Cholesterol Sulfate in Biological Membranes: A Biophysical Study in Cholesterol-Poor and Cholesterol-Rich Biomimetic Models
Abstract
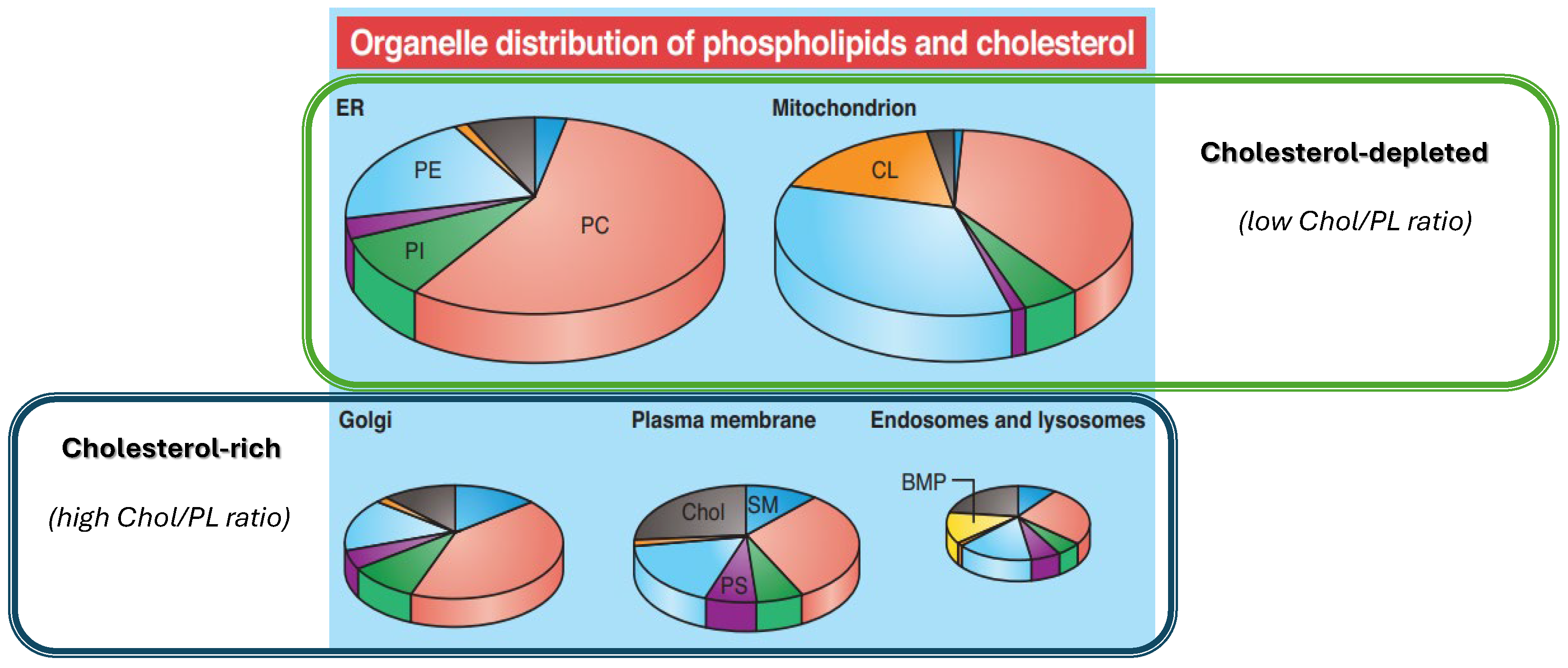
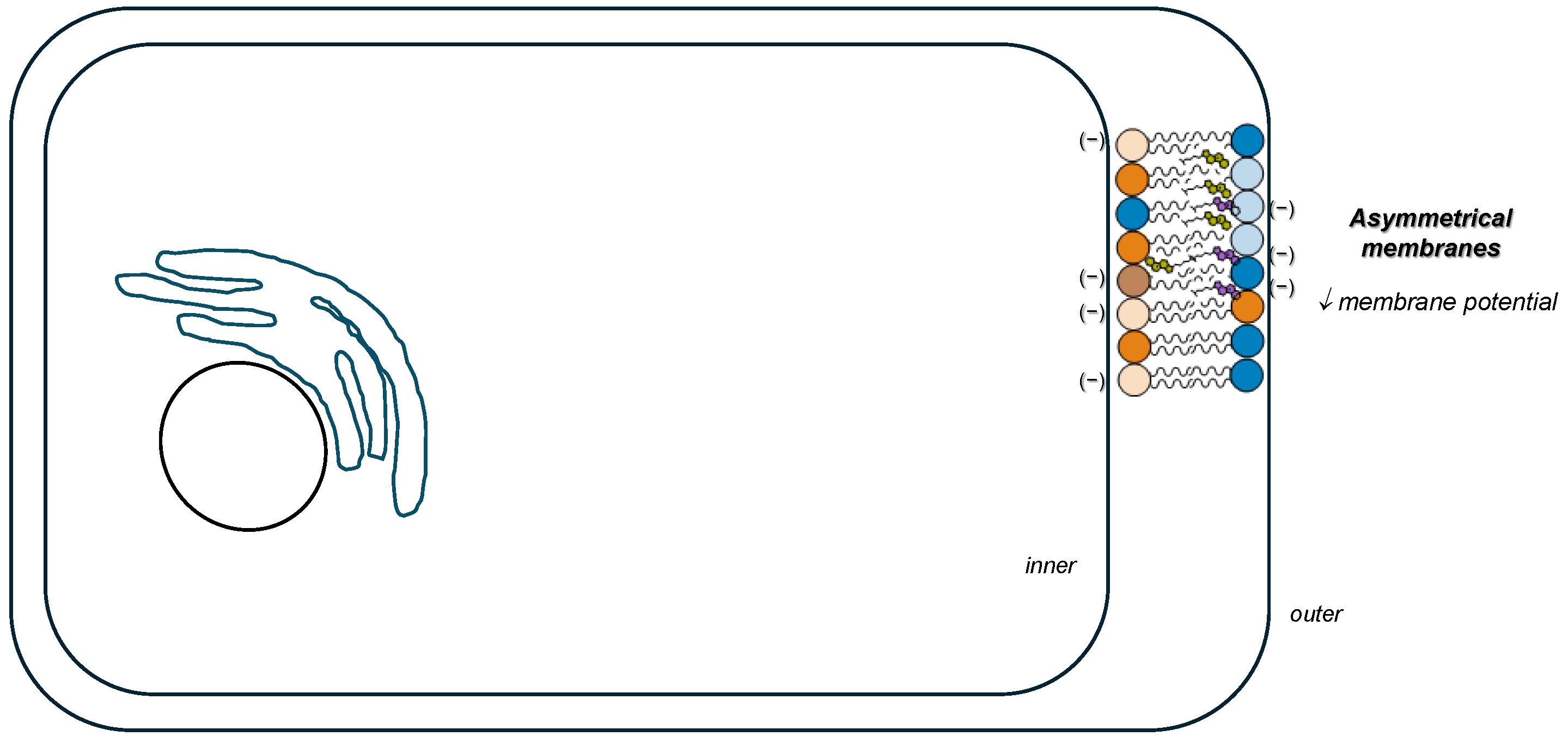
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Liposome Preparation
2.3. Fluorescence Measurements
2.4. Measurement of Surface Net Charge
2.5. Dynamic Light Scattering Measurements
2.6. Statistical Data Analysis
3. Results
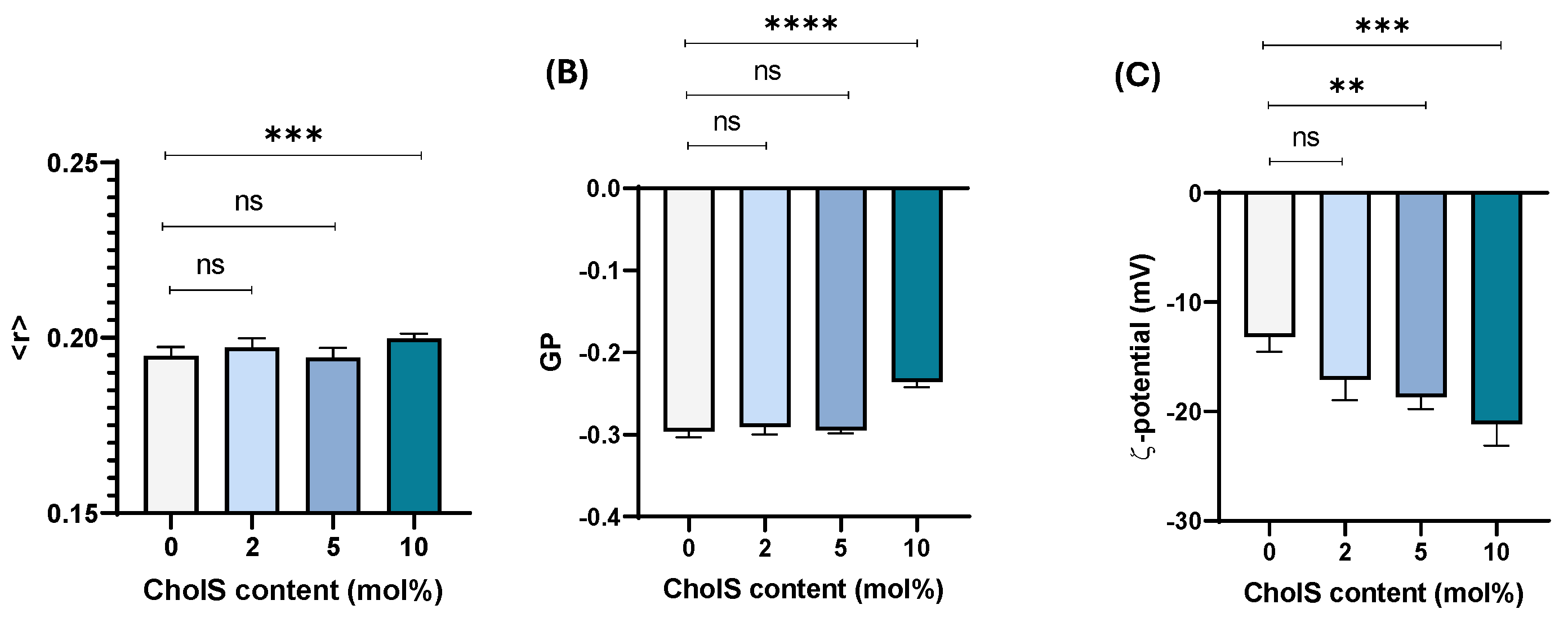
3.1. Effect of Chol Incorporation in Cholesterol-Poor Membrane Model
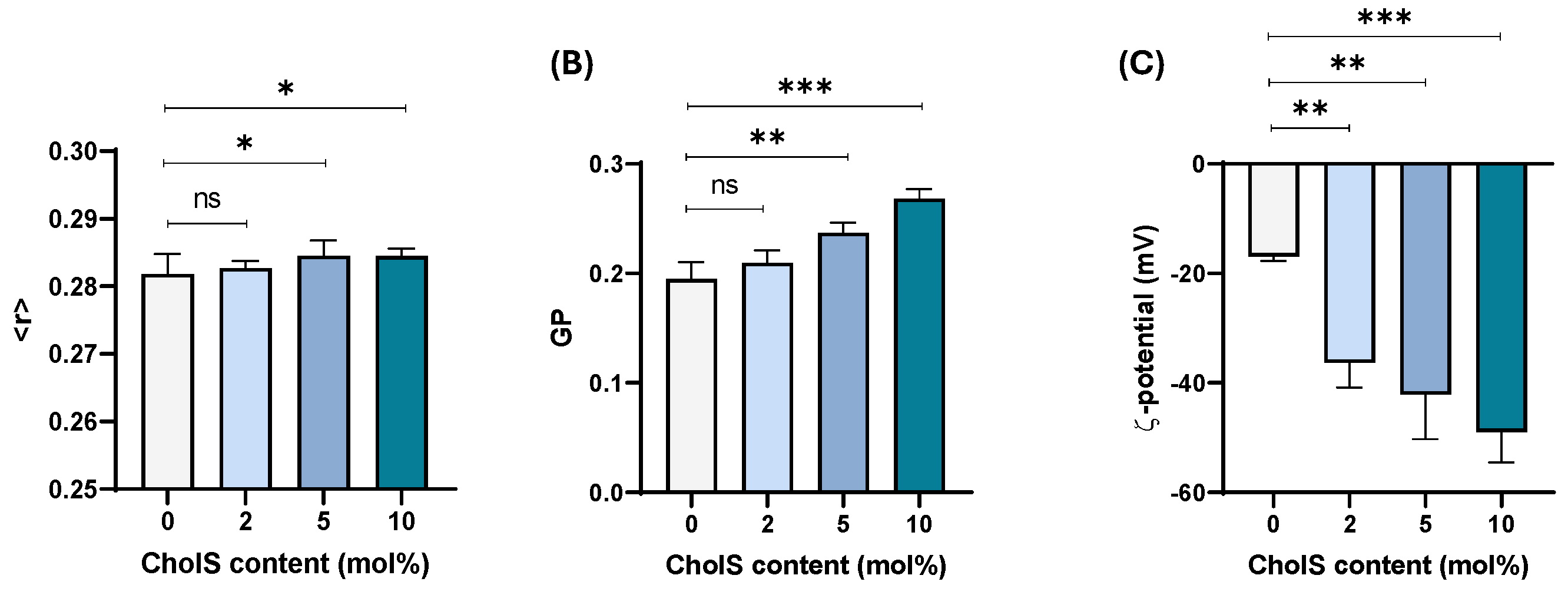
3.2. Effect of CholS in Cholesterol-Rich Epithelial-like Membrane Model
3.3. Thermotropic Behaviour of Cholesterol-Poor and Cholesterol-Rich Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Chol/PL | Cholesterol-to-Phospholipid ratio |
| SC | Directory of open access journals |
| STS | Steroid sulfatase |
| ESM | Egg sphingomyelin |
| ER | Endoplasmic Reticulum |
| GP | Generalised Polarisation |
| GSL | GlycoSphingoLipid |
| PC | Phosphatidylcholine lipids |
| PE | Phosphatidylethanolamine lipids |
| PL | Phospholipid |
| RLXI | Recessive X-linked ichthyosis disorder |
References
- Strott, C.A.; Higashi, Y. Cholesterol sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Dias, I.H.K. Oxysterol sulfates in fluids, cells and tissues: How much do we know about their clinical significance, biological relevance and biophysical implications? Essays Biochem. 2024, 68, 401–410. [Google Scholar] [PubMed]
- Dias, I.H.K.; Ferreira, R.; Gruber, F.; Vitorino, R.; Rivas-Urbina, A.; Sanchez-Quesada, J.L.; Vieira Silva, J.; Fardilha, M.; de Freitas, V.; Reis, A. Sulfate-based lipids: Analysis of healthy human fluids and cell extracts. Chem. Phys. Lipids 2019, 221, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.; Leyh, T.S. Sulfotransferase 2B1b, Sterol Sulfonation, and Disease. Pharmacol. Rev. 2023, 75, 521–531. [Google Scholar] [CrossRef]
- Foster, P.A.; Mueller, J.W. Insights into steroid sulfation and desulfation pathways. J. Mol. Endocrinol. 2018, 61, T271–T283. [Google Scholar] [CrossRef]
- Le, H.H.; Lee, M.T.; Besler, K.R.; Comrie, J.M.C.; Johnson, E.L. Characterization of interactions of dietary cholesterol with the murine and human gut microbiome. Nat. Microbiol. 2022, 7, 1390–1403. [Google Scholar] [CrossRef]
- Williams, M.L.; Elias, P.M. Stratum corneum lipids in disorders of cornification. Increased cholesterol sulfate content of stratum corneum in recessive X-linked ichthyosis. J. Clin. Investig. 1981, 68, 1404–1410. [Google Scholar] [CrossRef]
- Elias, P.M.; Williams, M.L.; Maloney, M.E.; Bonifas, J.A.; Brown, B.E.; Grayson, S.; Epstein, E.H., Jr. Stratum Corneum Lipids in Disorders of Cornification. JCI 1984, 74, 1414–1421. [Google Scholar] [CrossRef]
- Law, S.; Wertz, P.; Swartzendruber, D.; Squier, C. Regional variation in content, composition and organization of porcine epithelial barrier lipids revealed by thin-layer chromatography and transmission electron microscopy. Archs. Oral Biol. 1995, 40, 1085–1091. [Google Scholar] [CrossRef]
- Tamasawa, N.; Tamasawa, A.; Takebe, K. Higher levels of plasma cholesterol sulfate in patients with liver cirrhosis and hypercholesterolemia. Lipids 1993, 28, 833–836. [Google Scholar] [CrossRef]
- Eberlin, L.S.; Dill, A.L.; Costa, A.B.; Ifa, D.R.; Cheng, L.; Masterson, T.; Koch, M.; Ratliff, T.L.; Cooks, R.G. Cholesterol Sulfate Imaging in Human Prostate Cancer Tissue by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 3430–3434. [Google Scholar] [CrossRef]
- Elias, P.M.; Williams, M.L.; Choi, E.H.; Feingold, K.R. Role of cholesterol sulfate in epidermal structure and function: Lessons from X-linked ichthyosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, P.; Vitale, R.; Cho, Y.S.; Totaro, P.; Corcelli, A.; Lobasso, S. Alteration of Cholesterol Sulfate/Seminolipid Ratio in Semen Lipid Profile of Men With Oligoasthenozoospermia. Front. Physiol. 2019, 10, 1344. [Google Scholar] [CrossRef]
- Xu, D.; Ma, R.; Ju, Y.; Song, X.; Niu, B.; Hong, W.; Wang, R.; Yang, Q.; Zhao, Z.; Zhang, Y.; et al. Cholesterol sulfate alleviates ulcerative colitis by promoting cholesterol biosynthesis in colonic epithelial cells. Nat. Commun. 2022, 13, 4428. [Google Scholar] [CrossRef]
- Weerachatyanukul, W.; Probodh, I.; Kongmanas, K.; Tanphaichitr, N.; Johnston, L.J. Visualizing the localization of sulfoglycolipids in lipid raft domains in model membranes and sperm membrane extracts. Biochim. Biophys. Acta Biomembr. 2007, 1768, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Beck-Garcia, K.; Zorzin, C.; Schamel, W.W.; Davis, M.M. Inhibition of T cell receptor signalling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 2016, 17, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Merten, M.; Dong, J.F.; Lopez, J.A.; Thiagarajan, P. Cholesterol sulfate: A new adhesive molecule for platelets. Circulation 2001, 103, 2032–2034. [Google Scholar] [CrossRef]
- Mi-ichi, F.; Tsugawa, H.; Arita, M.; Yoshida, H. Pleiotropic Roles of Cholesteryl Sulfate during Entamoeba Encystation: Involvement in Cell Rounding and Development of Membrane Impermeability. mSphere 2022, 7, e00299-22. [Google Scholar] [CrossRef]
- Sjövall, P.; Skedung, L.; Gregoire, S.; Biganska, O.; Clément, F.; Luengo, G.S. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci. Rep. 2018, 8, 16683. [Google Scholar] [CrossRef]
- Morino, K.; Kunimura, K.; Sugiura, Y.; Izumi, Y.; Matsubara, K.; Akiyoshi, S.; Maeda, R.; Hirotani, K.; Sakata, D.; Mizuno, S.; et al. Cholesterol sulfate limits neutrophil recruitment and gut inflammation during mucosal injury. Front. Immunol. 2023, 14, 1131146. [Google Scholar] [CrossRef]
- Smondyrev, A.M.; Berkowitz, M.L. Molecular dynamics simulation of dipalmitoylphosphatidylcholine membrane with cholesterol sulfate. Biophys. J. 2000, 78, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Fandrei, F.; Engberg, O.; Opálka, L.; Jančálková, P.; Pullmannová, P.; Steinhart, M.; Kováčik, A.; Vávrová, K.; Huster, D. Cholesterol sulfate fluidizes the sterol fraction of the stratum corneum lipid phase and increases its permeability. J. Lipid Res. 2022, 63, 100177. [Google Scholar] [CrossRef]
- Przybylska, M.; Faber, M.; Zaborowski, A.; Świȩtosl, J.; Bryszewska, M. Morphological changes of human erythrocytes induced by cholesterol sulphate. Clin. Biochem. 1998, 31, 73–79. [Google Scholar] [CrossRef]
- Schofield, M.; Jenski, L.J.; Dumaual, A.C.; Stillwell, W. Cholesterol versus cholesterol sulfate: Effects on properties of phospholipid bilayers containing docosahexaenoic acid. Chem. Phys. Lipids 1998, 95, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Tranchant, J.F.; Dufourc, E.J. Comparative effects of cholesterol and cholesterol sulfate on hydration and ordering of dimyristoylphosphatidylcholine membranes. Biophys. J. 1996, 70, 1380–1390. [Google Scholar] [CrossRef]
- Van Meer, G.; De Kroon, A.I.P.M. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef]
- Sarmento, M.J.; Llorente, A.; Petan, T.; Khnykin, D.; Popa, I.; Perkovic, M.N.; Konjevod, M.; Jaganjac, M. The expanding organelle lipidomes: Current knowledge and challenges. Cell. Mol. Life Sci. 2023, 80, 237. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta Biomembr. 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Tiziana, P.; Gratton, E.; Yu, W.M.; Wilson, P.; Levi, M. Two-photon fluorescence microscopy of Laurdan generalized polarization domains in model and natural membranes. Biophys. J. 1997, 72, 2413–2429. [Google Scholar]
- Michel, N.; Fabiano, A.S.; Polidori, A.; Jack, R.; Pucci, B. Determination of phase transition temperatures of lipids by light scattering. Chem. Phys. Lipids 2006, 139, 11–19. [Google Scholar] [CrossRef]
- De Almeida, R.F.M.; Fedorov, A.; Prieto, M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M.; Alonso, A.; Bagatolli, L.A.; Brown, R.E.; Marsh, D.; Prieto, M.; Thewalt, J.L. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim. Biophys. Acta 2008, 1781, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Keller, S.L. Seeing spots: Complex phase behavior in simple membranes. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 172–185. [Google Scholar] [CrossRef]
- Heberle, F.A.; Feigenson, G.W. Phase separation in lipid membranes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004630. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.J.; Lingwood, D.; Levental, I.; Sampaio, J.L.; Kalvodova, L.; Rajendran, L.; Simons, K. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA 2009, 106, 16645–16650. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Fadeel, B.; Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 264–277. [Google Scholar] [CrossRef]
- Pabst, G.; Keller, S. Exploring membrane asymmetry and its effects on membrane proteins. Trends Biochem. Sci. 2024, 49, 333–345. [Google Scholar] [CrossRef]
- Le Grimellec, C.; Friedlander, G.; El Yandouzi, E.H.; Zlatkine, P.; Giocondi, M.C. Membrane fluidity and transport properties in epithelia. Kidney Int. 1992, 42, 825–836. [Google Scholar] [CrossRef]
- Sot, J.; Ibarguren, M.; Busto, J.V.; Montes, L.R.; Goñi, F.M.; Alonso, A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008, 582, 3230–3236. [Google Scholar] [CrossRef]
- Lopes, S.; Neves, C.S.; Eaton, P.; Gameiro, P. Cardiolipin, a key component to mimic the E. coli bacterial membrane in model systems revealed by dynamic light scattering and steady-state fluorescence anisotropy. Anal. Bioanal. Chem. 2010, 398, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Teixeira, J.P.F.; Silva, A.M.G.; Ferreira, M.; Gameiro, P.; de Freitas, V. Modelling Hyperglycaemia in an Epithelial Membrane Model: Biophysical Characterisation. Biomolecules 2022, 12, 1534–1548. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Żyłka, R.; Jurkiewicz, P.; Pruchnik, H.; Oszmiański, J.; Hof, M.; Kleszczyńska, H. Interaction of procyanidin B3 with membrane lipids–Fluorescence, DSC and FTIR studies. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- Chen, W.; Duša, F.; Witos, J.; Ruokonen, S.K.; Wiedmer, S.K. Determination of the Main Phase Transition Temperature of Phospholipids by Nanoplasmonic Sensing. Sci. Rep. 2018, 8, 14815. [Google Scholar] [CrossRef]
- Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta Biomembr. 2009, 1788, 97–121. [Google Scholar] [CrossRef]
- do Canto, A.M.T.M.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2647–2661. [Google Scholar] [CrossRef]
- Jurkiewicz, P.; Cwiklik, L.; Jungwirth, P.; Hof, M. Lipid hydration and mobility: An interplay between fluorescence solvent relaxation experiments and molecular dynamics simulations. Biochimie 2012, 94, 26–32. [Google Scholar] [CrossRef]
- Pokorna, S.; Ventura, A.E.; Santos, T.C.B.; Hof, M.; Prieto, M.; Futerman, A.H.; Silva, L.C. Laurdan in live cell imaging: Effect of acquisition settings, cell culture conditions and data analysis on generalized polarization measurements. J. Photochem. Photobiol. B Biol. 2022, 228, 112404. [Google Scholar] [CrossRef]
- Marquardt, D.; Heberle, F.A.; Greathouse, D.V.; Koeppe, R.E.; Standaert, R.F.; Van Oosten, B.J.; Harroun, T.A.; Kinnun, J.J.; Williams, J.A.; Wassall, S.R.; et al. Lipid bilayer thickness determines cholesterols location in model membranes. Soft Matter 2016, 12, 9417–9428. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Poole, K.; Goyette, J.; Gaus, K. Introducing membrane charge and membrane potential to T cell signaling. Front. Immunol. 2017, 8, 1513. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Vanlaere, I.; Van Hauwermeiren, F.; Van Wonterghem, E.; Wilson, C.; Libert, C. Pro-inflammatory effects of matrix metalloproteinase 7 in acute inflammation. Mucosal Immunol. 2014, 7, 579–588. [Google Scholar] [CrossRef]
- Prior, S.H.; Fulcher, Y.G.; Koppisetti, R.K.; Jurkevich, A.; Van Doren, S.R. Charge-Triggered Membrane Insertion of Matrix Metalloproteinase-7, Supporter of Innate Immunity and Tumors. Structure 2015, 23, 2099–2110. [Google Scholar] [CrossRef]
- Wertz, P.W.; Cox, P.S.; Squier, C.A.; Downing, D.T. Lipids of epidermis and keratinized and non-keratinized oral epithelia. Comp. Biochem. Physiol. Part B Biochem. 1986, 83, 529–531. [Google Scholar] [CrossRef]
- Brasitus, T.A.; Schachter, D. Lipid composition and fluidity of rat enterocyte basolateral membranes regional differences. Biochim. Biophys. Acta Biomembr. 1984, 774, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zehethofer, N.; Bermbach, S.; Hagner, S.; Garn, H.; Müller, J.; Goldmann, T.; Lindner, B.; Schwudke, D.; König, P. Lipid Analysis of Airway Epithelial Cells for Studying Respiratory Diseases. Chromatographia 2014, 78, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Gerl, M.J.; Sampaio, J.L.; Urban, S.; Kalvodova, L.; Verbavatz, J.M.; Binnington, B.; Lindemann, D.; Lingwood, C.A.; Shevchenko, A.; Schroeder, C.; et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell Biol. 2012, 196, 213–221. [Google Scholar] [CrossRef]
- Buwaneka, P.; Ralko, A.; Liu, S.L.; Cho, W. Evaluation of the available cholesterol concentration in the inner leaflet of the plasma membrane of mammalian cells. J. Lipid Res. 2021, 62, 100084. [Google Scholar] [CrossRef]
- Ingólfsson, H.I.; Melo, M.N.; Van Eerden, F.J.; Arnarez, C.; Lopez, C.A.; Wassenaar, T.A.; Periole, X.; De Vries, A.H.; Tieleman, D.P.; Marrink, S.J. Lipid organization of the plasma membrane. J. Am. Chem. Soc. 2014, 136, 14554–14559. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef]
- Koponen, K.K.; Salosensaari, A.; Ruuskanen, M.O.; Havulinna, A.S.; Männistö, S.; Jousilahti, P.; Palmu, J.; Salido, R.; Sanders, K.; Brennan, C.; et al. Associations of healthy food choices with gut microbiota profiles. Am. J. Clin. Nutr. 2021, 114, 605–616. [Google Scholar] [CrossRef] [PubMed]

| Models | Lipid Composition (mol%) | GP Value | Anisotropy (<r>) | Zeta-Potential (mV) |
|---|---|---|---|---|
| Cholesterol-poor | PLPC/Chol (95:5) | −0.296 ± 0.007 | 0.19 ± 0.02 | −13.2 ± 1.1 |
| Cholesterol-rich | PLPC/Chol/SM/DMPE (30:25:30:15) | 0.195 ± 0.013 | 0.282 ± 0.003 | −16.9 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, A.; Sarmento, M.J.; Ferreira, M.; Gameiro, P.; de Freitas, V. Cholesterol Sulfate in Biological Membranes: A Biophysical Study in Cholesterol-Poor and Cholesterol-Rich Biomimetic Models. Membranes 2025, 15, 159. https://doi.org/10.3390/membranes15060159
Reis A, Sarmento MJ, Ferreira M, Gameiro P, de Freitas V. Cholesterol Sulfate in Biological Membranes: A Biophysical Study in Cholesterol-Poor and Cholesterol-Rich Biomimetic Models. Membranes. 2025; 15(6):159. https://doi.org/10.3390/membranes15060159
Chicago/Turabian StyleReis, Ana, Maria João Sarmento, Mariana Ferreira, Paula Gameiro, and Victor de Freitas. 2025. "Cholesterol Sulfate in Biological Membranes: A Biophysical Study in Cholesterol-Poor and Cholesterol-Rich Biomimetic Models" Membranes 15, no. 6: 159. https://doi.org/10.3390/membranes15060159
APA StyleReis, A., Sarmento, M. J., Ferreira, M., Gameiro, P., & de Freitas, V. (2025). Cholesterol Sulfate in Biological Membranes: A Biophysical Study in Cholesterol-Poor and Cholesterol-Rich Biomimetic Models. Membranes, 15(6), 159. https://doi.org/10.3390/membranes15060159









