The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Obtaining Graphene Oxide Nanoparticles
2.3. Obtaining Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles
2.4. Obtaining Cellulose Acetate Membranes with Nanoparticles
2.5. Sample Characterization
2.6. Study of Membrane Filtration Performance
2.6.1. Establishing the Permeate Flow Rate
2.6.2. Establishing the Filtration Efficiency
3. Results
3.1. Scanning Electron Microscopy for Nanomaterials
3.2. Thermogravimetric Analysis for Nanomaterials
3.3. FTIR Analysis for Membranes
3.4. DSC Analysis for Membranes
3.5. TGA Analysis for Membranes
3.6. SEM Analysis for Membranes
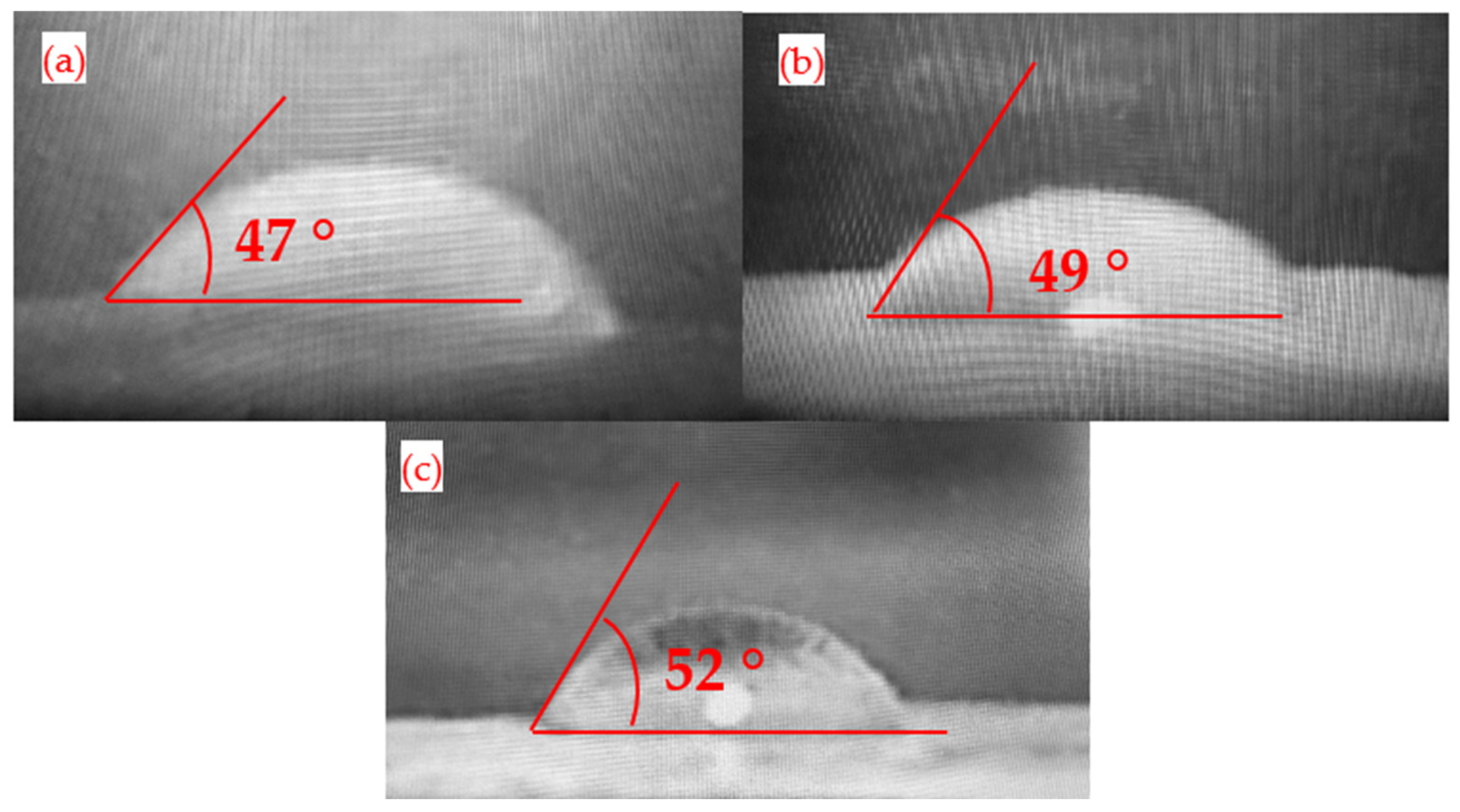
3.7. Goniometric Analysis for Membranes
3.8. Permeability Test for Membranes
3.9. Membrane Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; Mao, D.; Song, K.; Xiang, H.; Li, S.; Wang, Z. Effects of Landscape Changes on Water Quality: A Global Meta-Analysis. Water Res. 2024, 260, 121946. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.O.; Pow-Seng, Y.; Kingsley, O.I.; Chukwunonso, O.A.; Tianqi, L.; Kanika, D.; Felicitas, U.I.; Selvasembian, R. Adsorption of Persistent Organic Pollutants (POPs) from the Aqueous Environment by Nano-Adsorbents: A Review. Environ. Res. 2022, 212, 113123. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.M.; Zulhairun, A.K.; Ismail, A.F. Antifouling Property of Oppositely Charged Titania Nanosheet Assembled on Thin Film Composite Reverse Osmosis Membrane for Highly Concentrated Oily Saline Water Treatment. Membranes 2020, 10, 237. [Google Scholar] [CrossRef]
- Husaini, I.S.; Haddabi, M.H. Recent advances in functionalized electrospun nanofiber membranes for enhanced oily water treatment. J. Environ. Chem. Eng. 2025, 13, 115391. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, X.; Miao, D.; Song, J.; Fu, S.; Zhang, J. Faveolate cell-engineered and multi-heterostructured flexible ceramic nanofibrous membranes with confined coalescent nanochannels enable sustainable oily water remediation. Chem. Eng. J. 2024, 494, 152966. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, C.; Mao, Y. Silica-Nanocoated Membranes with Enhanced Stability and Antifouling Performance for Oil-Water Emulsion Separation. Membranes 2025, 15, 41. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, L.; Liu, L.; An, X.; Lan, H.; Liu, H.; Qu, J. Polymeric loose nanofiltration membranes based on water-soluble carbon nitride for efficient organics/salts separation. J. Membr. Sci. 2025, 718, 123687. [Google Scholar] [CrossRef]
- Chen, J.; Ren, J.; Yang, C.; Feng, B.; Dang, X.; Gong, Y. Enhanced antifouling durability of zwitterionic polymer brush grafted ceramic membrane for sustainable oil/water separation applications. Sep. Purif. Technol. 2025, 362, 131963. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Qu, Y.; Jia, H.; Xu, S.; Zhang, M. Fabrication of smart spherical three-dimensional covalent organic framework membranes with switchable oil–water separation via interfacial polymerization. Sep. Purif. Technol. 2025, 360, 131074. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhu, Y.; Wang, H.; Cui, Z.; Li, X.; Mo, J.; Li, J. An ultrathin Al2O3 ceramic membrane prepared by organic-inorganic blending with solvent evaporation and high-temperature sintering for highly efficient oil/water separation. J. Water Process Eng. 2025, 70, 107116. [Google Scholar] [CrossRef]
- Kayanja, O.; Abdel-Aty, A.A.R.; Hassan, M.A.; Hassanin, A.; Ohashi, H.; Khalil, A.S.G. A review on TMDCs nanomaterials and their surface engineered polymeric membrane nanocomposites for water remediation and wastewater treatment. Surf. Interfaces 2023, 43, 103578. [Google Scholar] [CrossRef]
- Ma, G.; Xu, X.; Tesfai, M.; Wang, H.; Xu, P. Developing anti-biofouling and energy-efficient cation-exchange membranes using conductive polymers and nanomaterials. J. Membr. Sci. 2020, 603, 118034. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.; Chen, W.; Wang, X.; Ji, H.; Fu, Y.; Lü, C. Synthesis, antibacterial activity and action mechanism of silver-based nanomaterials with thermosensitive polymer-decorated graphene oxide as a stable support. Mater. Today Commun. 2023, 36, 106598. [Google Scholar] [CrossRef]
- Băjan, M.; Cursaru, D.L. Study regarding the compatibility between cellulose acetate (CA) and ZnO nanoparticles in polymeric membrane structures using functionalized mphenylenediamine. Dig. J. Nanomater. Biostructures 2025, 20, 103–112. [Google Scholar] [CrossRef]
- Li, B.; Ansari, A.A.; Parchur, A.K.; Lv, R. Exploring the influence of polymeric and non-polymeric materials in synthesis and functionalization of luminescent lanthanide nanomaterials. Coord. Chem. Rev. 2024, 514, 215922. [Google Scholar] [CrossRef]
- Kima, N.; Lib, X.; Kimb, S.H.; Kima, J. Colloidally stable organic–inorganic hybrid nanoparticles prepared using alkoxysilane-functionalized amphiphilic polymer precursors and mechanical properties of their cured coating film. J. Ind. Eng. Chem. 2018, 68, 209–219. [Google Scholar] [CrossRef]
- Hao, M.; Tang, M.; Wang, W.; Tian, M.; Zhang, L.; Lu, Y. Silver-nanoparticle-decorated multiwalled carbon nanotubes prepared by poly(dopamine) functionalization and ultraviolet irradiation. Compos. Part B 2016, 95, 395–403. [Google Scholar] [CrossRef]
- Schiavi, S.; Taglietti, A.; Magni, A.; Galinetto, P.; Albini, B. Increasing SERS performance of silver nanoparticles with nanometric coating of polydopamine: A novel approach for methylene blue detection. Appl. Surf. Sci. 2025, 687, 162223. [Google Scholar] [CrossRef]
- Huisman, K.T.; Abdellah, M.H.; Sosa, D.S.A.; Simoes, F.R.F.; Blankert, B.; Vrouwenvelder, J.S.; Szekely, G. Improved cleaning performance of membrane modules using feed spacers modified with cold-plasma treatment and polydopamine and silver-nanoparticle coatings. Desalination 2024, 582, 117604. [Google Scholar] [CrossRef]
- Sahoo, B.; Panigrahi, L.L.; Jha, S.; Arakha, M. Polyethylene glycol functionalized zinc oxide nanoparticle with excellent photocatalytic performance and oxidative stress mediated bacterial cell death. Opt. Mater. 2024, 148, 11891. [Google Scholar] [CrossRef]
- Akbaria, A.; Yegania, R.; Pourabbasb, B.; Behboudia, A. Analysis of antifouling behavior of high dispersible hydrophilic poly (ethylene glycol)/vinyl functionalized SiO2 nanoparticles embedded polyethylene membrane. Desalination Water Treat. 2017, 76, 83–97. [Google Scholar] [CrossRef]
- Choopani, L.; Mohammadi, A.; Aliabadi, H.A.M.; Kashtiaray, A.; Keihan, R.E.; Maleki, A.; Mahdavi, M. Functionalization of zinc ferrites nanoparticles by cyclic aromatic polyimide chains as a novel star polymer with antibacterial activity and low toxicity. J. Ind. Eng. Chem. 2024, 137, 243–251. [Google Scholar] [CrossRef]
- Wajahat, M.; Lee, S.; Kim, J.H.; Ahn, J.; Sim, H.H.; Kim, J.H.; Bae, J.; Seong Hyeon Kim, S.H.; Pyo, J.; Seol, S.K. Three-dimensional printing of silver nanoparticle-decorated graphene microarchitectures. Addit. Manuf. 2022, 60, 103249. [Google Scholar] [CrossRef]
- Xin, F.; Li, L. Decoration of carbon nanotubes with silver nanoparticles for advanced CNT/polymer nanocomposites. Compos. Part A 2011, 42, 961–967. [Google Scholar] [CrossRef]
- Ranjbaran, N.; Akbari, A.; Yegani, R.; Mamaqani, H.R.; Chapalaghi, M. Graphene oxide decorated copper nanoparticles embedded polysulfone nanocomposite membrane: Anti-bacterial, organo-bio fouling evaluation in pharmaceutical wastewater treatment via MBR. J. Ind. Eng. Chem. 2025, 142, 293–308. [Google Scholar] [CrossRef]
- Rahman, N.; Bharti, M.; Nasir, M.; Azmi, S.N.H. Performance assessment of graphene oxide decorated with silver nanoparticles as adsorbent for removal of metformin from water: Equilibrium modeling, kinetic and thermodynamic studies. Next Mater. 2024, 3, 100046. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N.; Babatunde, B.N.; Tetteh, E.K.; Rathilal, S. Versatile Silver-Nanoparticle-Impregnated Membranes for Water Treatment: A Review. Membranes 2023, 13, 432. [Google Scholar] [CrossRef]
- Jung, R.; Kim, Y.; Kim, H.S.; Jin, H.J. Antimicrobial Properties of Hydrated Cellulose Membranes With Silver Nanoparticles. J. Biomater. Sci. 2009, 20, 311–324. [Google Scholar] [CrossRef]
- Lkhagvajav, N.; Koizhaiganova, M.; Yasa, I.; Çelik, E.; Sari, Ö. Characterization and antimicrobial performance of nano silver coatings on leather materials. Braz. J. Microbiol. 2015, 46, 41–48. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Yu, L.; Koh, K.Y.; Chen, J.P. Modification of polyvinylidene fluoride membrane by silver nanoparticles-graphene oxide hybrid nanosheet for effective membrane biofouling mitigation. Chemosphere 2021, 268, 129187. [Google Scholar] [CrossRef]
- Liew, K.B.; Leong, J.X.; Daud, W.R.W.; Ahmad, A.; Hwang, J.J. Incorporation of silver graphene oxide and graphene oxide nanoparticles in sulfonated polyether ether ketone membrane for power generation in microbial fuel cell. J. Power Sources 2020, 449, 227490. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ba-Abbad, M.M.; Mohammad, A.W. Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chem. Eng. J. 2015, 227, 1–10. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Ruman, U.E.; Shafiq, M.; Waqas, S.; Sabir, A. Intercalation of GO-Ag nanoparticles in cellulose acetate nanofiltration mixed matrix membrane for efficient removal of chromium and cobalt ions from wastewater. J. Environ. Chem. Eng. 2024, 12, 113713. [Google Scholar] [CrossRef]
- Sun, X.F.; Qin, J.; Xia, P.F.; Guo, B.B.; Yang, C.M.; Song, C.; Wang, S.G. Graphene oxide–silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, C.M.; Khirul, M.A.; Ahmad, I.; Jee, H.; Chuah, C.Y.; Park, J.; Chae, K.J.; Yang, E. Silver nanoparticle-decorated reduced graphene oxide/nanocrystalline titanium metal-organic frameworks composite membranes with enhanced nanofiltration performance and photocatalytic ability. Desalination Water Treat. 2024, 320, 100836. [Google Scholar] [CrossRef]
- Hoque, M.A.; Rahman, A.F.M.M.; Rahman, M.M.; Bhuiyan, M.N.I.; Jahan, S.A.; Shaikh, M.A.A.; Nurnabi, M. Effect of Successive Recycling and Reuse of Acid Liquor for the Synthesis of Graphene Oxides with Higher Oxygen-to-Carbon Ratios. Heliyon 2024, 10, e27639. [Google Scholar] [CrossRef]
- Wong-u-ra, O.; Ekgasit, S.; Wongravee, K. Phase transferring of silver nanoparticles to organic solvents using modified graphene oxide as carrier. Mater. Chem. Phys. 2017, 199, 348–355. [Google Scholar] [CrossRef]
- Alsulaiman, L.; Abdel-Naby, A.S.; Alharthi, S.; Alabdullatif, B.; Al-Dossary, A.; Al-Mughrabi, W.; Alqarni, Y. Synthesis and Characterization of Cellulose Acetate—HBA/Poly Sulfone Blend for Water Treatment Applications. Membranes 2025, 15, 38. [Google Scholar] [CrossRef]
- Vanumamalai, S.M.; Venkatachalam, S.; Srinivasan, N.; Dhinakar, G. Optimized hummer’s method for graphene oxide: Structural properties and electrochemical applications. J. Organomet. Chem. 2025, 1031, 123577. [Google Scholar] [CrossRef]
- Azman, N.H.N.; Lim, X.C.; Sulaiman, Y. Tuning the capacitance of graphene-based materials as negative electrode materials for supercapacitor applications. Diam. Relat. Mater. 2025, 152, 111952. [Google Scholar] [CrossRef]
- Cesaris, M.G.; Rodríguez, M.J.T.; Pasan, J.; Gentili, A.; Pino, V. Mixed matrix membranes based on cellulose acetate recycled from cigarette butts and metal-organic frameworks for thin film solid-phase microextraction: Determination of phenols in environmental waters. Adv. Sample Prep. 2025, 13, 100150. [Google Scholar] [CrossRef]
- Moraes, A.C.M.; Andrade, P.F.; Fariaa, A.F.; Simões, M.B.; Salomãoc, F.C.C.S.; Barros, E.B.; Goncalves, M.C.; Alves, O.L. Fabrication of transparent and ultraviolet shielding composite films based on graphene oxide and cellulose acetate. Carbohydr. Polym. 2015, 123, 217–227. [Google Scholar] [CrossRef]
- Ahmed, D.F.; Isawi, H.; Badway, N.A.; Elbayaa, A.A.; Shawky, H. Graphene oxide incorporated cellulose triacetate/cellulose acetate nanocomposite membranes for forward osmosis desalination. Arab. J. Chem. 2021, 14, 102995. [Google Scholar] [CrossRef]
- Chavan, V.D.; Pinjari, D.V.; Waghmare, N.G.; Juikar, V.G.; Sayyed, A.J. A critical review on technological development of cellulosic material as a sustainable alternative for superabsorbent polymers and its recent applications. Chem. Eng. J. 2024, 499, 156487. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Shidlovskiya, I.P.; Nikolaevab, E.D.; Sukovatiyb, A.G.; Vasiliev, A.D.; Shishatskaya, E.I. Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym. Test. 2018, 65, 54–68. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Shimizu, F.M.; Scagion, V.P.; Correa, D.S. Ternary nanocomposites based on cellulose nanowhiskers, silver nanoparticles and electrospun nanofibers: Use in an electronic tongue for heavy metal detection. Sens. Actuators B Chem. 2019, 290, 387–395. [Google Scholar] [CrossRef]
- Olasupo, A.; Mohammad, R.A.E.; Suah, F.B.M. A novel approach in remediation of diclofenac from environmental water samples using silver nanocomposite polymer inclusion membrane. J. Water Process Eng. 2023, 55, 104173. [Google Scholar] [CrossRef]
- Kouda, I.; Seddik, N.B.; Boumlasy, S.E.; Achache, M.; Zarki, Y.; Aghmiz, A.; Tahaikt, M.; Elmidaoui, A.; Draoui, K. Impact of solvent treatment on the adsorption efficiency of crystal violet dye using cellulose acetate-clay composite membranes: Experimental and molecular dynamics approaches. Carbohydr. Polym. 2025, 357, 123494. [Google Scholar] [CrossRef]
- Dugam, S.; Jain, R.; Dandekar, P. Silver nanoparticles loaded triple-layered cellulose-acetate based multifunctional dressing for wound healing. Int. J. Biol. Macromol. 2024, 276, 133837. [Google Scholar] [CrossRef]
- Benhalima, T.; Harrar, H.F.; Doufene, N.; Sadi, A. Silver decorated zeolite embedded in bionanocomposite hydrogels based on cross-linked carboxymethyl cellulose for excellent catalytic hydrogenation of azo dyes. Int. J. Biol. Macromol. 2024, 279, 135556. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmada, I.; Khana, S.B.; Asir, A.M. Synthesis and catalytic properties of silver nanoparticles supported on porous cellulose acetate sheets and wet-spun fibers. Carbohydr. Polym. 2017, 157, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Domke, A.; Przysiecka, L.; Jancelewicz, M.; Jarek, M.; Coy, E.; Iatsunskyi, I.; Richardson, J.J.; Staszak, K.; Wozniak-Budych, M. Improving the bioactivity of cellulose acetate hemodialysis membranes through nanosilver modification. Biomater. Adv. 2025, 169, 214180. [Google Scholar] [CrossRef]
- Ali, A.S.M.; Soliman, M.M.; Kandil, S.H.; Ebrahim, S.; Khalil, M. Tailoring nanocomposite membranes of cellulose acetate/silica nanoparticles for desalination. J. Mater. 2022, 8, 1122–1130. [Google Scholar] [CrossRef]
- Afzal, A.; Rafique, M.S.; Iqbal, S.S.; Butt, S.H.; Kalsoom, U.; Rafique, M. Idiosyncratic cellulose acetate nanocomposite membranes: Synthesis and performance control study for desalination. Environ. Technol. 2021, 42, 1336–1352. [Google Scholar] [CrossRef]
- Penkova, A.; Trchova, M.; Toikka, A.M.; Slouf, M. Structure and Pervaporation Properties of Poly(phenylene-iso-phthalamide) Membranes Modified by Fullerene C60. Macromol. Mater. Eng. 2009, 294, 432–440. [Google Scholar] [CrossRef]
- Elaissaoui, I.; Sayeb, S.; Ounif, I.; Ferhi, M.; Karima, H.; Ennigrou, D.J. Preparation and characterization of acetate cellulose electrospun nanofibers membrane: Potential application on wastewater treatment. Heliyon 2024, 10, e32552. [Google Scholar] [CrossRef]
- Faria, A.F.; Moraes, A.C.M.; Andrade, P.F.; Silva, D.S.; Goncalves, M.C.; Alves, O.L. Cellulose acetate membrane embedded with graphene oxidesilver nanocomposites and its ability to suppress microbial proliferation. Cellulose 2017, 24, 781–796. [Google Scholar] [CrossRef]
- Spricka, C.; Chedea, S.; Craverb, V.O.; Escobar, I.C. Bio-inspired immobilization of casein-coated silver nanoparticles on cellulose acetate membranes for biofouling control. J. Environ. Chem. Eng. 2018, 6, 2480–2491. [Google Scholar] [CrossRef]
- Bothun, G.D. Hydrophobic silver nanoparticles trapped in lipid bilayers: Size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnol. 2008, 6, 13. [Google Scholar] [CrossRef]
- Bakirdere, S.; Yilmaz, M.T.; Tornuk, F.; Keyf, S.; Yilmaz, A.; Sagdic, O.; Kocabas, B. Molecular characterization of silver–stearate nanoparticles (AgStNPs): A hydrophobic and antimicrobial material against foodborne pathogens. Food Res. Int. 2015, 76, 439–448. [Google Scholar] [CrossRef]
- Khalili, P.; Abdolmaleki, A.; Riazi, M.; Davarpanah, A. Enhanced desulfurization Pervaporate via tailored Polypyrrolidone membranes with functionalized graphene oxide nanoparticles and silver ions. Sep. Purif. Technol. 2025, 364, 132562. [Google Scholar] [CrossRef]
- Valenzuela, P.G.T.; Sánchez, A.; Isiordia, G.E.D.; Ontiveros, M.M.A.; Macias, M.R.M.; Sicairos, S.P.; Duarte, R.G.S.; Weihs, G.A.F. Modifcation and characterization of TFC membranes with Ag nanoparticles: Application in seawater desalination. Polym. Bull. 2023, 80, 6285–6306. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, J.; Dong, J.; Wang, M.; Liu, S.; Tang, H.; Hong, R.; He, Q.; Wada, M.; Teng, B.; et al. Highly flexible free-standing bacterial cellulose-based filter membrane with tunable wettability for high-performance water purification. Int. J. Biol. Macromol. 2025, 293, 139419. [Google Scholar] [CrossRef]
- Yuksel, M.S.; Tas, B.; Imer, D.Y.K.; Koyuncu, I. Effect of silver nanoparticle (AgNP) location in nanocomposite membrane matrix fabricated with different polymer type on antibacterial mechanism. Desalination 2014, 347, 120–130. [Google Scholar] [CrossRef]
- Wang, R.; Li, N.; Liu, H.; Li, R.; Zhang, L.; Liu, S.; Peng, Q.; Ren, L.; Liu, J.; Li, B.; et al. Construction of cellulose acetate-based composite nanofiber films with effective antibacterial and filtration properties. Int. J. Biol. Macromol. 2024, 254, 128102. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef]
- Jose, J.K.; Mishra, B.; Kootery, K.P.; Cherian, C.T.; Tripathi, B.T.; Sarojini, S.; Balachand, M. Fabrication of silver nanoparticle decorated graphene oxide membranes for water purification, antifouling and antibacterial applications. Mater. Sci. Eng. B 2023, 297, 116789. [Google Scholar] [CrossRef]
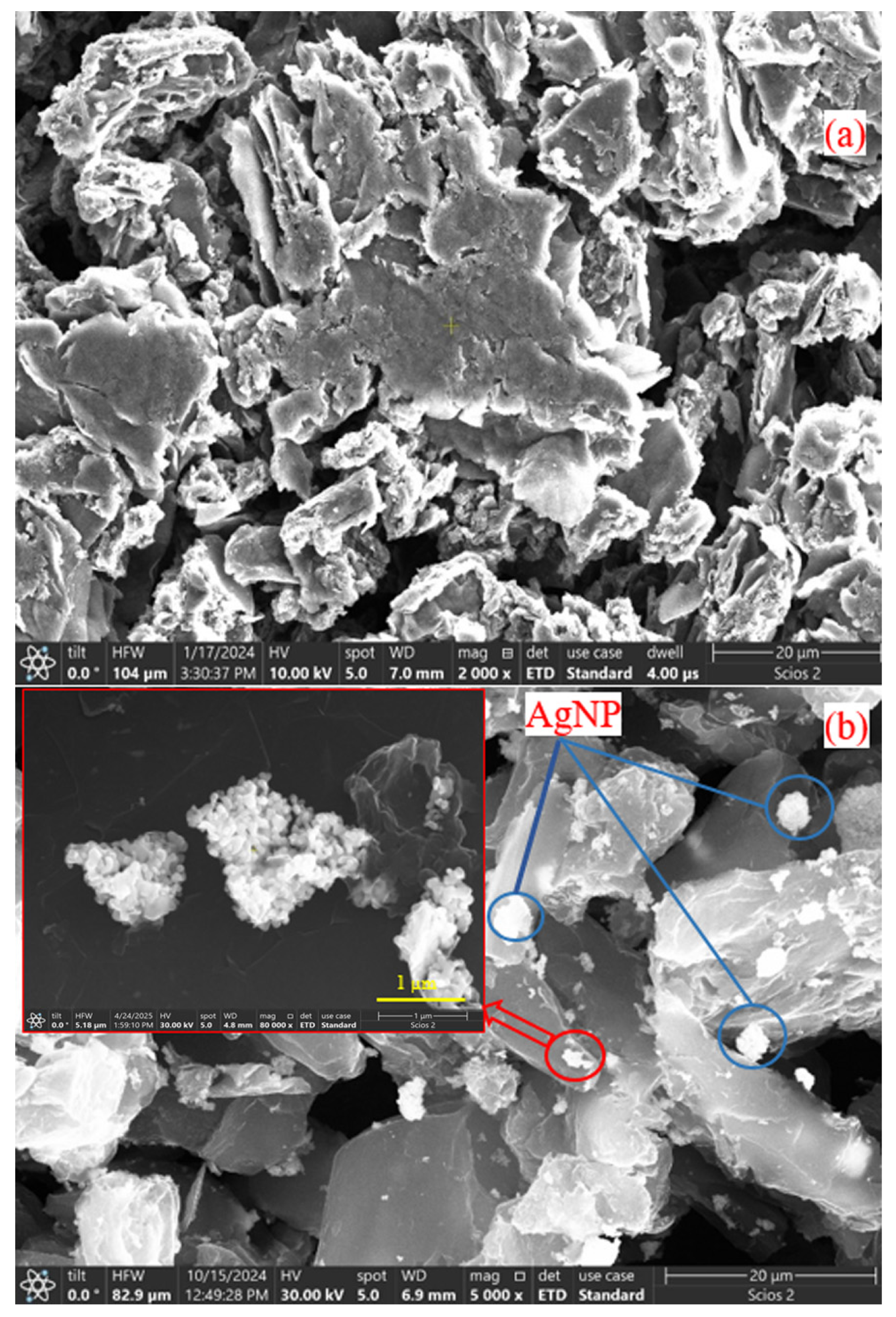
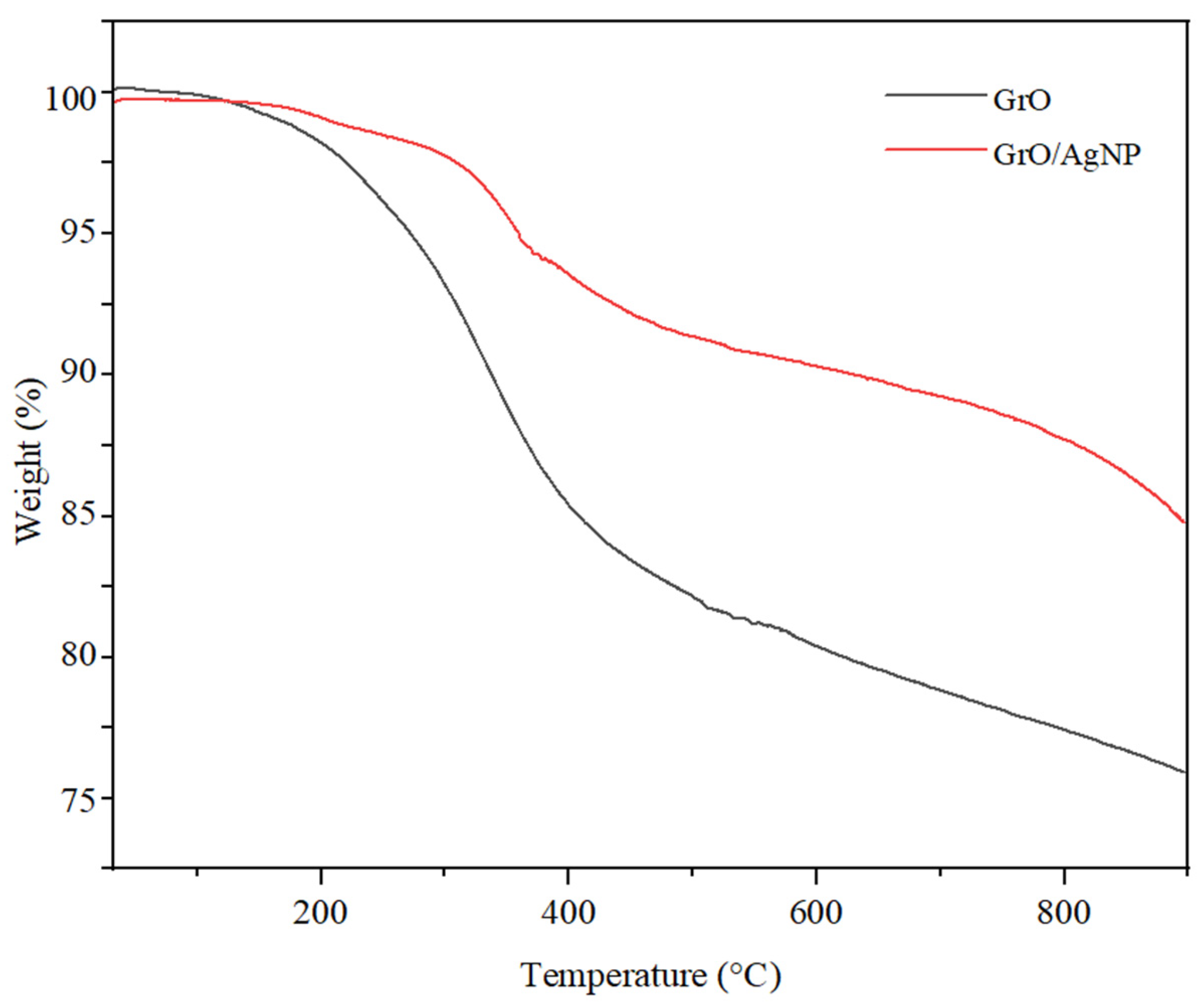

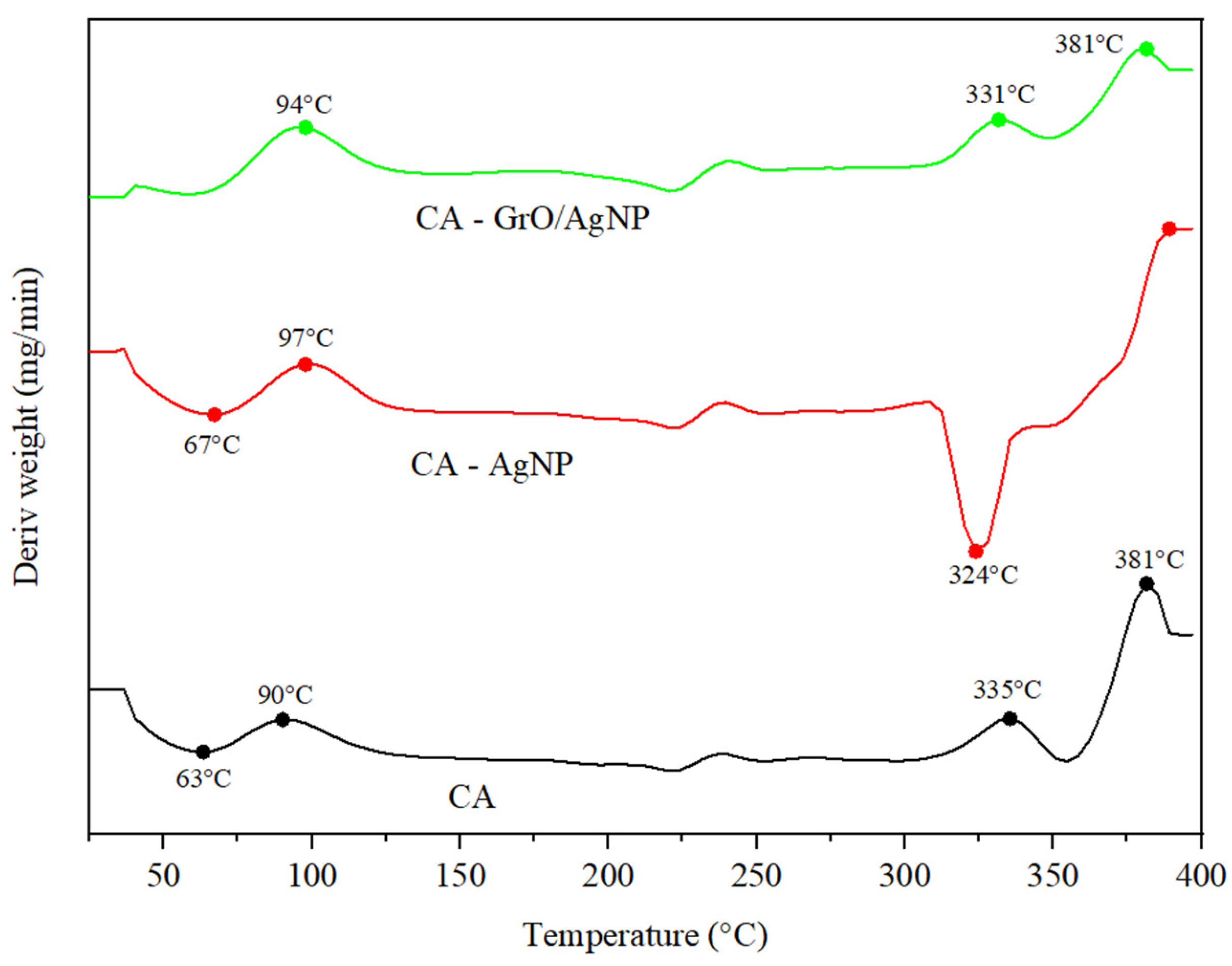
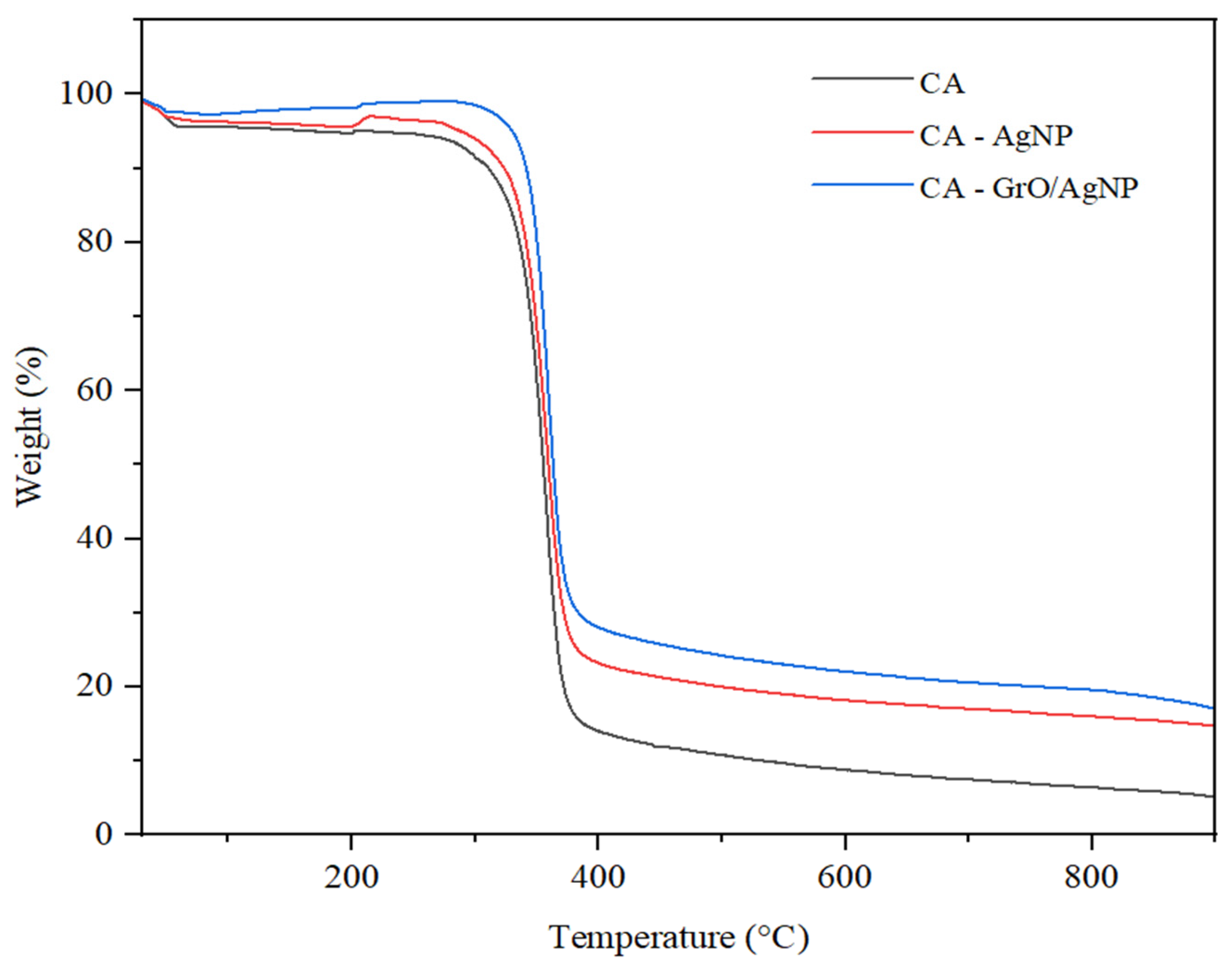



| Membrane Type | CA (g) | AgNP (g) | GrO/AgNP (g) | DMF (mL) |
|---|---|---|---|---|
| CA | 0.75 | - | - | 5 |
| CA, AgNP | 0.75 | 0.1 | - | 5 |
| CA, GrO/AgNP | 0.75 | - | 0.1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băjan, M.; Cursaru, D.L.; Mihai, S. The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water. Membranes 2025, 15, 158. https://doi.org/10.3390/membranes15060158
Băjan M, Cursaru DL, Mihai S. The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water. Membranes. 2025; 15(6):158. https://doi.org/10.3390/membranes15060158
Chicago/Turabian StyleBăjan, Marian, Diana Luciana Cursaru, and Sonia Mihai. 2025. "The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water" Membranes 15, no. 6: 158. https://doi.org/10.3390/membranes15060158
APA StyleBăjan, M., Cursaru, D. L., & Mihai, S. (2025). The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water. Membranes, 15(6), 158. https://doi.org/10.3390/membranes15060158







