Abstract
In sorption cooling systems, an important stage of the thermodynamic cycle is the separation of the refrigerant fluid from the absorbent mixture. This process is called “regeneration” or “desorption,” and it is similar to thermal desalination, where water is separated from an aqueous saline solution. However, since sorption systems utilize high salt concentration solutions, conventional desalination techniques such as reverse osmosis are not suitable. In this regard, membrane devices can enhance heat and mass transfer processes in compact sizes. In the present paper, a membrane device with an air gap membrane distillation configuration was evaluated, operating with the H2O/LiBr + LiCl solution (with a mass ratio of 2:1, LiBr:LiCl), to assess the produced distilled water flux. Among the operating parameters analyzed (solution temperature, cooling water temperature, salt concentration, and membrane pore size), solution temperature had the highest impact on the distilled water flux, while the membrane pore size had the lowest impact. The maximum distilled water flux was 7.63 kg/h·m2 with a solution temperature of 95.3 °C, a cooling water temperature of 25.1 °C, a salt concentration of 44.99% w/w, and a membrane pore size of 0.45 μm. On the other hand, the minimum distilled water flux was 0.28 kg/h·m2 with a solution temperature of 80.3 °C, a cooling water temperature of 40.1 °C, a salt concentration of 50.05% w/w, and with a membrane pore size of 0.22 μm.
1. Introduction
Thermally driven systems related to thermal comfort (such as liquid desiccant–air contact and absorption chillers) have been widely studied, as they offer promising and eco-friendly alternatives to conventional vapor compression devices, and can help reduce global warming. In addition, neither chlorofluorocarbons (CFCs) nor hydrochlorofluorocarbons (HCFCs) are used as working fluids, nor are fossil fuels used as a heat source, and relatively low electricity consumption is required [1]. In this technology, an important step is the separation of the refrigerant fluid from the absorbent mixture. This process is called “regeneration” or “desorption,” and it is similar to thermal desalination, where water is separated from an aqueous saline solution. The H2O/LiBr (water–lithium bromide) solution is the most used saline solution for brine–water sorption cooling systems; however, LiBr crystallization reduces the operational temperature range [2]. Increasing the LiBr solubility means the crystallization risk is reduced; in this regard, the use of additives such as organic salts [3,4,5], alcohols [6,7,8,9,10], ionic liquids [11,12,13], or inorganic salts [14,15,16] has been proposed and analyzed in the literature. To widen the operating range of a sorption cooling system, a working mixture with a low crystallization temperature is desirable [17], and the use of hygroscopic salts can help achieve this condition. Increasing the absorbent salt concentration in the working mixture reduces the water vapor pressure, which improves the refrigeration process efficiency [11]. Lithium chloride (LiCl) is a common absorbent used in sorption cycles. Additionally, it is less expensive than LiBr and exhibits long-term stability and good hygroscopic properties [18]. Therefore, the advantages of individual absorbents (LiBr and LiCL) can produce an interesting ternary mixture (H2O/LiBr + LiCl) for sorption cooling cycles. This mixture was proposed and analyzed by Patil et al. [19], who used a mixture with a mass ratio of 1:1 (LiBr:LiCl) in an absorption heat pump. The authors noted the advantages of this ternary mixture, which includes lower corrosivity due to the addition of LiCl, reducing the amount of LiBr. Consequently, the solubility of the absorbent salts increases, thereby reducing the crystallization risk, and the solution cost is lower than that of the conventional H2O/LiBr mixture. According to the authors’ results, the use of a ternary mixture can enhance the interaction between the working fluid and the absorbent. Grover et al. [20] used the same ternary mixture in an absorption cooler. The authors concluded that the generator heat load was lower with the ternary mixture than with the H2O/LiCl mixture. Pataskar et al. [21] evaluated an absorption heat transformer operating with the H2O/LiBr, H2O/LiBr + LiCl (with a mass ratio of 1:1, LiBr:LiCl), and the H2O/LiCl mixtures. According to the authors’ results, the Coefficient of Performance (COP) values for the ternary mixture were higher. However, the temperature lifts were lower compared to those for the H2O/LiBr mixture under similar operating conditions. Recently, Arabi and Dehghani [22] proposed the H2O/LiBr+NaHCO2 and H2O/LiBr + LiCl mixtures (with a mass ratio of 2:1) as alternatives for absorption cooling systems. The authors reported the solubility and density, and according to their results, the solubility of the H2O/LiBr + LiCl mixture is higher. In contrast, the density is lower than the conventional H2O/LiBr mixture. Based on these results, Aktemur and Öztürk [23,24] carried out an energy analysis of a solar-driven absorption cooling system with 50 kW of cooling capacity. The authors concluded that, compared with the H2O/LiBr mixture, the H2O/LiBr + LiCl mixture increased the COP and exergy efficiency by 8.81% and 8.96%, respectively. Meanwhile, the circulation ratio, solar collector area, and storage tank volume were reduced by 48.93%, 8.92%, and 8.91%, respectively. From the literature reviewed, it is clear that the H2O/LiBr + LiCl mixture is a viable alternative for sorption systems since several advantages have been reported.
Besides the impact of the selected working mixture on the sorption device’s performance, compact components are desirable for small-scale applications. Typically, the generator or regenerator used in sorption heat pumps is large and heavy; additionally, the heat and mass transfer of these key components are deficient, and the capital costs are higher than those of vapor compression systems [25,26]. Since sorption systems use high salt concentration solutions, conventional desalination techniques such as reverse osmosis (RO) are not suitable. Therefore, water separation is carried out by boiling at low operating pressure, which requires auxiliary devices. In this regard, membrane separation technologies, such as membrane distillation (MD), are emerging alternatives for treating high salt concentration solutions. In membrane distillation (MD), the water in the vapor phase is separated from a saline solution due to the vapor pressure difference caused by a temperature difference. Since this desorption process occurs below the boiling point of the working mixture, low-grade heat sources are suitable [27]. MD modules can enhance heat and mass transfer processes in reduced sizes, as the area-to-volume ratio is higher than that of conventional boiling components [25]. Several authors have reported the application of membrane devices in sorption heat pumps [25,28,29].
In this paper, an experimental evaluation of a membrane device using the H2O/LiBr + LiCl ternary mixture (with a mass ratio of 2:1, LiBr:LiCl) is presented and compared with other working mixtures reported in the literature. The effect on the distilled water flux of operation parameters such as salt concentration, pore size, saline solution temperature, and condensation temperature, was analyzed. The membrane configuration used was air gap membrane distillation (AGMD), as it achieves two purposes (desorber or regenerator/condenser) with this configuration.
2. Materials and Methods
2.1. Membrane Device Description
The membrane device has been described in previous reports [30,31] and comprises two support plates made of a polymeric material 300 mm in length, 200 mm wide, and 25.4 mm thick; neoprene gaskets and heat-resistant silicon gaskets with 1 and 3 mm thicknesses, respectively; a metallic mesh to support the membrane to reduce the deformation of the membrane by the solution flow; an aluminum plate with a 0.4 mm thickness for vapor condensation; and 12 bolts and screws. The solution channel was created by the junction of the support plate, the heat-resistant silicon gasket, and the membrane, measuring 180 mm in length, 80 mm in width, and 3 mm in thickness. The air gap, with a thickness of 3 mm, was integrated by the opposite membrane face, two neoprene gaskets, a metallic mesh, one silicon gasket, and one side of the condensing plate. Finally, the cooling water channel included the posterior side of the condenser plate, the heat-resistant silicon gasket, and the second support plate. Polytetrafluoroethylene (PTFE) hydrophobic membranes were used. Due to the high porosity and high hydrophobicity of the PTFE membranes, they are suitable for the MD process. In addition, the permeate flux is higher than that of the polyvinylidene difluoride (PVDF) and polypropylene (PP) membranes in the AGMD configuration [32]. Figure 1 shows the components of the experimental membrane device.
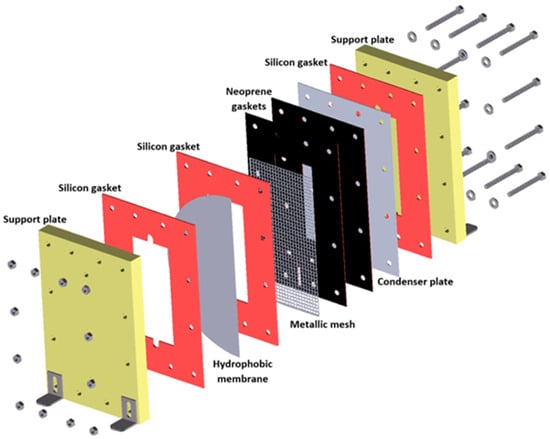
Figure 1.
Experimental membrane device configuration.
2.2. Experimental Setup
The experimental setup was integrated by the membrane device, heating and cooling systems, and the instrumental devices required to quantify the operating parameters. The heat load required for the membrane distillation process was supplied by a heating circulator with temperature control (by Julabo, Seelbach, Germany), which heated a fluid used to transfer sensible heat to the saline solution inside a plate heat exchanger (PHE) manufactured by Alfa Laval (Lund, Sweden). The latent heat load produced by the condensation of water in the vapor phase on the cooling plate was removed by cooling water refrigerated with a cooling circulator that features an integrated pump and temperature control (by Julabo, Seelbach, Germany). A Coriolis mass flowmeter (by Emerson Electric Co., St. Louis, MO, USA) was used to measure the mass flow of the saline solution, while the heating fluid stream and the cooling water stream were measured with analog flowmeters (by Cole Parmer, Vernon Hills, IL, USA). The solution and the heating fluid were pumped using gear pumps (by Cole Parmer, Vernon Hills, IL, USA) with a power rating of 32 W. An electronic weighing scale (by Optima Scale, Rancho Cucamonga, CA, USA) was used to measure the weight of the distilled water. The inlet and outlet temperatures of the membrane device and the PHE were measured with RTD pt100 temperature sensors (by Omega Engineering, Stamford, CT, USA). An Agilent data acquisition unit (by Agilent Technologies, Santa Clara, CA, USA) recorded the temperatures and the mass flow data of the solution. The H2O/LiBr + LiCl solution was prepared by mixing an aqueous LiBr solution with a concentration of 54% w/w and a density of 1.57 kg/L (at 25 °C), and crystalline LiCl powder, both chemical compounds were provided by Sigma Aldrich (Burlington, MA, USA). Since the mass ratio was 2:1 (LiBr:LiCL), to prepare 1 kg of saline solution with a concentration of 45% w/w, 0.556 kg of LiBr solution was mixed with 0.150 kg of LiCl and 0.294 kg of distilled water. To prepare 1 kg of saline solution with a concentration of 50% w/w, 0.333 kg of LiBr solution, 0.167 kg of LiCl, and 0.216 kg of distilled water were required. The salt concentration was calculated with the correlation reported by Arabi and Dehghani [22]. Table 1 presents the uncertainties associated with the devices used to measure the operating variables in the experimental tests. The experimental setup is shown in Figure 2.

Table 1.
Uncertainty of the measured variables.
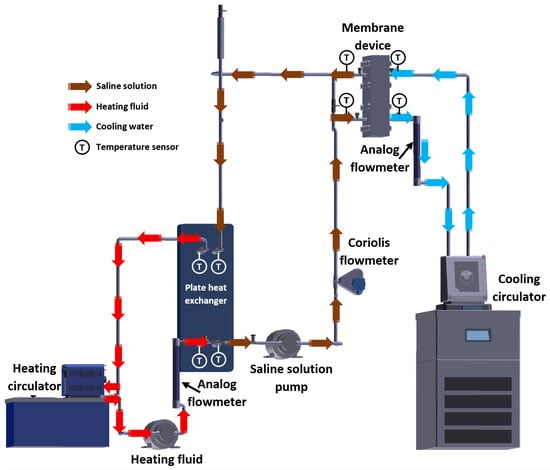
Figure 2.
Experimental setup diagram.
2.3. Experimental Operating Conditions
Different solution temperatures, salt concentrations, cooling water temperatures, and membrane pore sizes were used to analyze their effects on the distilled water flux. Table 2 shows the experimental operating conditions.

Table 2.
Experimental operating conditions.
3. Results
To evaluate the membrane device performance with the H2O/LiBr + LiCl mixture, the saline solution and cooling water temperatures were varied, together with the salt concentration and the membrane pore size. The results are present in the following subsections.
3.1. Operation Temperatures
To mitigate the effects of global warming, utilizing renewable energy sources for cooling sorption systems is desirable. Since solar energy is suitable for driving thermal separation processes such as MD, the salt solution temperatures were selected based on a possible scenario involving the integration of thermal solar technologies [33]. Additionally, if waste heat is utilized as a complementary thermal source (which is relatively inexpensive), an energy cost reduction can be achieved [34]. The cooling water temperatures were selected considering a wide environmental temperature range, allowing for the convenient selection of auxiliary systems to remove the heat load in the condenser. Figure 3 and Figure 4 show the distilled water flux for membrane pore sizes of 0.22 μm and 0.45 μm, respectively, with a salt concentration of 44.99% w/w and the operation temperatures shown in Table 1. In these figures, the effect of the operational temperatures can be observed. The impact of the saline solution temperature on the distilled water flux is higher than the cooling water temperature. For instance, in Figure 3, with a membrane pore size of 0.22 μm, increasing the solution temperature from 80.3 to 95.3 °C at a cooling water temperature of 25.1 °C results in a 128% increase in the distilled water flux. On the other hand, by decreasing the temperature of the cooling water from 40.1 °C to 25.1 °C, at a solution temperature of 95.3 °C, the distilled water flux increases by 43%. Similar behavior is observed in Figure 4, where a membrane pore size of 0.45 μm results in an increase in distilled water flux of 124% and 41% when the solution temperature increases from 80.3 to 95.3 °C and the cooling water temperature is reduced from 40.1 to 25.1 °C, respectively. Since membrane distillation is a thermally driven separation process, the temperature difference between the hot and cold sides of the membrane increases the distilled water flux because the vapor partial pressure at the membrane interphase increases exponentially with the solution temperature [35]. Additionally, the viscosity of the saline solution decreases as the solution temperature increases, which reduces the thickness of the boundary layer at the membrane, thereby enhancing the mass transfer coefficient [36]. Because there is no information about the viscosity and vapor pressure of the analyzed mixture, to illustrate the effect of the solution temperature on viscosity and the vapor mass transfer, the reported data with the H2O/LiBr solution with a salt concentration of 49.96% w/w was used to make an analogy. For this solution, the contribution of the boundary layer mass transfer resistance, which is related to the solution viscosity, was reduced by 22% when the solution temperature increased from 75.2 °C to 95 °C [37].
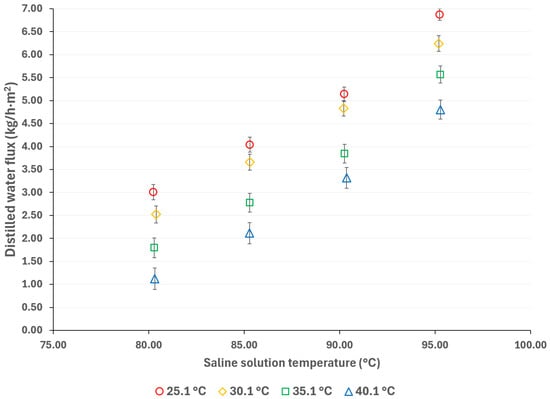
Figure 3.
Distilled water flux for different operating temperatures with a membrane pore size of 0.22 μm.
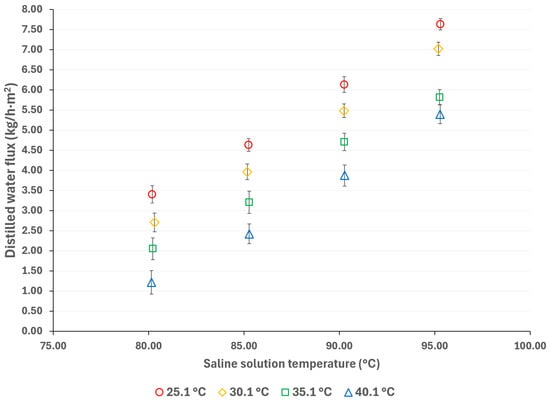
Figure 4.
Distilled water flux for different operating temperatures with a membrane pore size of 0.45 μm.
3.2. Membrane Pore Size
Generally, higher membrane pore sizes are desirable since they improve mass transfer and reduce heat transfer by conduction; however, as the membrane pore size increases, the liquid entry pressure (LEP) decreases, which can lead to membrane wetting and solute contamination of the distilled water [38,39]. Figure 5 compares the distilled water flux with membrane pore sizes of 0.22 µm and 0.45 µm, at a cooling water temperature of 25 °C, and with a salt concentration of 44.99% w/w. Figure 6 shows the same comparison with a salt concentration of 50.05% w/w. It is expected that, as the pore size increases, the distilled water flux will also increase; however, this behavior is observed at the lowest salt concentration. According to Khalifa et al. [40], the increase in distilled water flux is not a linear function of the pore size. In addition, the vapor partial pressure, which is independent of membrane pore size, is mainly influenced by the temperature and salt concentration, thus, the effect of these parameters on the mass transport driving force is greater than that of the membrane pore size [41]. For instance, with a solution temperature of 95.3 °C and cooling water at 25.1 °C, the distilled water flux increased 11% when using a membrane with a pore size of 0.45 μm compared to a membrane with a pore size of 0.22 μm, with the lower salt concentration. On the other hand, with the highest salt concentration, at the same operation temperatures, the distilled water flux difference between the two pore sizes is negligible (0.3%). This effect has been reported by other authors in high salt concentration solutions [41,42,43].
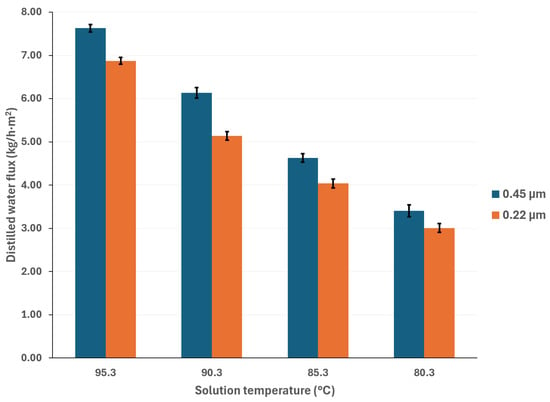
Figure 5.
Distilled water flux comparison between membrane pore sizes of 0.22 μm and 0.45 μm, with salt concentration of 44.99% w/w and cooling water temperature of 25.1 °C.
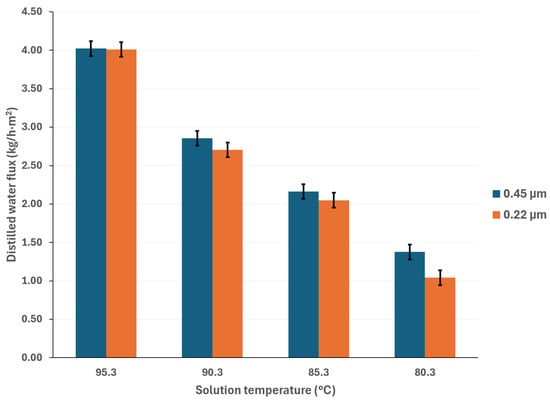
Figure 6.
Distilled water flux comparison between membrane pore sizes of 0.22 μm and 0.45 μm, with salt concentration of 50.05% w/w and cooling water temperature of 25.1 °C.
3.3. Salt Concentration
Compared to pressure-driven desalination technologies (such as RO), AGMD is more suitable for treating high-salinity solutions; however, thermodynamic properties, including vaporization heat, surface tension, boiling point, and viscosity, increase with salt concentration, thereby affecting the flux of the distilled water [44]. The influence of salt concentration can be observed in Figure 7 and Figure 8. As expected, a decreasing salt concentration increases the distilled water flux. According to the results presented in Figure 7, at a solution temperature of 95.3 °C and a cooling water at 25.1 °C, the distilled water flux increased by 71%, resulting in a decrease in salt concentration from 50.05% w/w to 44.99% w/w. Similarly, as shown in Figure 8, the distilled water flux increased by 90% at similar operation temperatures.
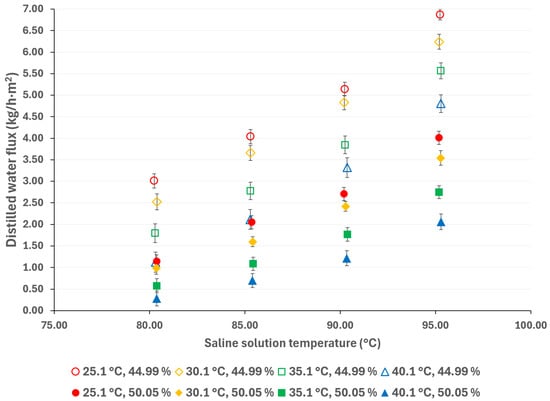
Figure 7.
Distilled water flux for different operating conditions with a membrane pore size of 0.22 μm.
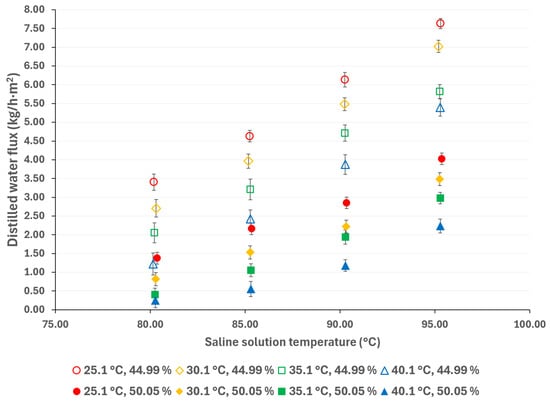
Figure 8.
Distilled water flux for different operating conditions with a membrane pore size of 0.45 μm.
According to the results presented in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8, the operation parameters that improve the distilled water flux, in order of importance, are as follows: the solution temperature, the salt concentration, the cooling water temperature, and, ultimately, the membrane pore size.
3.4. Comparison with Respect to the Conventional H2O/LiBr and H2O/LiCl Solutions
As previously mentioned, H2O/LiBr and H2O/LiCl are the most commonly used saline solutions in sorption systems. Thus, a performance comparison was conducted based on previous results available in the literature, using similar operating conditions as described in Table 2 and the same membrane device. Table 3 and Table 4 present the experimental conditions reported with the H2O/LiBr and H2O/LiCl solutions, respectively.

Table 3.
Experimental operating conditions for the H2O/LiBr solution [30].

Table 4.
Experimental operating conditions for the H2O/LiCl solution [31].
In Figure 9, the distilled water flux of the H2O/LiBr and H2O/LiBr + LiCl solutions is presented; the empty markers are for the H2O/LiBr saline solution, while the solid markers are for the H2O/LiBr + LiCl saline solution. As can be seen, under similar operating conditions, the performance of the H2O/LiBr solution is superior to that of the H2O/LiBr + LiCl solution. For instance, at a solution temperature of 95.3 °C and a cooling water temperature of 30.1 °C, the distilled water flux is 71% higher.
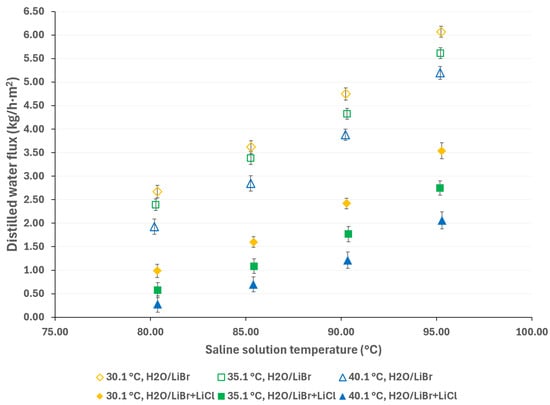
Figure 9.
Distilled water flux comparison between the H2O/LiBr and H2O/LiBr + LiCl solutions.
On the other hand, according to Figure 10, the distilled water flux with the H2O/LiBr + LiCl solution is 38% higher than that with the H2O/LiCl solution, with a saline solution temperature of 90.3 °C and a cooling water temperature of 25.1 °C. In this figure, the empty markers represent the H2O/LiCl saline solution, while the solid markers represent the H2O/LiBr + LiCl saline solution.
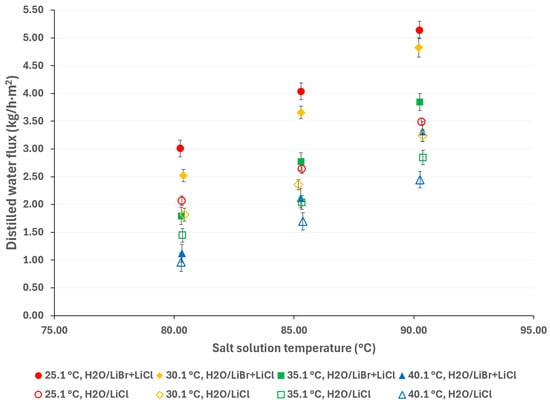
Figure 10.
Distilled water flux comparison between the H2O/LiCl and H2O/LiBr + LiCl solutions.
Even though the solution mass flow used with the H2O/LiCl solution was lower than reported in Table 1, this condition slightly impacts the distilled water flux [45] and, based on the data reported by Ibarra-Bahena et al. [31], increases the solution mass flow from 3.50 × 10−2 to 4.00 × 10−2 kg/s, with H2O/LiBr only increasing the distilled water flux by 3%. In addition, the salt concentration of the H2O/LiBr + LiCl solution was higher (44.99% w/w) than that of the H2O/LiCl solution (41.05% w/w).
According to Chen and Xu [46], the diffusion coefficient for water molecules in the H2O/LiBr solution is around 40% higher than that in the H2O/LiCl solution. This leads to an overall increase in the trans-membrane flux, indicating better vapor mass transfer performance. In addition, the authors indicated that lithium-based solutions are suitable, as cations with fewer charges and smaller volumes improve membrane distillation performance. In Figure 9 and Figure 10, it can clearly be observed that the distilled water flux of H2O/LiBr was higher than that of the H2O/LiCl solution, but the H2O/LiBr + LiCl solution is in the middle.
Since LiCl exhibits properties as a long-term stability, lower cost and better hygroscopic performance [47], the ternary mixture H2O/LiBr + LiCl offers an alternative that could reduce the crystallization risk, the required mass transfer area, and the operational costs for sorption systems. In particular, low corrosivity and high salt solubility are desirable properties for long-term operation, as observed in previous tests with LiBr-based solutions, where membrane fouling by rust and salt depositions was noted [43]. This can lead to membrane wetting, reduced distilled water flux, and membrane degradation [48]. In addition, according to Arabi and Dehghani [22], the density of the H2O/LiBr + LiCl solution is lower than that of the LiBr aqueous solution; therefore, the costs associated with pumping could be lower as well and, if thermal renewable sources are used to power the AGMD, the economics of the process can be remarkably improved [34].
4. Conclusions
A membrane device with the AGMD configuration was assessed, operating with the H2O/LiBr + LiCl (with a mass ratio of 2:1, LiBr:LiCl) mixture, to evaluate the produced distilled water flux. To quantify the effect of the operating parameters (solution temperature, cooling water temperature, salt concentration, and membrane pore size), 64 experimental conditions were tested. The maximum distilled water flux was 7.63 kg/h·m2 with a solution temperature of 95.3 °C, a cooling water temperature of 25.1 °C, a salt concentration of 44.99% w/w, and a membrane pore size of 0.45 μm. On the other hand, the minimum distilled water flux was 0.28 kg/h·m2 at a solution temperature of 80.3 °C, a cooling water temperature of 40.1 °C, salt concentration of 50.05% w/w, and with a membrane pore size of 0.22 μm. Based on the results, solution temperature is the most significant parameter affecting the distilled water flux because membrane distillation is a thermally driven separation process. As the solution temperature increases, vapor mass transfer is improved; conversely, the effect of membrane pore size is slight. A comparison of the distilled water flux produced with the H2O/LiBr + LiCl solution with that of the conventional H2O/LiBr and H2O/LiCl solutions was conducted. The distilled water flux was higher with the H2O/LiBr solution than with the H2O/LiBr + LiCl solution, but its performance was still higher than that of the H2O/LiCl mixture. These results could lead to the development of new saline solutions for sorption systems based on membrane separation devices, providing a new background for desalinating non-conventional brines with membrane distillation.
Author Contributions
Conceptualization, J.I.-B., U.D.-C., Y.R.G.-L., and W.R.; methodology, J.I.-B., U.D.-C., Y.R.G.-L., I.L.M.-C., and W.R.; formal analysis, J.I.-B., U.D.-C., Y.R.G.-L., I.L.M.-C., and W.R.; writing—original draft preparation, J.I.-B., U.D.-C., Y.R.G.-L., and W.R.; writing—review and editing, J.I.-B., U.D.-C., Y.R.G.-L., and W.R.; supervision, J.I.-B., U.D.-C., Y.R.G.-L., and W.R.; project administration, W.R.; funding acquisition, W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UNAM-PAPIIT-IT102224 project.
Data Availability Statement
The data recorders presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the support from the UNAM-PAPIIT-IT102224 project. Jonathan Ibarra-Bahena thanks the SECIHTI for the post-doctoral fellowship by the “Estancias Posdoctorales por México para la Formación y Consolidación de las y los Investigadores por México” program. Ulises Dehesa-Carrasco thanks El Colegio de Chihuahua for the academic support provided.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AGMD | Air Gap Membrane Distillation |
| CFCs | Chlorofluorocarbons |
| COP | Coefficient of Performance |
| HCFCs | Hydrochlorofluorocarbons |
| LEP | Liquid Entry Pressure |
| MD | Membrane Distillation |
| PHE | Plate Heat Exchanger |
| PP | Polypropylene |
| PVDF | Polyvinylidene difluoride |
| RO | Reverse Osmosis |
| RTD | Resistance Temperature Detector |
| LiBr | Lithium Bromide |
| LiCl | Lithium Chloride |
References
- Amin, M. Hybrid Thermally Driven Sorption–Ejector Systems: A Comprehensive Overview. Arab. J. Sci. Eng. 2023, 48, 11211–11235. [Google Scholar] [CrossRef]
- Izquierdo, M.; Venegas, M.; Rodríguez, P.; Lecuona, A. Crystallization as a limit to develop solar air-cooled LiBr–H2O absorption systems using low-grade heat. Sol. Energy Mater. Sol. Cells 2004, 81, 205–216. [Google Scholar] [CrossRef]
- Królikowska, M.; Nedzi, F. Experimental data on the physicochemical and thermodynamic properties of the aqueous lithium bromide modified by the addition of lithium salt. J. Chem. Thermodyn. 2021, 161, 106514. [Google Scholar] [CrossRef]
- Kim, J.-S.; Park, Y.; Lee, H.J. Solubilities and Vapor Pressures of the Water + Lithium Bromide + Ethanolamine System. Chem. Eng. Data 1996, 41, 279–281. [Google Scholar] [CrossRef]
- Donate, M.; Rodriguez, L.; De Lucas, A.; Rodríguez, J.F. Thermodynamic evaluation of new absorbent mixtures of lithium bromide and organic salts for absorption refrigeration machines. Int. J. Refrig. 2006, 29, 30–35. [Google Scholar] [CrossRef]
- Królikowska, M.; Romańska, K.; Paduszyński, K.; Skonieczny, M. The study on the influence of glycols on the vapor pressure, density and dynamic viscosity of lithium bromide aqueous solution. Fluid Phase Equilib. 2020, 519, 112640. [Google Scholar] [CrossRef]
- Królikowska, M.; Romańska, K. The experimental study on the influence of crown ethers and glycols on the mutual solubility of lithium bromide in water. Fluid Phase Equilib. 2019, 483, 175–181. [Google Scholar] [CrossRef]
- Inada, T.; Tomita, H.; Takemura, F.; Tsubouchi, O.; Hihara, E. Crystallization temperature, vapor pressure, density and viscosity of lithium bromide + lithium iodide + ethylene glycol + water system for absorption refrigerators for automotive use. Int. J. Refrig. 2019, 100, 274–283. [Google Scholar] [CrossRef]
- Rivera, W.; Cerezo, J. Experimental study of the use of additives in the performance of a single-stage heat transformer operating with water–lithium bromide. Int. J. Energy Res. 2005, 29, 121–130. [Google Scholar] [CrossRef]
- Rivera, W.; Romero, R.J.; Best, R.; Heard, C.L. Experimental evaluation of a single-stage heat transformer operating with the water/CarrolTM mixture. Energy 1999, 24, 317–326. [Google Scholar] [CrossRef]
- Królikowska, M.; Skonieczny, M.; Paduszyński, K.; Zawadzki, M. Vapor pressure and physicochemical properties of {libr + IL-based additive + water} mixtures: Experimental data and COSMO-RS predictions. J. Solut. Chem. 2021, 50, 473–502. [Google Scholar] [CrossRef]
- Xuan, Y.; Fang, K.; Duan, B.; Gao, N.; Chen, G. Vapor pressure measurement of ternary systems LiBr + [Emim]I + H2O and LiBr + [Dmim]I + H2O. J. Chem. Eng. Data 2020, 65, 487–494. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Y.; Liu, S.; Lv, H.; Luo, C. Thermodynamic Properties of a Ternary AHP Working Pair: Lithium Bromide + 1Ethyl-3-methylimidazolium Chloride + H2O. J. Chem. Eng. Data 2019, 64, 574–583. [Google Scholar] [CrossRef]
- Bourouis, M.; Vallès, M.; Medrano, M.; Coronas, A. Performance of air-cooled absorption air-conditioning systems working with water-(LiBr + LiI + LiNO3 + LiCl). Proc. Inst. Mech. Eng. J. Process Mech. Eng. Part E 2005, 219, 205–213. [Google Scholar] [CrossRef]
- Salavera, D.; Esteve, X.; Patil, K.R.; Mainar, A.M.; Coronas, A. Solubility, heat capacity, and density of Lithium Bromide + Lithium Iodide + Lithium Nitrate + Lithium Chloride Aqueous Solutions at Several Compositions and Temperatures. J. Chem. Eng. Data 2004, 49, 613–619. [Google Scholar] [CrossRef]
- Saravanan, R.; Maiya, M.P. Thermodynamic comparison of water-based working fluid combinations for a vapour absorption refrigeration system. Appl. Therm. Eng. 1998, 18, 553–568. [Google Scholar] [CrossRef]
- Lee, H.R.; Koo, K.K.; Jeong, S.; Kim, J.S.; Lee, H.; Oh, Y.S. Thermodynamic design data and performance evaluation of the H2O + LiBr + lithium iodide + lithium nitrate + lithium chloride system for absorption chiller. Appl. Therm. Eng. 2000, 20, 707–720. [Google Scholar] [CrossRef]
- Parham, K.; Atikol, U.; Yari, M.; Agboola, O.P. Evaluation and Optimization of Single Stage Absorption Chiller Using (LiCl + H2O) as the Working Pair. Adv. Mech. Eng. 2013, 5, 683157. [Google Scholar] [CrossRef]
- Patil, K.R.; Kim, M.N.; Eisa, M.A.R.; Holland, F.A. Experimental evaluation of aqueous lithium halides as single- and double-salt systems in absorption heat-pumps. Appl. Energy 1989, 34, 99–111. [Google Scholar] [CrossRef]
- Grover, G.S.; Devotta, S.; Holland, F.A. Performance of an experimental absorption cooler using aqueous LiCl and LiCl/LiBr solutions. Ind. Eng. Chem. Res. 1989, 28, 250–253. [Google Scholar] [CrossRef]
- Pataskar, S.G.; Adyanthaya, S.D.; Devotta, S.; Holland, F.A. Performance of an experimental absorption heat transformer using aqueous LiBr, LiCl, and LiBr/LiCl solutions. Ind. Eng. Chem. Res. 1990, 29, 1658–1662. [Google Scholar] [CrossRef]
- Arabi, M.; Dehghani, M.R. Measurement of solubility and density of water + lithium bromide + lithium chloride and water + lithium bromide + sodium formate systems. Int. J. Refrig. 2015, 56, 99–104. [Google Scholar] [CrossRef]
- Aktemur, C.; öztürk, İ.T. Energetic and exergetic analysis of a solar-driven single-effect absorption refrigeration system using LiBr + LiCl/H2O solution mixture. ASME J. Sol. Energy Eng. 2022, 144, 061007. [Google Scholar] [CrossRef]
- Aktemur, C.; öztürk, İ.T. Thermodynamic optimization of utilization of LiBr + LiCl/H2O solution mixture on a single-effect absorption chiller driven by solar energy. ASME J. Sol. Energy Eng. 2023, 145, 051003. [Google Scholar] [CrossRef]
- Asfand, F.; Bourouis, M. A review of membrane contactors applied in absorption refrigeration systems. Renew. Sustain. Energy Rev. 2015, 45, 173–191. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; You, T.; Wang, B.; Shi, W. Combining ground source absorption heat pump with ground source electrical heat pump for thermal balance, higher efficiency and better economy in cold regions. Renew. Energy 2015, 84, 74–88. [Google Scholar] [CrossRef]
- Zhai, C.; Wu, W.; Coronas, A. Membrane-based absorption cooling and heating: Development and perspectives. Renew. Energy 2021, 177, 663–688. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Raman, S.; Galindo-Luna, Y.R.; Rodríguez-Martínez, A.; Rivera, W. Role of membrane technology in absorption heat pumps: A comprehensive review. Membranes 2020, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Wu, W. A comprehensive review of membrane-based absorbers/desorbers towards compact and efficient absorption refrigeration systems. Renew. Energy 2022, 201, 563–593. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Rivera, W.; Nanco-Mejía, S.D.; Romero, R.J.; Venegas-Reyes, E.; Dehesa-Carrasco, U. Experimental performance of a membrane desorber operating under simulated warm weather condensation temperatures. Membranes 2021, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Bahena, J.; Dehesa-Carrasco, U.; Galindo-Luna, Y.R.; Medina-Caballero, I.L.; Rivera, W. Experimental performance of a membrane desorber with a H2O/LiCl mixture for absorption chiller applications. Membranes 2022, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hwang, H.J.; Moon, I.S. Air gap membrane distillation on the different types of membrane. Korean J. Chem. Eng. 2011, 28, 770–777. [Google Scholar] [CrossRef]
- Atmaca, I.; Yigit, A. Simulation of solar-powered absorption cooling system. Renew. Energy 2003, 28, 1277–1293. [Google Scholar] [CrossRef]
- Usman, H.S.; Touati, K.; Rahaman, M.S. An economic evaluation of renewable energy-powered membrane distillation for desalination of brackish water. Renew. Energy 2021, 169, 1294–1304. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Transport analysis of air-gap membrane distillation. J. Membr. Sci. 2005, 255, 239–253. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Q.; Lu, X.; Zhao, L.; Wu, S.; Ma, Z.; Zhang, H. Study on the performance of double-pipe air gap membrane distillation module. Desalination 2016, 396, 48–56. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Dehesa-Carrasco, U.; Romero, R.; Rivas-Herrera, B.; Rivera, W. Experimental assessment of a hydrophobic membrane-based desorber/condenser with H2O/LiBr mixture for absorption systems. Exp. Therm. Fluid Sci. 2017, 88, 145–159. [Google Scholar] [CrossRef]
- Eykens, L.; Reyns, T.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How to select a membrane distillation configuration? Process conditions and membrane influence unraveled. Desalination 2016, 399, 105–115. [Google Scholar] [CrossRef]
- Geng, H.; He, Q.; Wu, H.; Li, P.; Zhang, C.; Chang, H. Experimental study of hollow fiber AGMD modules with energy recovery for high saline water desalination. Desalination 2014, 344, 55–63. [Google Scholar] [CrossRef]
- Khalifa, A.; Lawal, D.; Antar, M.; Khayet, M. Experimental and theoretical investigation on water desalination using air gap membrane distillation. Desalination 2015, 376, 94–108. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Treatment of high salinity solutions: Application of air gap membrane distillation. Desalination 2012, 287, 55–60. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Air gap membrane distillation: A detailed study of high saline solution. Desalination 2017, 403, 179–186. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Dehesa-Carrasco, U.; Montiel-González, M.; Romero, R.J.; Basurto-Pensado, M.A.; Hernández-Cristóbal, O. Experimental evaluation of a membrane contactor unit used as a desorber/condenser with water/Carrol mixture for absorption heat transformer cycles. Exp. Therm. Fluid Sci. 2016, 76, 193–204. [Google Scholar] [CrossRef]
- Shahu, V.T.; Thombre, S.B. Air gap membrane distillation: A review. J. Renew. Sustain. Energy 2019, 11, 045901. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Al-Amri, F.; Khalifa, A.E.; Al-Zoubi, A.; Abid, M.; Younis, E.; Mallick, T.K. A comprehensive review of air gap membrane distillation process. Desalin. Water Treat. 2018, 110, 27–64. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X. Molecular dynamics study on the influence of working pairs on membrane distillation process applied in absorption refrigeration systems. J. Mol. Liq. 2025, 433, 127796. [Google Scholar] [CrossRef]
- Altamirano, A.; Le Pierrès, N.; Stutz, B. Review of small-capacity single-stage continuous absorption systems operating on binary working fluids for cooling: Theoretical, experimental and commercial cycles. Int. J. Refrig. 2019, 106, 350–373. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).