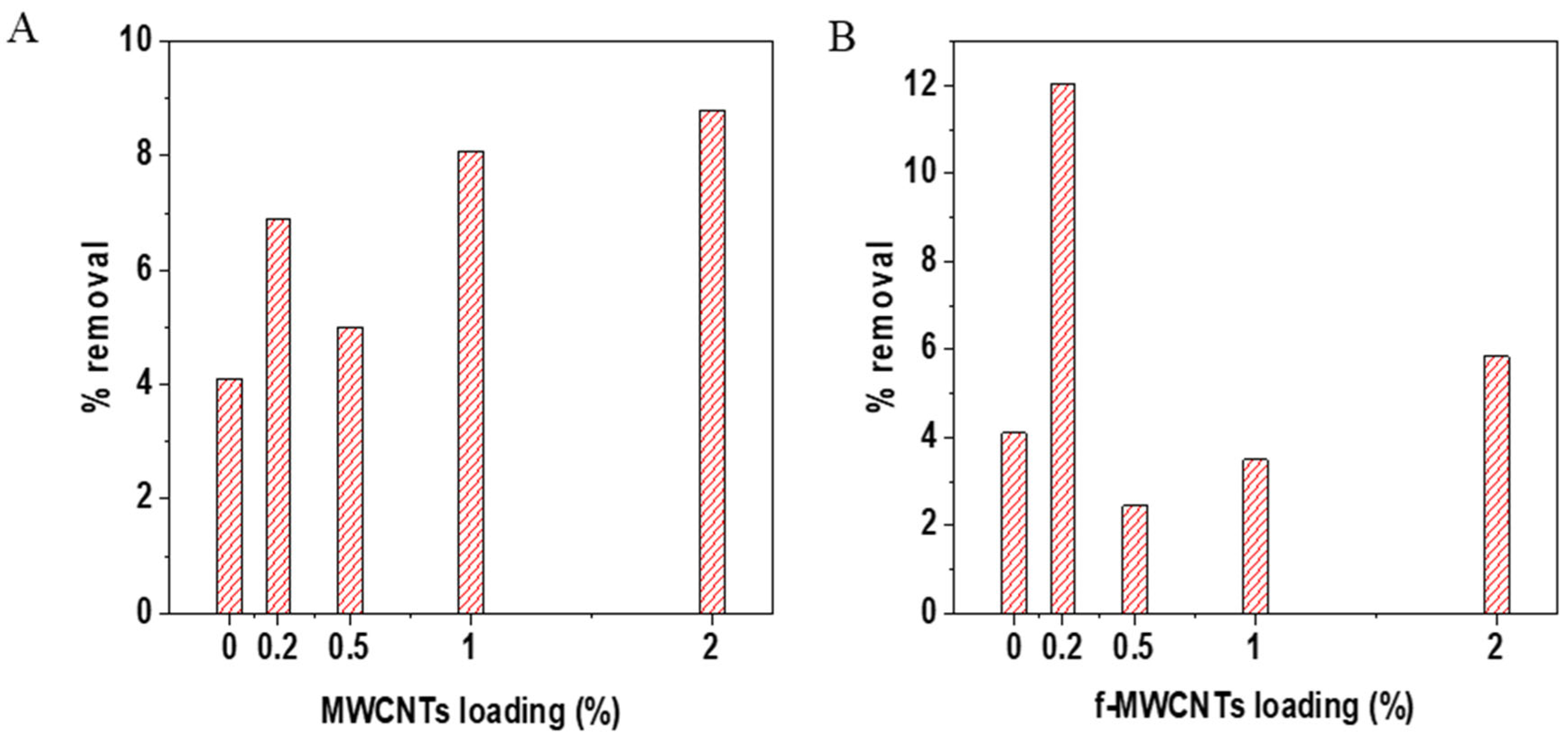
3.4. TOC Removal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVDF | Poly (vinylidene) fluoride |
| PVP | Polyvinylpyrrolidone |
| MWCNTs | Multi-walled carbon nanotubes |
| f-MWCNTS | Functionalized multi-walled carbon nanotubes |
| p-MWCNTs | Pristine multi-walled carbon nanotubes |
| FTIR | Fourier Transform Infrared Spectroscopy |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| SEM | Scanning electron microscopy |
| TOC | Total organic carbon |
| WIC | water intake capacity |
| KHP | Potassium hydrogen phthalate |
References
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Dhingra, A.; Hussain, A.; Changani, F. Applications of nanotechnology in water and wastewater treatment: A review. Asian J. Water Environ. Pollut. 2019, 16, 81–86. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research progress of high-salinity wastewater treatment technology. Water 2023, 15, 684. [Google Scholar] [CrossRef]
- Panagopoulos, A. Study and evaluation of the characteristics of saline wastewater (brine) produced by desalination and industrial plants. Environ. Sci. Pollut. Res. 2021, 29, 23736–23749. [Google Scholar] [CrossRef] [PubMed]
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of shale gas development on regional water quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Michailidis, P. Membrane technologies for sustainable wastewater treatment: Advances, challenges, and applications in zero liquid discharge (zld) and minimal liquid discharge (mld) systems. Membranes 2025, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Petrik, L.; Fatoba, O.; Fey, M.; Ndlela, R.; Ndlovu, N.; Omoniyi, E.; Bent, D.; Nell, P. Industrial Brine Minimization: Determining the Physical Chemical Parameters That Affect Evaporation Rates on Multi-Component Hyper-Saline Effluents: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2015. [Google Scholar]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef] [PubMed]
- El Zayat, H.; Nasr, P.; Sewilam, H. Investigating sustainable management of desalination brine through concentration using forward osmosis. Environ. Sci. Pollut. Res. 2021, 28, 39938–39951. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and water treatment application of carbon nanotubes (CNTs)-based composite membranes: A review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Criscuoli, A. Water–Energy Nexus: Membrane Engineering Towards a Sustainable Development. Membranes 2025, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Jarvis, P.; Carra, I.; Jafari, M.; Judd, S.J. Ceramic vs polymeric membrane implementation for potable water treatment. Water Res. 2022, 215, 118269. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-polymer composite membranes for water and wastewater treatment: Bridging the big gap between ceramics and polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef] [PubMed]
- Berber, M.R. Current advances of polymer composites for water treatment and desalination. J. Chem. 2020, 2020, 7608423. [Google Scholar] [CrossRef]
- Arundhathi, B.; Pabba, M.; Raj, S.S.; Sahu, N.; Sridhar, S. Advancements in Mixed-Matrix Membranes for Various Separation Applications: State of the Art and Future Prospects. Membranes 2024, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, V. Membrane fouling and cleaning in food and bioprocessing. In Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 213–254. [Google Scholar]
- Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 2008, 112, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Kumar, S.R.; Nguyen, C.H.; Lee, J.W.; Tsai, H.-A.; Hsieh, C.-H.; Lue, S.J. High-permeability graphene oxide and poly (vinyl pyrrolidone) blended poly (vinylidene fluoride) membranes: Roles of additives and their cumulative effects. J. Membr. Sci. 2021, 619, 118773. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Regmi, C.; Kshetri, Y.K.; Wickramasinghe, S.R. Carbon-Based Nanocomposite Membranes for Membrane Distillation: Progress, Problems and Future Prospects. Membranes 2024, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Mukherjee, A.; Maddela, N.R.; Chakraborty, S.; Shen, B.; Li, M.; Du, D.; Peng, Y.; Lu, F.; García Cruzatty, L.C. Outlook on the bottleneck of carbon nanotube in desalination and membrane-based water treatment—A review. J. Environ. Chem. Eng. 2020, 8, 103572. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Guan, M.; Zhang, H.; Xu, X.; Shi, H.; Liu, Q. Effects of modification groups and defects on the desalination performance of multi-walled carbon nanotube (MWNT) membranes. Sep. Purif. Technol. 2022, 297, 121444. [Google Scholar] [CrossRef]
- Lavagna, L.; Nisticò, R.; Musso, S.; Pavese, M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: A review. Mater. Today Chem. 2021, 20, 100477. [Google Scholar] [CrossRef]
- Alosime, E.M. A review on surface functionalization of carbon nanotubes: Methods and applications. Discov. Nano 2023, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Sianipar, M.; Kim, S.H.; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Abd Hamid, S.B.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Tijing, L.D.; Shim, W.-G.; Choi, J.-S.; Kim, S.-H.; He, T.; Drioli, E.; Shon, H.K. Water desalination using graphene-enhanced electrospun nanofiber membrane via air gap membrane distillation. J. Membr. Sci. 2016, 520, 99–110. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, H.; Liang, C.; Song, P.; Han, Y.; Han, Y.; Gu, J.; Kong, J.; Pan, D.; Guo, Z. Electromagnetic interference shielding MWCNT-Fe3O4@ Ag/epoxy nanocomposites with satisfactory thermal conductivity and high thermal stability. Carbon 2019, 141, 506–514. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Lang, Q.; Yan, B.; Kuang, S.; Mao, D.; Shu, L.; Zhang, Y. Anion exchange membrane organic fouling and mitigation in salt valorization process from high salinity textile wastewater by bipolar membrane electrodialysis. Desalination 2019, 465, 94–103. [Google Scholar] [CrossRef]
- Chai, Y.; Qin, P.; Wu, Z.; Bai, M.; Li, W.; Pan, J.; Cao, R.; Chen, A.; Jin, D.; Peng, C. A coupled system of flow-through electro-Fenton and electrosorption processes for the efficient treatment of high-salinity organic wastewater. Sep. Purif. Technol. 2021, 267, 118683. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.; Zhang, C.; Luan, J. Exploration and optimization of two-stage vacuum membrane distillation process for the treatment of saline wastewater produced by natural gas exploitation. Desalination 2016, 385, 117–125. [Google Scholar] [CrossRef]
- Avid, A.; Jafari, S.H.; Khonakdar, H.A.; Ghaffari, M.; Krause, B.; Pötschke, P. Surface modification of MWCNT and its influence on properties of paraffin/MWCNT nanocomposites as phase change material. J. Appl. Polym. Sci. 2020, 137, 48428. [Google Scholar] [CrossRef]
- Alwan, S.H.; Alshamsi, H.A. In situ synthesis NiO/F-MWCNTs nanocomposite for adsorption of malachite green dye from polluted water. Carbon Lett. 2022, 32, 1073–1084. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, K.; Shahi, V.K. Side-chain grafted functional groups poly (vinylidene fluoride-hexafluoropropylene) anti-fouling fluorinated polymer membrane with tuneable hydrophobicity for distillation. Desalination 2022, 525, 115501. [Google Scholar] [CrossRef]
- Jaber, L.; Almanassra, I.W.; Backer, S.N.; Kochkodan, V.; Shanableh, A.; Atieh, M.A. A comparative analysis of the effect of carbonaceous nanoparticles on the physicochemical properties of hybrid polyethersulfone ultrafiltration membranes. Membranes 2022, 12, 1143. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lu, D.; Harris, T.A.; Escobar, I.C. Polymers and solvents used in membrane fabrication: A review focusing on sustainable membrane development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Yaragalla, S.; Anilkumar, G.; Kalarikkal, N.; Thomas, S. Structural and optical properties of functionalized multi-walled carbon nanotubes. Mater. Sci. Semicond. Process. 2016, 41, 491–496. [Google Scholar] [CrossRef]
- Ramoraswi, N.O.; Ndungu, P.G. Photo-catalytic properties of TiO2 supported on MWCNTs, SBA-15 and silica-coated MWCNTs nanocomposites. Nanoscale Res. Lett. 2015, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Lin, W.-H.; Chang, Y.-C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Zhou, R.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of multi-walled carbon nanotubes (MWCNTs) and integrated MWCNTs/SiO2 nano-additives on PVDF polymeric membranes for vacuum membrane distillation. Sep. Purif. Technol. 2019, 217, 154–163. [Google Scholar] [CrossRef]
- Hoa, L.T.M. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4. Diam. Relat. Mater 2018, 89, 43–51. [Google Scholar]
- Dhall, S.; Jaggi, N.; Nathawat, R. Functionalized multiwalled carbon nanotubes based hydrogen gas sensor. Sens. Actuators A Phys. 2013, 201, 321–327. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suárez, G. Surface modification of multiwall carbon nanotubes by sulfonitric treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Theodore, M.; Hosur, M.; Thomas, J.; Jeelani, S. Influence of functionalization on properties of MWCNT–epoxy nanocomposites. Mater. Sci. Eng. A 2011, 528, 1192–1200. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsu, I.-K.; Aykol, M.; Hung, W.-H.; Chen, C.-C.; Cronin, S.B. A new lower limit for the ultimate breaking strain of carbon nanotubes. Acs Nano 2010, 4, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Gatabi, M.P.; Moghaddam, H.M.; Ghorbani, M. Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J. Mol. Liq. 2016, 216, 117–125. [Google Scholar] [CrossRef]
- Bourchak, M.; Juhany, K.; Salah, N.; Ajaj, R.; Algarni, A.; Scarpa, F. Determining the tensile properties and dispersion characterization of CNTs in epoxy using Tem and Raman spectroscopy. Mech. Compos. Mater. 2020, 56, 215–226. [Google Scholar] [CrossRef]
- Yuan, X.-T.; Xu, C.-X.; Geng, H.-Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO mixed matrix membranes for ultrafiltration and fouling detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef] [PubMed]
- Bobenko, N.; Bolotov, V.; Egorushkin, V.; Korusenko, P.; Melnikova, N.; Nesov, S.; Ponomarev, A.; Povoroznyuk, S. Experimental and theoretical study of electronic structure of disordered MWCNTs. Carbon 2019, 153, 40–51. [Google Scholar] [CrossRef]
- Sharma, M.; Rani, S.; Pathak, D.K.; Bhatia, R.; Kumar, R.; Sameera, I. Manifestation of anharmonicities in terms of phonon modes’ energy and lifetime in multiwall carbon nanotubes. Carbon 2021, 171, 568–574. [Google Scholar] [CrossRef]
- Santangelo, S.; Messina, G.; Faggio, G.; Abdul Rahim, S.; Milone, C. Effect of sulphuric–nitric acid mixture composition on surface chemistry and structural evolution of liquid-phase oxidised carbon nanotubes. J. Raman Spectrosc. 2012, 43, 1432–1442. [Google Scholar] [CrossRef]
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S. Enhanced ibuprofen adsorption and desorption on synthesized functionalized magnetic multiwall carbon nanotubes from aqueous solution. Materials 2020, 13, 3329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, K.; Verma, V.; Bhatti, H. Low-temperature hydrothermal synthesis and functionalization of multiwalled carbon nanotubes. Indian J. Phys. 2016, 90, 139–148. [Google Scholar] [CrossRef]
- Kaskun, S.; Akinay, Y.; Kayfeci, M. Improved hydrogen adsorption of ZnO doped multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2020, 45, 34949–34955. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Oxidative acid treatment of carbon nanotubes. Surf. Interfaces 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Shabaan, O.A.; Jahin, H.S.; Mohamed, G.G. Removal of anionic and cationic dyes from wastewater by adsorption using multiwall carbon nanotubes. Arab. J. Chem. 2020, 13, 4797–4810. [Google Scholar] [CrossRef]
- Abbas, A.; Yi, Y.M.; Saleem, F.; Jin, Z.; Veksha, A.; Yan, Q.; Lisak, G.; Lim, T.M. Multiwall carbon nanotubes derived from plastic packaging waste as a high-performance electrode material for supercapacitors. Int. J. Energy Res. 2021, 45, 19611–19622. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Huang, S.-C.; Chou, P.-H.; Den, W.; Hou, C.-H. Application of a multiwalled carbon nanotube-chitosan composite as an electrode in the electrosorption process for water purification. Chemosphere 2016, 146, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Grant, D.; Hou, X.; Xu, F. High rhodamine B and methyl orange removal performance of graphene oxide/carbon nanotube nanostructures. Mater. Today Proc. 2021, 34, 184–193. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Zhang, Q.; Li, T.; Chen, H.; Chen, X. Preparation and microwave absorption properties of asphalt carbon coated reduced graphene oxide/magnetic CoFe2O4 hollow particles modified multi-wall carbon nanotube composites. J. Alloys Compd. 2017, 723, 912–921. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Selek, A.; Turhan, O.; Kızılduman, B.K.; Bicil, Z. Different functional groups functionalized hexagonal boron nitride (h-BN) nanoparticles and multi-walled carbon nanotubes (MWCNT) for hydrogen storage. Fuel 2021, 303, 121335. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Beygmohammdi, F.; Kazerouni, H.N.; Jafarzadeh, Y.; Hazrati, H.; Yegani, R. Preparation and characterization of PVDF/PVP-GO membranes to be used in MBR system. Chem. Eng. Res. Des. 2020, 154, 232–240. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly (vinylylidenefluoride)(PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, X.; Xiang, Y.; Lu, S.; Jiang, S.P. New anhydrous proton exchange membranes for high-temperature fuel cells based on PVDF–PVP blended polymers. J. Mater. Chem. A 2015, 3, 148–155. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Effect of reduced graphene oxide/TiO2 nanocomposite with different molar ratios on the performance of PVDF ultrafiltration membranes. Sep. Purif. Technol. 2015, 140, 32–42. [Google Scholar] [CrossRef]
- Begum, S.; Ullah, H.; Kausar, A.; Siddiq, M.; Aleem, M.A. Fabrication of epoxy functionalized MWCNTs reinforced PVDF nanocomposites with high dielectric permittivity, low dielectric loss and high electrical conductivity. Compos. Sci. Technol. 2018, 167, 497–506. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, M.; Mao, M.; Zhang, Q.; Wang, H. Enhanced power output of an electrospun PVDF. Nanotechnology 2013, 24, 405401. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kang, W.; Han, Z.; Yan, J.; Cheng, B.; Liu, Y. Hierarchical MnOx@ PVDF/MWCNTs tree-like nanofiber membrane with high catalytic oxidation activity. J. Alloys Compd. 2019, 780, 805–815. [Google Scholar] [CrossRef]
- Hou, C.; Pang, Z.; Xie, S.; Yang, Z.; Wong, N.H.; Sunarso, J.; Peng, Y. Dual PVP roles for preparing PVDF hollow fiber membranes with bicontinuous structures via the complex thermally induced phase separation (c-TIPS). Sep. Purif. Technol. 2024, 332, 125766. [Google Scholar] [CrossRef]
- Kartohardjono, S.; Salsabila, G.M.K.; Ramadhani, A.; Purnawan, I.; Lau, W.J. Preparation of PVDF-PVP Composite Membranes for Oily Wastewater Treatment. Membranes 2023, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotubes for improving the surface properties and performance of polyethersulfone (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, T.; Yang, X. Facile pore structure control of poly(vinylidene fluoride) membrane for oil/water separation. Sep. Purif. Technol. 2020, 251, 117305. [Google Scholar] [CrossRef]
- Yang, S.; Fang, P.; Li, X.; Li, Q.; Zhou, Y.; Fan, E. Preparation and properties of patterned P-MWCNTs/PVDF functional membranes. J. Polym. Res. 2025, 32, 134. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, X.; Feng, X.; Ma, Y.; Zou, H. Fabrication of super-hydrophobic film from PMMA with intrinsic water contact angle below 90. Polymer 2007, 48, 7455–7460. [Google Scholar] [CrossRef]
- Zhenyu, S.; Zhanqiang, L.; Hao, S.; Xianzhi, Z. Prediction of contact angle for hydrophobic surface fabricated with micro-machining based on minimum Gibbs free energy. Appl. Surf. Sci. 2016, 364, 597–603. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Zhan, Y.; Zhang, L.; Pan, Y.; Zhang, C.; Yu, Z. Novel polyvinylidene fluoride nanofiltration membrane blended with functionalized halloysite nanotubes for dye and heavy metal ions removal. J. Hazard. Mater. 2016, 317, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, N.; Ahmed, Z.; Mahar, R.B.; Khatri, Z. Development and application of electrospun modified polyvinylidene fluoride (PVDF) nanofibers membrane for biofouling control in membrane bioreactor. Desalin. Water Treat. 2021, 217, 74–82. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Silva, T.L.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M. Multi-walled carbon nanotube/PVDF blended membranes with sponge-and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Bilal, A.; Yasin, M.; Akhtar, F.H.; Gilani, M.A.; Almohamadi, H.; Younas, M.; Mushtaq, A.; Aslam, M.; Hassan, M.; Nawaz, R. Enhancing water purification by integrating titanium dioxide nanotubes into polyethersulfone membranes for improved hydrophilicity and anti-fouling performance. Membranes 2024, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.K.; Zhou, Y.; Jiao, Y.; Cheng, X.Q.; Zhang, Y.; Wang, P.; Liang, H.; Yang, X.; Drioli, E.; Figoli, A. Multi-hydrophilic functional network enables porous membranes excellent anti-fouling performance for highly efficient water remediation. J. Membr. Sci. 2020, 608, 118191. [Google Scholar] [CrossRef]
- Shan, M.; Kang, H.; Xu, Z.; Li, N.; Jing, M.; Hu, Y.; Teng, K.; Qian, X.; Shi, J.; Liu, L. Decreased cross-linking in interfacial polymerization and heteromorphic support between nanoparticles: Towards high-water and low-solute flux of hybrid forward osmosis membrane. J. Colloid Interface Sci. 2019, 548, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. Development of hydrophilic microporous PES ultrafiltration membrane containing CuO nanoparticles with improved antifouling and separation performance. Mater. Chem. Phys. 2019, 222, 338–350. [Google Scholar] [CrossRef]
- Athanasekou, C.; Sapalidis, A.; Katris, I.; Savopoulou, E.; Beltsios, K.; Tsoufis, T.; Kaltzoglou, A.; Falaras, P.; Bounos, G.; Antoniou, M. Mixed matrix PVDF/graphene and composite-skin PVDF/graphene oxide membranes applied in membrane distillation. Polym. Eng. Sci. 2019, 59, E262–E278. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, C.; Zhang, S.; Li, P.; Hou, D. Preparation of graphene oxide modified poly (m-phenylene isophthalamide) nanofiltration membrane with improved water flux and antifouling property. Appl. Surf. Sci. 2017, 394, 149–159. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Dykstra, J.E.; Porada, S.; Biesheuvel, P.; Elimelech, M. Salt and water transport in reverse osmosis membranes: Beyond the solution-diffusion model. Environ. Sci. Technol. 2021, 55, 16665–16675. [Google Scholar] [CrossRef] [PubMed]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Yang, G.; Xie, Z.; Cran, M.; Ng, D.; Gray, S. Enhanced desalination performance of poly (vinyl alcohol)/carbon nanotube composite pervaporation membranes via interfacial engineering. J. Membr. Sci. 2019, 579, 40–51. [Google Scholar] [CrossRef]
- Fornasiero, F.; In, J.B.; Kim, S.; Park, H.G.; Wang, Y.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. pH-tunable ion selectivity in carbon nanotube pores. Langmuir 2010, 26, 14848–14853. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Hong, S.K.; Li, L.; Kim, J.-M.; Kim, S.H.; Rhee, K.Y.; Lee, H.W. Fabrication of robust, ultrathin and light weight, hydrophilic, PVDF-CNT membrane composite for salt rejection. Compos. Part B Eng. 2019, 160, 632–643. [Google Scholar] [CrossRef]
- Kim, S.; Ou, R.; Hu, Y.; Li, X.; Zhang, H.; Simon, G.P.; Wang, H. Non-swelling graphene oxide-polymer nanocomposite membrane for reverse osmosis desalination. J. Membr. Sci. 2018, 562, 47–55. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Jiao, C.; Huang, M.; Wang, G.-H.; Jiang, H. Microstructure regulation of polyamide nanocomposite membrane by functional mesoporous polymer for high-efficiency desalination. J. Membr. Sci. 2020, 597, 117783. [Google Scholar] [CrossRef]
- Liu, S.; Low, Z.-X.; Hegab, H.M.; Xie, Z.; Ou, R.; Yang, G.; Simon, G.P.; Zhang, X.; Zhang, L.; Wang, H. Enhancement of desalination performance of thin-film nanocomposite membrane by cellulose nanofibers. J. Membr. Sci. 2019, 592, 117363. [Google Scholar] [CrossRef]
- Chong, C.-Y.; Lau, W.-J.; Yusof, N.; Lai, G.-S.; Ismail, A.F. Roles of nanomaterial structure and surface coating on thin film nanocomposite membranes for enhanced desalination. Compos. Part B Eng. 2019, 160, 471–479. [Google Scholar] [CrossRef]
- Epsztein, R.; Cheng, W.; Shaulsky, E.; Dizge, N.; Elimelech, M. Elucidating the mechanisms underlying the difference between chloride and nitrate rejection in nanofiltration. J. Membr. Sci. 2018, 548, 694–701. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Negative rejection of ions in pressure-driven membrane processes. Adv. Colloid Interface Sci. 2008, 139, 150–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lee, L.Y.; Ong, S.L.; Chowdhury, P.; Siah, K.B.; Ng, H.Y. Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep. Purif. Technol. 2019, 213, 339–347. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.Y.; Noh, J.H.; Bae, Y.H.; Lee, J.W.; Maeng, S.K. A shift from chemical oxygen demand to total organic carbon for stringent industrial wastewater regulations: Utilization of organic matter characteristics. J. Environ. Manag. 2022, 305, 114412. [Google Scholar] [CrossRef] [PubMed]






| Sample | 2θ/° | FWHM/° | Interplanar Spacing d(002)/nm |
|---|---|---|---|
| MWCNTs | 25.52 | 2.958 | 0.3486 |
| f-MWCNTs | 25.31 | 4.034 | 0.3515 |
| Sample | D | G | |||
|---|---|---|---|---|---|
| Position | FWHM | Position | FWHM | ID/IG | |
| MWCNTs | 1449.33 | 22.015 | 1601 | 11.31 | 0.26 |
| f-MWCNTs | 1347.61 | 58.38 | 1591.07 | 85.48 | 0.85 |
| Sample | Surface Area (m2/g) | Pore Volume (m3/g) | Pore Size (nm) |
|---|---|---|---|
| MWCNTs | 202.31 | 1.12 | 22.26 |
| f-MWCNTs | 244.51 | 1.95 | 31.96 |
| Sample | Contact Angle | Water Intake Capacity (WIC)% |
|---|---|---|
| PVDF/PVP | 85.45 | 75.51 |
| PVDF/PVP/0.2% MWCNTs | 79.41 | 79.52 |
| PVDF/PVP/0.5% MWCNTs | 77.43 | 73.72 |
| PVDF/PVP/1% MWCNTs | 75.76 | 77.06 |
| PVDF/PVP/2% MWCNTs | 72.63 | 76.77 |
| PVDF/PVP/0.2% f-MWCNTs | 72.57 | 78.39 |
| PVDF/PVP/0.5% f-MWCNTs | 71.46 | 73.80 |
| PVDF/PVP/1% f-MWCNTs | 70.55 | 78.71 |
| PVDF/PVP/2% f-MWCNTs | 68.26 | 78.10 |
| Membrane | Salt Rejection | Pressure (kPa) | Target Source | Concentration (ppm) | Ref |
|---|---|---|---|---|---|
| PVDF/PVP/1%MWNCTs | 41.8% | 100 | Real industrial brine | 1080–14,000 | This work |
| GO/polymer (PES) | 98.5% | 1000 | Synthetic NaCl | 2000 | [106] |
| NMPS/PA | 98.7% | 1600 | Synthetic NaCl | 2000 | [107] |
| TEMPO/CFNs/PA | 96.2% | 1500 | Synthetic NaCl | 2000 | [108] |
| TFN-f-nTiO2/PA | 98.4 | 1500 | Synthetic NaCl | 2000 | [109] |
| 54.8 | 1500 | Synthetic Boric acid | 5 | ||
| PSf-TiO2 | 72.8% | 250 | Synthetic NaCl | 1160 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshauambea, T.T.; Malinga, S.P.; Ndungu, P.G. Treatment of Industrial Brine Using a Poly (Vinylidene Fluoride) Membrane Modified with Carbon Nanotubes. Membranes 2025, 15, 220. https://doi.org/10.3390/membranes15080220
Tshauambea TT, Malinga SP, Ndungu PG. Treatment of Industrial Brine Using a Poly (Vinylidene Fluoride) Membrane Modified with Carbon Nanotubes. Membranes. 2025; 15(8):220. https://doi.org/10.3390/membranes15080220
Chicago/Turabian StyleTshauambea, Tshifhiwa T., Soraya P. Malinga, and Patrick G. Ndungu. 2025. "Treatment of Industrial Brine Using a Poly (Vinylidene Fluoride) Membrane Modified with Carbon Nanotubes" Membranes 15, no. 8: 220. https://doi.org/10.3390/membranes15080220
APA StyleTshauambea, T. T., Malinga, S. P., & Ndungu, P. G. (2025). Treatment of Industrial Brine Using a Poly (Vinylidene Fluoride) Membrane Modified with Carbon Nanotubes. Membranes, 15(8), 220. https://doi.org/10.3390/membranes15080220






