Preparation of Tannic Acid-Pectin Coated PVDF Membrane for High-Efficiency Separation of Oil and Water Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
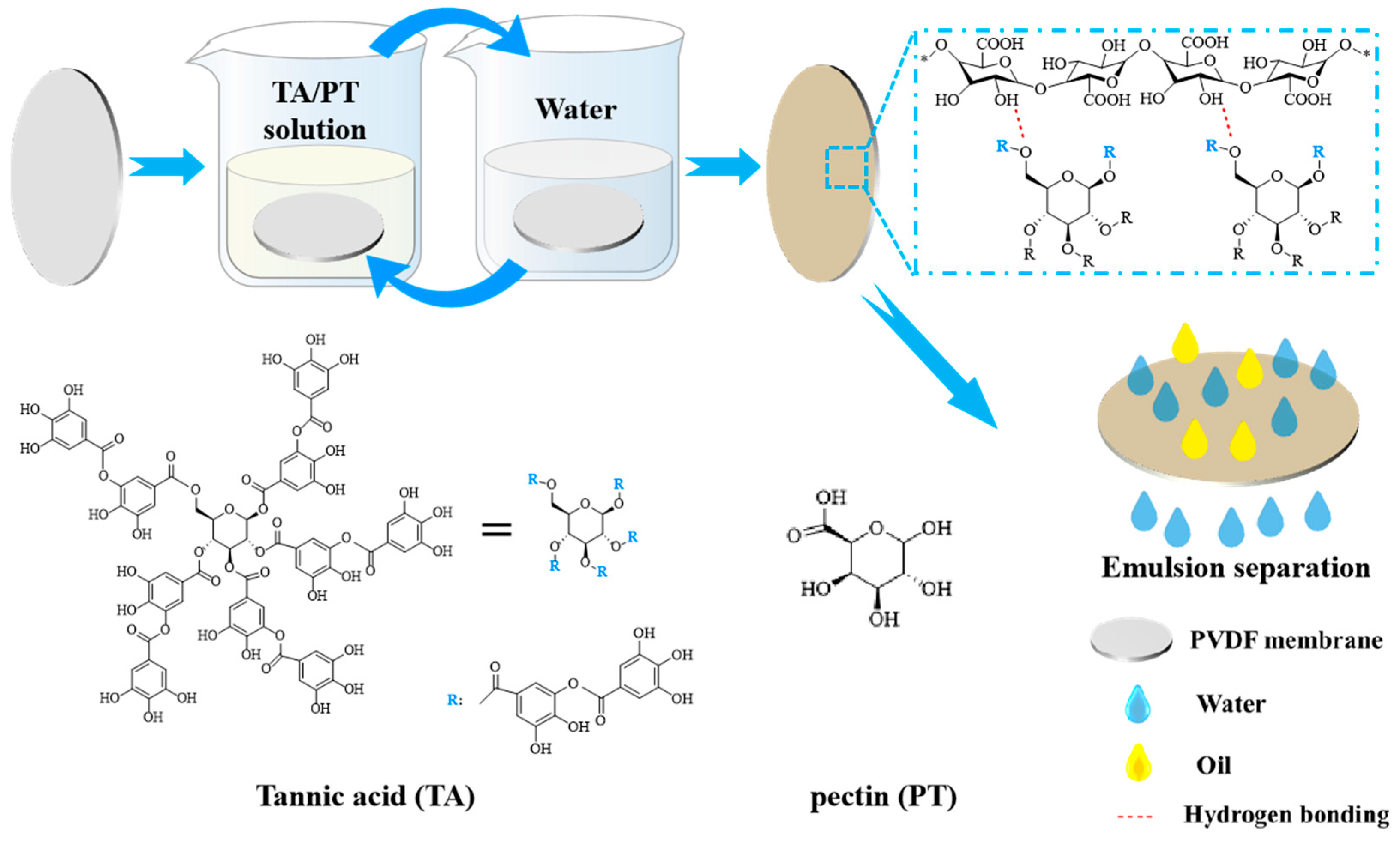
2.2. Synthesis of PVDF@TA/PT Membrane
2.3. Characterizations
2.4. Emulsion Separation Performance
2.5. Stability of Membrane
3. Results and Discussions
3.1. Characterization of Membranes
3.2. Surface Wettability
3.3. Separation Performance of Membrane
3.4. Stability of Membranes
3.5. Separation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TA | Tannic Acid |
| PT | Pectin |
References
- Deng, Y.; Dai, M.; Wu, Y.; Peng, C. Emulsion system, demulsification and membrane technology in oil–water emulsion separation: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1254–1278. [Google Scholar] [CrossRef]
- Liang, H.; Xie, A.; Nie, S.; Rui, J.; Li, C.; Xue, C.; Cui, J.; Pan, J. Low-pressure driving Co3O4/PAN nanofiber membrane with peroxymonosulfate activation self-cleaning for efficient wastewater purification. J. Membr. Sci. 2024, 693, 122380. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.R.; Ismadji, S.; Wentenet, I.G. Membrane fouling and fouling mitigation in oil–water separation: A review. Environ. Chem. Eng. 2022, 10, 107532. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Zhang, M.; Gao, S.; Cui, J.; Huang, C.; Fu, G. Biomimetic durable multifunctional self-cleaning nanofibrous membrane with outstanding oil/water separation, photodegradation of organic contaminants, and antibacterial performances. ACS Appl. Mater. Interfaces 2020, 12, 34999–35010. [Google Scholar] [CrossRef]
- Xie, A.; Wu, Y.; Liu, Y.; Xue, C.; Ding, G.; Cheng, G.; Cui, J.; Pan, J. Robust antifouling NH2-MIL-88B coated quartz fibrous membrane for efficient gravity-driven oil-water emulsion separation. J. Membr. Sci. 2022, 644, 120093. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membranebased separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qi, B.; Guo, Z.; Wang, D.; Jiao, T. Recent developments in the application of membrane separation technology and its challenges in oil-water separation: A review. Chemosphere 2023, 327, 138528. [Google Scholar] [CrossRef]
- Cui, J.; Xie, A.; Yan, Z.; Yan, Y. Fabrication of crosslinking modified PVDF/GO membrane with acid, alkali and salt resistance for efficient oil-water emulsion separation. Sep. Purif. Technol. 2021, 265, 118528. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.A.; Ismail, F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Olabintan, A.B.; Ahmed, E.; Abdulgader, H.A.; Saleh, T.A. Hydrophobic and oleophilic amine-functionalised graphene/polyethylene nanocomposite for oil–water separation. Environ. Technol. Innov. 2022, 27, 102391. [Google Scholar] [CrossRef]
- Meng, J.; Teng, J.; Li, F.; Li, T.; Greco, R.; Cao, W. Sm-MOF decorated cotton for efficient on-demand oil-water separation and organic pollutants removal. Sep. Purif. Technol. 2025, 358, 130248. [Google Scholar] [CrossRef]
- Xiong, Z.; He, Z.; Mahmud, S.; Yang, Y.; Zhou, L.; Hu, C.; Zhao, S. Simple amphoteric charge strategy to reinforce superhydrophilic polyvinylidene fluoride membrane for highly efficient separation of various surfactant-stabilized oil-in-water emulsions. ACS Appl. Mater. Interfaces 2020, 12, 47018–47028. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; Wang, N. Fabrication of modified PVDF membranes with PAA network polymer for highly efficient oil/water emulsion separation. Sep. Purif. Technol. 2025, 357, 130138. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Zuo, J.; Cheng, P.; Chen, X.; Yan, X.; Guo, Y.; Lang, W. Ultrahigh flux of polydopamine-coated PVDF membranes quenched in air via thermally induced phase separation for oil/water emulsion separation. Sep. Purif. Technol. 2018, 192, 348–359. [Google Scholar] [CrossRef]
- Zin, G.; Wu, J.; Rezzadori, K.; Petrus, J.; Luccio, M.; Li, Q. Modification of hydrophobic commercial PVDF microfiltration membranes into superhydrophilic membranes by the mussel-inspired method with dopamine and polyethyleneimine. Sep. Purif. Technol. 2019, 212, 641–649. [Google Scholar] [CrossRef]
- Cheng, X.; Li, T.; Yan, L.; Jiao, Y.; Zhang, Y.; Wang, K.; Cheng, Z.; Ma, J.; Shao, L. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil-water separation. Sci. Adv. 2023, 9, eadh8195. [Google Scholar] [CrossRef]
- Zeng, S.; Gao, X.; Chen, H.; Wang, Q.; Si, J.; Cui, Z. Phytic acid metal complex as precursor for fabrication of superhydrophilic membrane with photo-Fenton self-cleaning property for microalgae dewatering and oil/water emulsion separation. Sep. Purif. Technol. 2024, 350, 127802. [Google Scholar] [CrossRef]
- Huang, H.; Xie, W.; Wu, C.; Zhang, Z.; Qu, J.; Jiang, R.; Huang, J.; Zhang, B.; Hou, Y.; Yu, Z. Regulating coordinatively unsaturated Co active sites in TA@ ZIF-L (Co) via tannic acid for efficient photoelectrochemical water oxidation. Sep. Purif. Technol. 2025, 354, 129246. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Qu, Y.; Yang, B.; Zhang, Q.; Wang, J.; Lin, Y.; Chen, Z.; Lu, G. Bio-based tannic acid as a raw material for membrane surface modification. Desalination 2023, 555, 116535. [Google Scholar] [CrossRef]
- Xu, L.; Neoh, K.G.; Kang, E.T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Zuo, Y.; Long, X.; Zheng, Y.; Zhang, J.; Wang, L.; Hu, J.; Jiao, F. Gelatin-tannic acid coating for high flux oil-water separation. J. Environ. Chem. Eng. 2022, 10, 107992. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S.; Zhang, J.; Liu, Q.; He, F.; Peng, S.; Li, Y. Tannic acid encountering ovalbumin: A green and mild strategy for superhydrophilic and underwater superoleophobic modification of various hydrophobic membranes for oil/water separation. J. Mater. Chem. A 2018, 6, 13959–13967. [Google Scholar] [CrossRef]
- Xie, H.; Shen, L.; Xu, Y.; Hong, H.; Yang, L.; Li, R.; Lin, H. Tannic acid (TA)-based coating modified membrane enhanced by successive inkjet printing of Fe3+ and sodium periodate (SP) for efficient oil-water separation. J. Membr. Sci. 2022, 660, 120873. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, B.; Xi, W.; Liu, X.; Tang, S.; Tan, X.; Li, G.; Huang, L.; Liu, Y.; Bai, J. Modification methods, biological activities and applications of pectin: A review. Int. J. Biol. Macromol. 2023, 253, 127523. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, L.; Lin, N.; Wang, J.; Wang, Y.; Wu, T.; Song, P. Highly transparent, healable, and durable anti-fogging coating by combining hydrophilic pectin and tannic acid with poly (ethylene terephthalate). Green Chem. 2019, 21, 5405–5413. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Effect of different cation in situ cross-linking on the properties of pectin-thymol active film. Food Hydrocoll. 2022, 128, 107594. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Z.; Guo, H.; Yao, Z.; Ma, X.; Song, X.; Feng, S.; Tang, C. Tannic acid/Fe3+ nanoscaffold for interfacial polymerization: Toward enhanced nanofiltration performance. Environ. Sci. Technol. 2018, 52, 9341–9349. [Google Scholar] [CrossRef]
- Ong, C.; Chang, Y.J.; Alduraiei, F.; Wehbe, N.; Ahmed, Z.; Wang, P. Tannin-inspired robust fabrication of superwettability membranes for highly efficient separation of oil-in-water emulsions and immiscible oil/water mixtures. Sep. Purif. Technol. 2019, 227, 115657. [Google Scholar] [CrossRef]
- Zou, D.; Kim, H.W.; Jeon, S.M.; Lee, Y.M. Fabrication and modification of PVDF/PSF hollow-fiber membranes for ginseng extract and saline water separations via direct contact membrane distillation. J. Membr. Sci. 2022, 644, 120101. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Dai, J.; Lang, J.; Li, C.; Yan, Y. Photo-Fenton self-cleaning membranes with robust flux recovery for an efficient oil/water emulsion separation. J. Mater. Chem. A 2019, 7, 8491–8502. [Google Scholar] [CrossRef]










| Membranes | Flux (L m−2 h−1) | Separation Efficiency (%) |
|---|---|---|
| PVDF@TA3 | 143.1 | 99.05 |
| PVDF@PT3 | 129.5 | 98.75 |
| PVDF@TA/PT1 | 139.3 | 99.20 |
| PVDF@TA/PT3 | 156.3 | 99.35 |
| PVDF@TA/PT5 | 149.5 | 99.23 |
| PVDF@TA/PT3 (1 wt%) | 150.6 | 99.27 |
| PVDF@TA/PT3 (2 wt%) | 153.8 | 99.20 |
| PVDF@TA/PT3 (3 wt%) | 149.9 | 99.24 |
| PVDF@TA/PT3 (4 wt%) | 150.0 | 99.30 |
| PVDF@TA/PT3 (5 wt%) | 155.0 | 99.18 |
| PVDF@TA/PT3 (pH = 1) | 146.2 | 99.15 |
| PVDF@TA/PT3 (pH = 3) | 150.4 | 99.10 |
| PVDF@TA/PT3 (pH = 5) | 153.1 | 99.20 |
| PVDF@TA/PT3 (pH = 9) | 148.3 | 99.00 |
| PVDF@TA/PT3 (pH = 11) | 110.2 | 98.95 |
| PVDF@TA/PT3 (pH = 13) | 84.9 | 99.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, L.; Cui, J.; Lu, L.; Wang, H.; Wei, C.; Luo, J.; Xie, A. Preparation of Tannic Acid-Pectin Coated PVDF Membrane for High-Efficiency Separation of Oil and Water Emulsions. Membranes 2025, 15, 155. https://doi.org/10.3390/membranes15050155
Zhai L, Cui J, Lu L, Wang H, Wei C, Luo J, Xie A. Preparation of Tannic Acid-Pectin Coated PVDF Membrane for High-Efficiency Separation of Oil and Water Emulsions. Membranes. 2025; 15(5):155. https://doi.org/10.3390/membranes15050155
Chicago/Turabian StyleZhai, Liangku, Jiuyun Cui, Lei Lu, Hailong Wang, Can Wei, Jirong Luo, and Atian Xie. 2025. "Preparation of Tannic Acid-Pectin Coated PVDF Membrane for High-Efficiency Separation of Oil and Water Emulsions" Membranes 15, no. 5: 155. https://doi.org/10.3390/membranes15050155
APA StyleZhai, L., Cui, J., Lu, L., Wang, H., Wei, C., Luo, J., & Xie, A. (2025). Preparation of Tannic Acid-Pectin Coated PVDF Membrane for High-Efficiency Separation of Oil and Water Emulsions. Membranes, 15(5), 155. https://doi.org/10.3390/membranes15050155






