Microplasma-Mediated Enhancement of FD-150 Uptake in HL-60 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Medium Preparation
2.2. Cell Culture
2.3. Preparation of Cells for FD-150 Treatment
2.4. Microplasma Treatment
2.5. Quantification of FD-150 Uptake
2.6. Investigation of Transmembrane Potential
2.7. Evaluating Membrane Lipid Order
2.8. Statistical Analysis
3. Results and Discussion
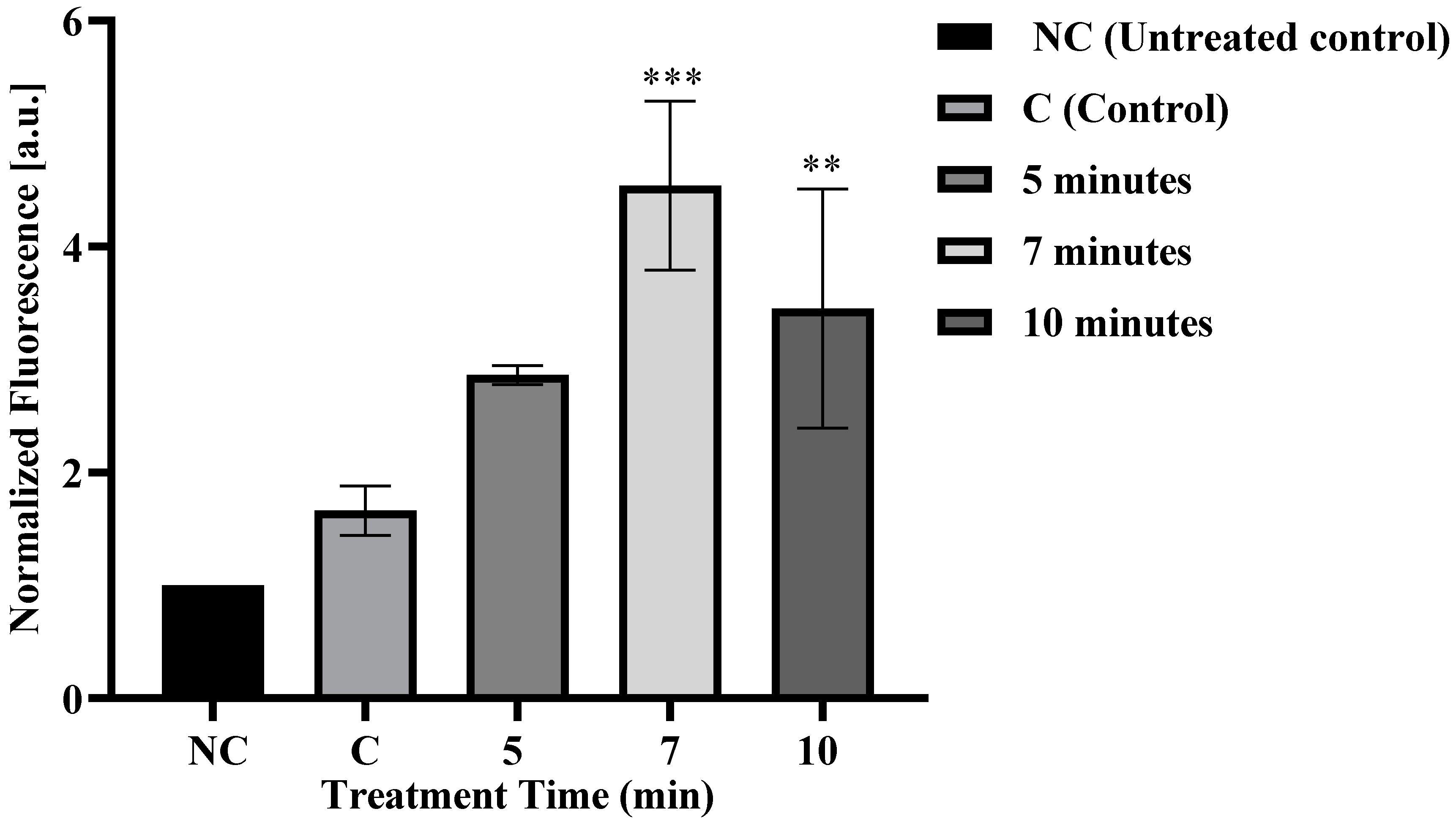
3.1. Microplasma Irradiation Induced FD-150 Uptake in Cells
3.2. Cell Viability
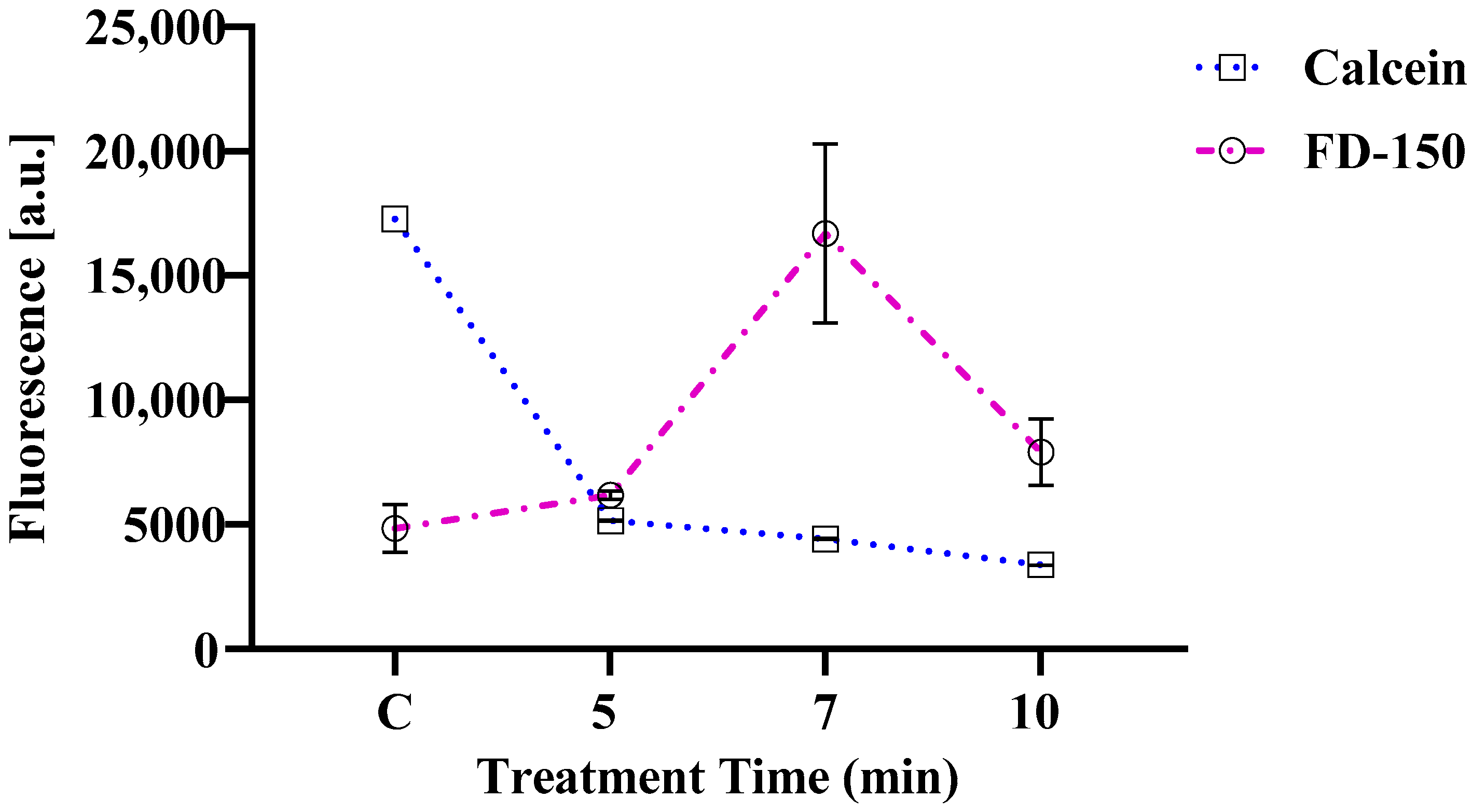
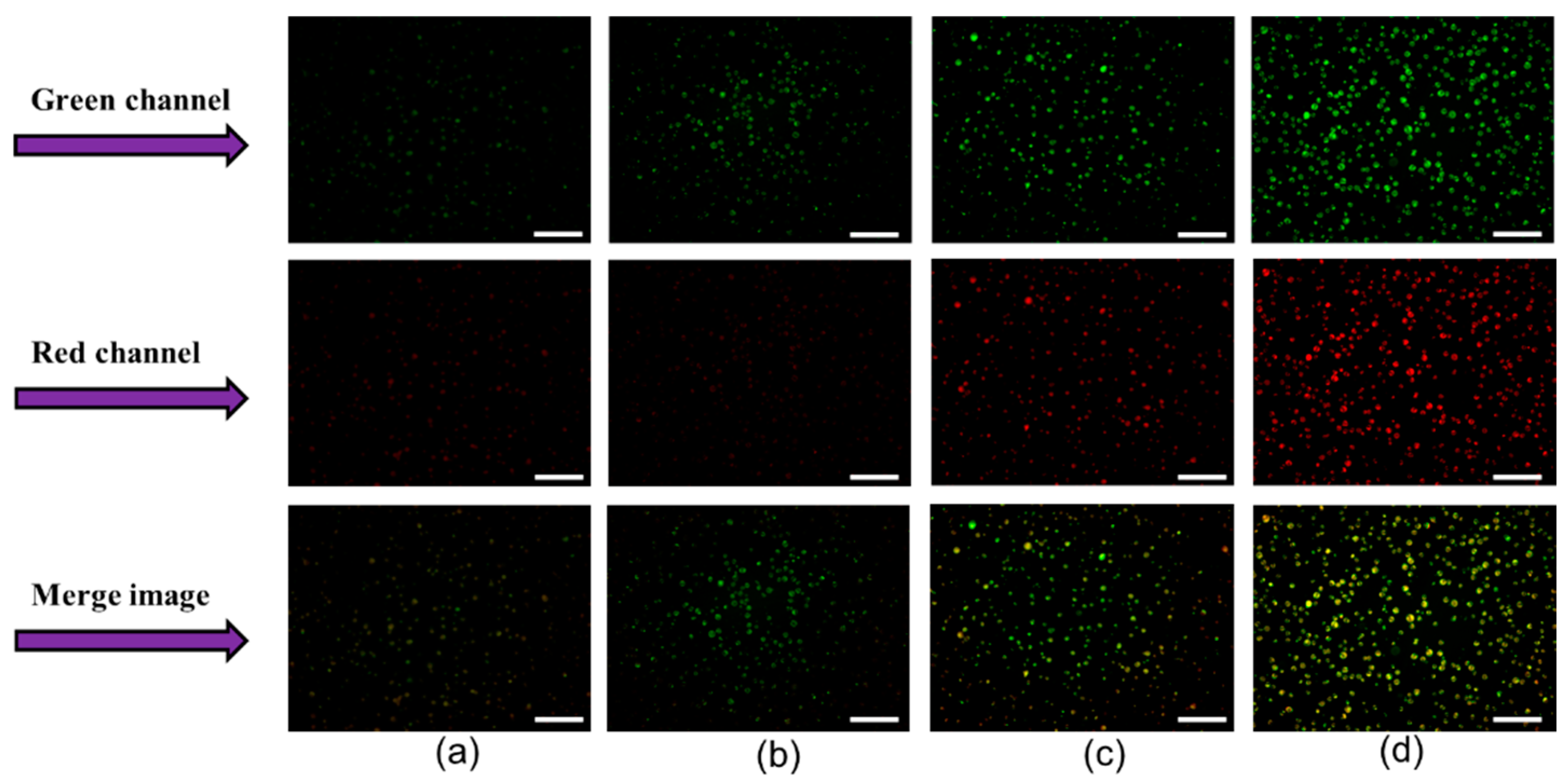
3.3. Changes in Calcein Violet 450 AM Fluorescence and FD-150 Internalization in Cells
3.4. Changes in Transmembrane Potential in Live Cells
3.5. Changes in Membrane Lipid Order
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Davis, M. Clinically relevant concentrations of anticancer drugs: A guide for nonclinical studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Mount, N.M.; Ward, S.J.; Kefalas, P.; Hyllner, J. Cell-based therapy technology classifications and translational challenges. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150017. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer. 2015, 15, 361–370. [Google Scholar] [CrossRef]
- Maeder, M.L.; Gersbach, C.A. Genome-editing technologies for gene and cell therapy. Mol. Ther. 2016, 24, 430–446. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020, 27, 1425–1437. [Google Scholar] [CrossRef]
- Lutz, H.; Hu, S.; Dinh, P.U.; Cheng, K. Cells and cell derivatives as drug carriers for targeted delivery. Med. Drug Discov. 2019, 3, 100014. [Google Scholar] [CrossRef]
- Rattan, V.I.; Shen, Y.A.; Sultana, C.H.; Kumar, D.I.; Kalra, V.K. Diabetic RBC-induced oxidant stress leads to transendothelial migration of monocyte-like HL-60 cells. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, E369–E375. [Google Scholar] [CrossRef]
- Reynwar, B.J.; Illya, G.; Harmandaris, V.A.; Müller, M.M.; Kremer, K.; Deserno, M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature. 2007, 447, 461–464. [Google Scholar] [CrossRef]
- Sharma, A.; Zhu, Y.; Spangler, E.J.; Laradji, M. Modes of adhesion of spherocylindrical nanoparticles to tensionless lipid bilayers. J. Chem. Phys. 2022, 156, 234901. [Google Scholar] [CrossRef]
- Zimmerberg, J.; McLaughlin, S. Membrane curvature: How BAR domains bend bilayers. Curr. Biol. 2004, 14, R250-2. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, C.; De Rosa, E.; Martinez, J.O.; Liu, X.; Steele, J.; Stevens, M.M.; Tasciotti, E. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat. Mater. 2015, 14, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Han, Y.; Liu, Y.; Zhou, Y.; Yu, J.; von Brunn, A.; Lei, J. Viral vector-based cancer treatment and current clinical applications. MedComm–Oncol. 2023, 2, e55. [Google Scholar] [CrossRef]
- Du, X.; Zhao, M.; Jiang, L.; Pang, L.; Wang, J.; Lv, Y.; Yao, C.; Wu, R. A mini-review on gene delivery technique using nanoparticles-mediated photoporation induced by nanosecond pulsed laser. Drug Deliv. 2024, 31, 2306231. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.K.; Bose, S.; Ghosh, T.K. Iontophoresis and electroporation: Comparisons and contrasts. Int. J. Pharm. 1999, 179, 1–19. [Google Scholar] [CrossRef]
- Cheng, X.; Rajjoub, K.; Sherman, J.; Canady, J.; Recek, N.; Yan, D.; Bian, K.; Murad, F.; Keidar, M. Cold plasma accelerates the uptake of gold nanoparticles into glioblastoma cells. Plasma Process. Polym. 2015, 12, 1364–1369. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Isozaki, Y.; Kido, Y.; Satoh, S. Synergistic effect of electrical and chemical factors on endocytosis in micro-discharge plasma gene transfection. Plasma Sources Sci. Technol. 2017, 26, 065016. [Google Scholar] [CrossRef]
- Tero, R.; Yamashita, R.; Hashizume, H.; Suda, Y.; Takikawa, H.; Hori, M.; Ito, M. Nanopore formation process in artificial cell membrane induced by plasma-generated reactive oxygen species. Arch. Biochem. Biophys. 2016, 605, 26–33. [Google Scholar] [CrossRef]
- Tero, R.; Suda, Y.; Kato, R.; Tanoue, H.; Takikawa, H. Plasma irradiation of artificial cell membrane system at solid–liquid interface. Appl. Phys. Express 2014, 7, 077001. [Google Scholar] [CrossRef]
- Hasan, M.; Nishimoto, A.; Ohgita, T.; Hama, S.; Kashida, H.; Asanuma, H.; Kogure, K. Faint electric treatment-induced rapid and efficient delivery of extraneous hydrophilic molecules into the cytoplasm. J. Control. Release 2016, 228, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Tarashima, N.; Fujikawa, K.; Ohgita, T.; Hama, S.; Tanaka, T.; Saito, H.; Minakawa, N.; Kogure, K. The novel functional nucleic acid iRed effectively regulates target genes following cytoplasmic delivery by faint electric treatment. Sci. Technol. Adv. Mater. 2016, 17, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Kristof, J.; Yokoyama, R.; Yahaya, A.G.; Rimi, S.A.; Blajan, M.; Shimizu, K. Absorption of FD-150 into intestinal cells by microplasma. Plasma Med. 2022, 12, 11–28. [Google Scholar] [CrossRef]
- Dolezalova, E.; Malik, M.A.; Heller, L.; Heller, R. Delivery and expression of plasmid DNA into cells by a novel non-thermal plasma source. Bioelectrochemistry 2021, 140, 107816. [Google Scholar] [CrossRef] [PubMed]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef]
- Szili, E.J.; Bradley, J.W.; Short, R.D. A ‘tissue model’to study the plasma delivery of reactive oxygen species. J. Phys. D Appl. Phys. 2014, 47, 152002. [Google Scholar] [CrossRef]
- Choi, J.H.; Nam, S.H.; Song, Y.S.; Lee, H.W.; Lee, H.J.; Song, K.; Hong, J.W.; Kim, G.C. Treatment with low-temperature atmospheric pressure plasma enhances cutaneous delivery of epidermal growth factor by regulating E-cadherin-mediated cell junctions. Arch. Dermatol. Res. 2014, 306, 635–643. [Google Scholar] [CrossRef]
- Kristof, J.; Aoshima, T.; Blajan, M.; Shimizu, K. Effect of microplasma treatment on stratum corneum lipid molecule. Jpn. J. Appl. Phys. 2020, 59, SHHF06. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S.W.; Maher, M.; Tiwari, B.; Byrne, H.J.; Bourke, P.; et al. Cold atmospheric plasma stimulates clathrin-dependent endocytosis to repair oxidised membrane and enhance uptake of nanomaterial in glioblastoma multiforme cells. Sci. Rep. 2020, 10, 6985. [Google Scholar] [CrossRef]
- Ghysels, A.; Krämer, A.; Venable, R.M.; Teague, W.E., Jr.; Lyman, E.; Gawrisch, K.; Pastor, R.W. Permeability of membranes in the liquid ordered and liquid disordered phases. Nat. Commun. 2019, 10, 5616. [Google Scholar] [CrossRef]
- Khan, F.; Elgeti, M.; Grandfield, S.; Paz, A.; Naughton, F.B.; Marcoline, F.V.; Althoff, T.; Ermolova, N.; Wright, E.M.; Hubbell, W.L.; et al. Membrane potential accelerates sugar uptake by stabilizing the outward facing conformation of the Na/glucose symporter vSGLT. Nat. Commun. 2023, 14, 7511. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Kok, Z.Y.; Koh, C.H.; Zhong, W.; Ng, J.T.; Mu, Y.; Chan-Park, M.B.; Gan, Y.-H. Membrane Potential-Dependent Uptake of Cationic Oligoimidazolium Mediates Bacterial DNA Damage and Death. Antimicrob. Agents Chemother. 2023, 67, e00355-23. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 6, 21464. [Google Scholar] [CrossRef] [PubMed]
- Kristof, J.; Hasan, M.; Rimi, S.A.; Begum, F.; Sadiq, A.H.; Jahangir, A.M.; Shimizu, K. Absorption of FD-150 into White Blood Cells by Microplasma. Plasma Med. 2024, 14, 53–68. [Google Scholar] [CrossRef]
- Sasaki, S.; Zheng, Y.; Mokudai, T.; Kanetaka, H.; Tachikawa, M.; Kanzaki, M.; Kaneko, T. Continuous release of O2−/ONOO− in plasma-exposed HEPES-buffered saline promotes TRP channel-mediated uptake of a large cation. Plasma Process. Polym. 2020, 17, 1900257. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Kido, Y.; Satoh, S. Investigation of plasma induced electrical and chemical factors and their contribution processes to plasma gene transfection. Arch. Biochem. Biophys. 2016, 605, 59–66. [Google Scholar] [CrossRef]
- Isozaki, Y.; Ikeda, Y.; Kido, Y.; Satoh, S.; Jinno, M. Reactive Oxygen Species and Intracellular Ca2+ Contribution to Micro-Discharge Plasma Gene Transfection. Plasma Med. 2017, 7, 321–332. [Google Scholar] [CrossRef]
- Jinno, M.; Satoh, S.; Ikeda, Y.; Motomura, H. The new technology of molecular and gene introduction method using discharge plasma: Plasma brings features of random genome integration-free and damage-free to cells, genomic-DNA and external introducing molecules. Jpn. J. Appl. Phys. 2021, 60, 30502. [Google Scholar] [CrossRef]
- Kono, Y.; Onishi, K.; Kitamura, H.; Ogawara, K.I. Development of liposome-loaded macrophages as tumor-targeted drug delivery carriers: Impact of macrophage phenotypes on their liposome-loading capacity and tumor-homing potential. J. Drug Deliv. Sci. Technol. 2025, 108, 106866. [Google Scholar] [CrossRef]
- Müller-Greven, G.; Carlin, C.R.; Burgett, M.E.; Ahluwalia, M.S.; Lauko, A.; Nowacki, A.S.; Herting, C.J.; Qadan, M.A.; Bredel, M.; Toms, S.A.; et al. Macropinocytosis of bevacizumab by glioblastoma cells in the perivascular niche affects their survival. Clin. Cancer Res. 2017, 23, 7059–7071. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Khajoee, V.; Ogawa, Y.; Kusuhara, K.; Katayama, Y.; Hara, T. A novel transfection method for mammalian cells using gas plasma. J. Biotechnol. 2006, 121, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakellis, P.; Faubert, F.; Wartel, M.; Gogolides, E.; Pellerin, S. Plasma surface modification of epoxy polymer in air DBD and gliding arc. Processes. 2022, 10, 104. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Kido, Y.; Tachibana, K.; Satoh, S. The necessity of radicals for gene transfection by discharge plasma irradiation. J. Photopolym. Sci. Technol. 2014, 27, 399–404. [Google Scholar] [CrossRef]
- Ikeda, Y.; Motomura, H.; Kido, Y.; Satoh, S.; Jinno, M. Effects of molecular size and chemical factor on plasma gene transfection. Jpn. J. Appl. Phys. 2016, 55, 07LG06. [Google Scholar] [CrossRef]
- Cavalli, V.; Vilbois, F.; Corti, M.; Marcote, M.J.; Tamura, K.; Karin, M.; Arkinstall, S.; Gruenberg, J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI: Rab5 complex. Mol. Cell. 2001, 7, 421–432. [Google Scholar] [CrossRef]
- Sundqvist, T.; Liu, S.M. Hydrogen peroxide stimulates endocytosis in cultured bovine aortic endothelial cells. Acta Physiol. Scand. 1993, 149, 127–131. [Google Scholar] [CrossRef]
- Hatz, S.; Lambert, J.D.; Ogilby, P.R. Measuring the lifetime of singlet oxygen in a single cell: Addressing the issue of cell viability. Photochem. Photobiol. Sciences. 2007, 6, 1106–1116. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Harada, M.; Handa, T.; Funakoshi, S.; Fujii, N.; Yajima, H.; Miyajima, K. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 1989, 981, 130–134. [Google Scholar] [CrossRef]
- Chemical Book. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0173162.htm (accessed on 30 April 2025).
- Jinno, M.; Tachibana, K.; Motomura, H.; Saeki, N.; Satoh, S. Improvement of efficiency and viability in plasma gene transfection by plasma minimization and optimization electrode configuration. Jpn. J. Appl. Phys. 2016, 55, 07LG09. [Google Scholar] [CrossRef]
- Ben-Dov, N.; Korenstein, R. Proton-induced endocytosis is dependent on cell membrane fluidity, lipid-phase order and the membrane resting potential. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2672–2681. [Google Scholar] [CrossRef] [PubMed]
- Juffermans, L.J.; Kamp, O.; Dijkmans, P.A.; Visser, C.A.; Musters, R.J. Low-intensity ultrasound-exposed microbubbles provoke local hyperpolarization of the cell membrane via activation of BKCa channels. Ultrasound Med. Biol. 2008, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, A.G.; Okuyama, T.; Kristof, J.; Blajan, M.G.; Shimizu, K. Direct and indirect bactericidal effects of cold atmospheric-pressure microplasma and plasma jet. Molecules 2021, 26, 2523. [Google Scholar] [CrossRef]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.M.; Robert, E.; Pichon, C. New insights on molecular internalization and drug delivery following plasma jet exposures. Int. J. Pharm. 2020, 589, 119874. [Google Scholar] [CrossRef]
- Valanciunaite, J.; Kempf, E.; Seki, H.; Danylchuk, D.I.; Peyriéras, N.; Niko, Y.; Klymchenko, A.S. Polarity mapping of cells and embryos by improved fluorescent solvatochromic pyrene probe. Anal. Chem. 2020, 92, 6512–6520. [Google Scholar] [CrossRef]
- Rimi, S.A.; Kristof, J.; Alam, M.J.; Oishi, K.; Okada, T.; Sadiq, A.H.; Shimizu, K. Changes in Membrane Lipid Order of Cells by Optimized Microplasma Irradiation for High-Molecule Drug Absorption. Plasma Med. 2023, 13, 15–32. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Cordeiro, R.M. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 438–444. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. In situ investigation of the oxidation of a phospholipid monolayer by reactive oxygen species. Biophys. J. 2023, 122, 2007–2022. [Google Scholar] [CrossRef]



| Sample | Fred/Fgeen Ratio (Flow Cytometric) | Fred/Fgreen Ratio (Microscopic) |
|---|---|---|
| Control | 0.65 ± 0.01 | 0.78 ± 0.44 |
| 5 min | 0.72 ± 0.02 *** | 1.00 ± 0.15 ** |
| 7 min | 0.70 ± 0.01 *** | 1.13 ± 0.33 *** |
| 10 min | 0.80 ± 0.04 *** | 1.34 ± 0.53 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Kristof, J.; Sadiq, A.H.; Alam, M.J.; Rimi, S.A.; Begum, F.; Shimizu, K. Microplasma-Mediated Enhancement of FD-150 Uptake in HL-60 Cells. Membranes 2025, 15, 156. https://doi.org/10.3390/membranes15050156
Hasan M, Kristof J, Sadiq AH, Alam MJ, Rimi SA, Begum F, Shimizu K. Microplasma-Mediated Enhancement of FD-150 Uptake in HL-60 Cells. Membranes. 2025; 15(5):156. https://doi.org/10.3390/membranes15050156
Chicago/Turabian StyleHasan, Mahedi, Jaroslav Kristof, Abubakar Hamza Sadiq, Md Jahangir Alam, Sadia Afrin Rimi, Farhana Begum, and Kazuo Shimizu. 2025. "Microplasma-Mediated Enhancement of FD-150 Uptake in HL-60 Cells" Membranes 15, no. 5: 156. https://doi.org/10.3390/membranes15050156
APA StyleHasan, M., Kristof, J., Sadiq, A. H., Alam, M. J., Rimi, S. A., Begum, F., & Shimizu, K. (2025). Microplasma-Mediated Enhancement of FD-150 Uptake in HL-60 Cells. Membranes, 15(5), 156. https://doi.org/10.3390/membranes15050156







