Toluidine Blue for the Determination of Binding of Anionic Polysaccharides to Lipid Raft Domains by Absorption Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
- Chemicals
- Preparation of Liposomes
- Absorption Spectroscopy
- Titration with Liposomes at Fixed Concentration of Toluidine Blue and PSA
- Titration with PSA at a Constant Toluidine Blue and Liposome Concentration
- Titration with Toluidine Blue at Fixed Liposome and PSA Concentrations
- Titration with Toluidine Blue at Fixed Liposome and PGA Concentrations
- Titration with PGA at Fixed Toluidine Blue and Liposome Concentrations
- Titration with Liposomes at Fixed Toluidine Blue and PGA Concentrations
- Parameters Used for Calculations
3. Results
3.1. Binding of Polysialic Acid (PSA) to Liposomes Evaluated Using Toluidine Blue Absorption
3.1.1. Titration with Toluidine Blue at Fixed Liposome and Polysialic Acid Concentrations
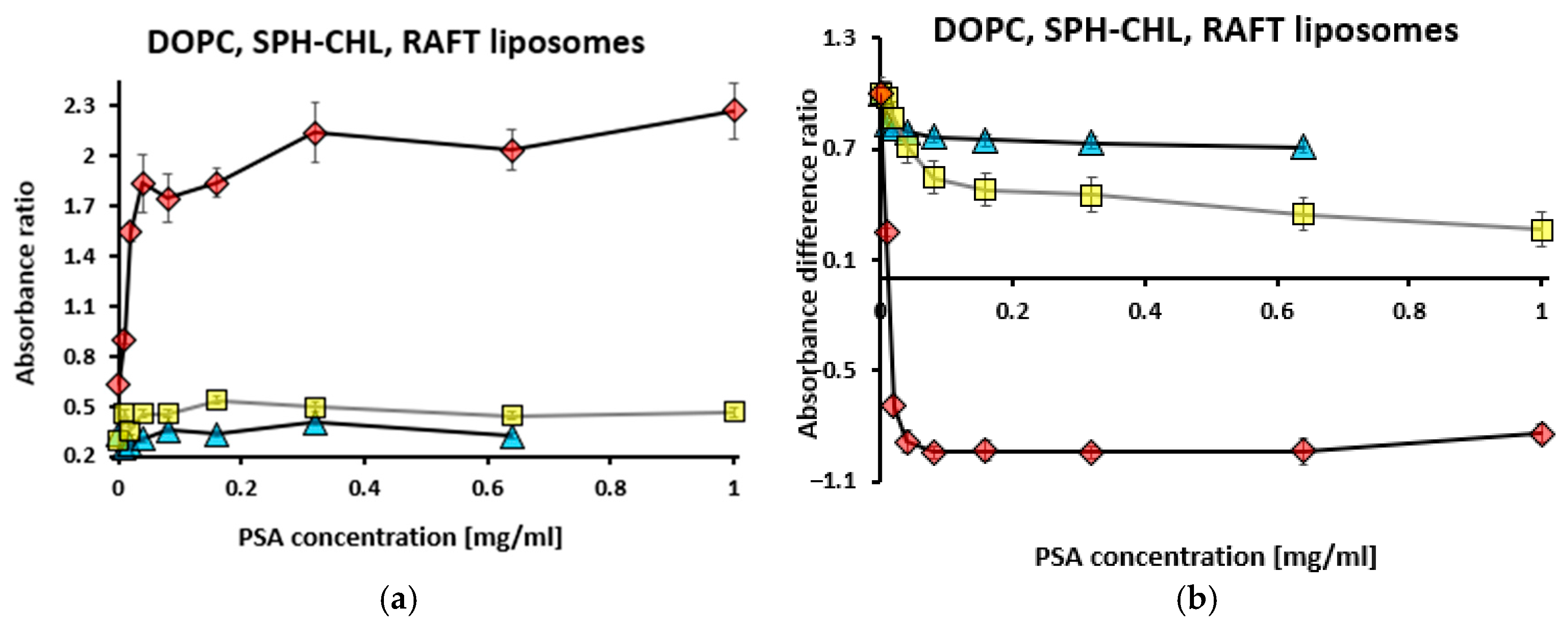
3.1.2. Titration with Polysialic Acid at Fixed Toluidine Blue and Liposome Concentrations
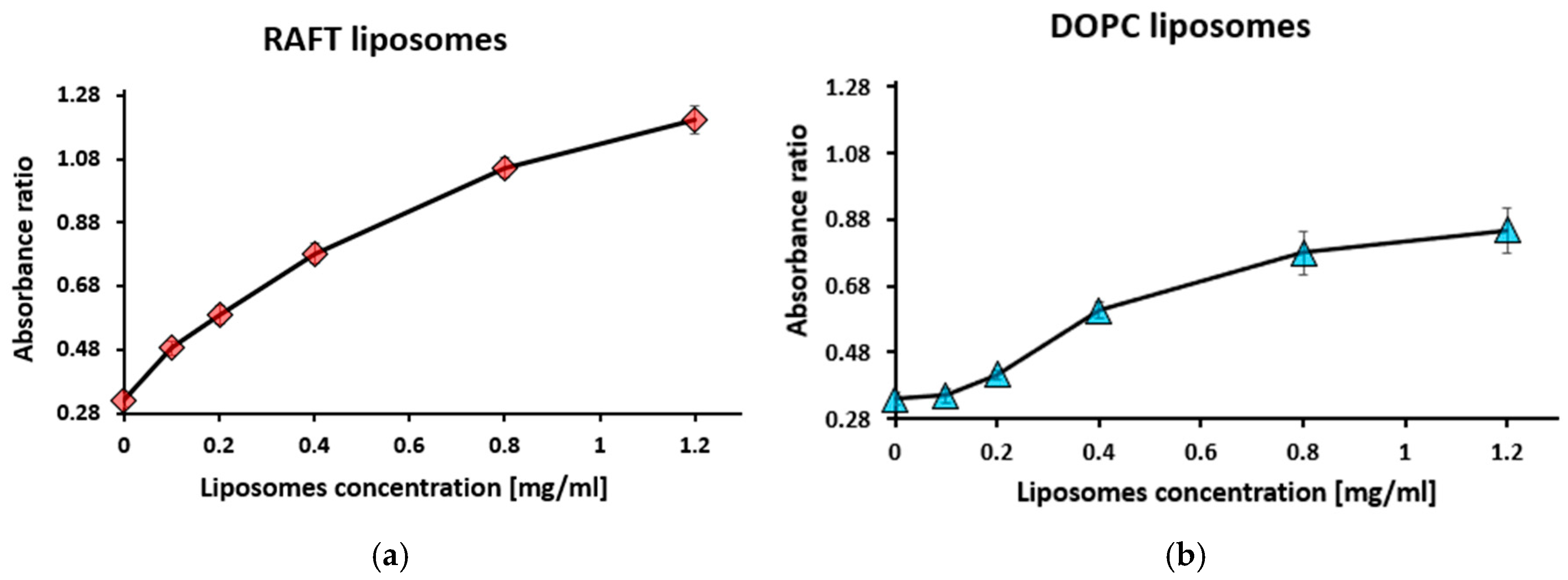
3.1.3. Titration with Liposomes at Fixed Toluidine Blue and Polysialic Acid Concentrations
3.2. Binding of Polygalacturonic Acid (PGA) to Liposomes Measured Using Toluidine Blue Absorption
3.2.1. Titration with Toluidine Blue at Constant Liposome and Polygalacturonic Acid Concentrations
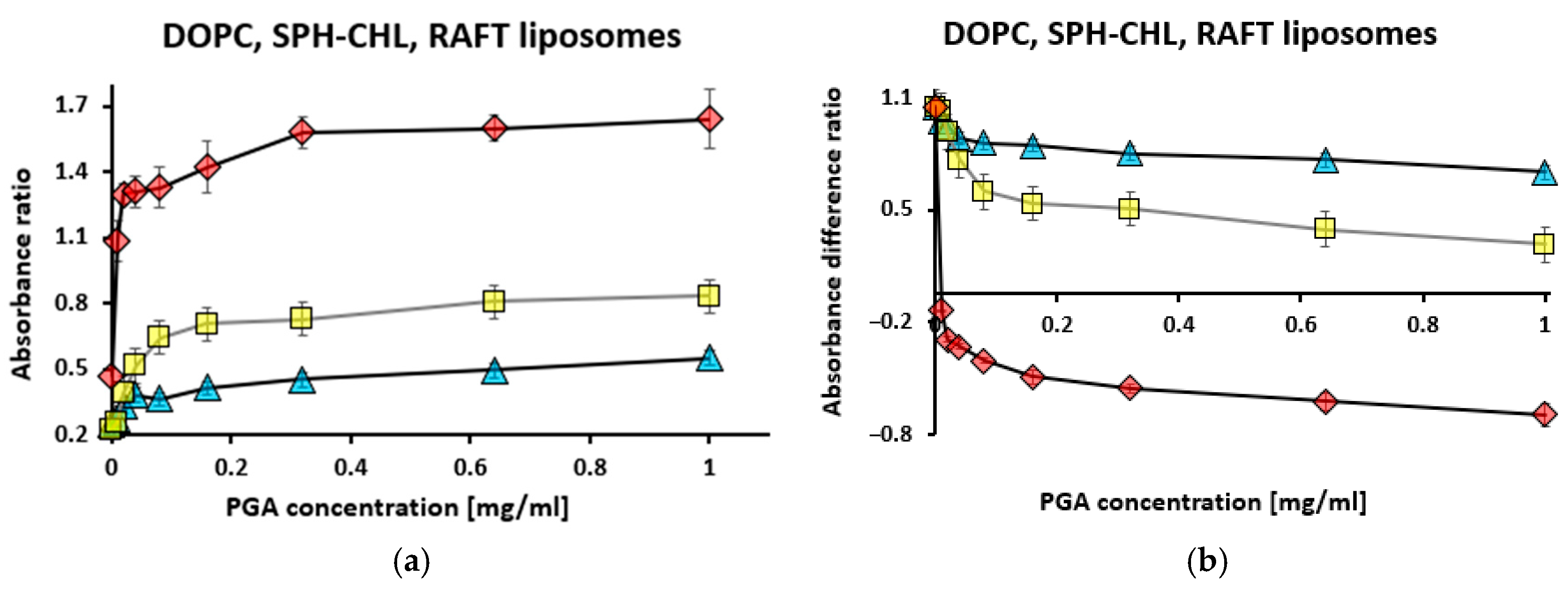
3.2.2. Titration with Polygalacturonic Acid at Constant Toluidine Blue and Liposome Concentrations
3.2.3. Titration with Liposomes at Constant Toluidine Blue and Polygalacturonic Acid Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trucillo, P. Biomaterials for drug delivery and human applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhadi, S.R.; Mai, N.; Taylor, C.; Roy, J.W.; Barnett, D.A.; Lewis, S.M.; Ghosh, A.; Ouellette, R.J. The polysaccharide chitosan facilitates the isolation of small extracellular vesicles from multiple biofluids. J. Extracell. Vesicles 2021, 10, e12138. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Saied, H.E.; Kamel, S. Polysaccharides Based Biosensors for Medical Applications: Prospective and Future Aspects. Starch Stärke 2023, 75, 2300037. [Google Scholar] [CrossRef]
- Barenholz, A.; Fishel, F.; Yakir, E.; Gatt, S.; Barenholz, Y.; Bercovier, H. Liposomes Enhance Bioremediation of Oil-Contaminated Soil. J. Liposome Res. 2003, 13, 173–186. [Google Scholar] [CrossRef]
- Gomez-Guzman, L.A.; Vallejo-Cardona, A.A.; Rodriguez-Campos, J.; Garcia-Carvajal, Z.Y.; Patrón-Soberano, O.A.; Contreras-Ramos, S.M. Slow-release microencapsulates containing nanoliposomes for bioremediation of soil hydrocarbons contaminated. Environ. Technol. 2024, 45, 5406–5418. [Google Scholar] [CrossRef]
- Barenholz, A.; Barenholz, Y.; Bercovier, H. Nano-Liposomes of Crude Soy Lecithin are Effective for Cleaning Fuel-Contaminated Sands and Soils. Expert Opin. Environ. Biol. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Wilschut, J.; Hoekstra, D. Membrane fusion: From liposomes to biological membranes. Trends Biochem. Sci. 1984, 9, 479–483. [Google Scholar] [CrossRef]
- Carstensen, J.; Stamou, D.; Kockelkoren, G.; Shuttle, C.G. 3D imaging reveals nanodomains of transmembrane proteins at the plasma membrane of live unperturbed cells. Biophys. J. 2024, 123, 303a–304a. [Google Scholar] [CrossRef]
- Mindler, K.; Ostertag, E.; Stehle, T. The polyfunctional polysialic acid: A structural view. Carbohydr. Res. 2021, 507, 108376. [Google Scholar] [CrossRef]
- Zlatina, K.; Saftenberger, M.; Kühnle, A.; Galuska, C.E.; Gärtner, U.; Rebl, A.; Oster, M.; Vernunft, A.; Galuska, S.P. Polysialic acid in human plasma can compensate the cytotoxicity of histones. Int. J. Mol. Sci. 2018, 19, 1679. [Google Scholar] [CrossRef] [PubMed]
- Sapoń, K.; Gawrońska, I.; Janas, T.; Sikorski, A.F.; Janas, T. Exosome-associated polysialic acid modulates membrane potentials, membrane thermotropic properties, and raft-dependent interactions between vesicles. FEBS Lett. 2020, 594, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Sapoń, K.; Janas, T.; Janas, T. Biophysical Characterization of Polysialic Acid—Membrane Nanosystems. In Thermodynamics and Biophysics of Biomedical Nanosystems; Demetzos, C., Pippa, N., Eds.; Springer: Singapore, 2019; pp. 365–396. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; He, L.; Feng, X. A brief review of polysialic acid-based drug delivery systems. Int. J. Biol. Macromol. 2023, 230, 123151. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, S.; Hu, L.; Peng, B.; Liu, Y.; Luo, X.; Song, Y.; Liu, X.; Deng, Y. Polysialic acid-modifying liposomes for efficient delivery of epirubicin, in-vitro characterization and in-vivo evaluation. Int. J. Pharm. 2016, 515, 449–459. [Google Scholar] [CrossRef]
- Fan, C.; Li, C.; Lu, S.; Lai, X.; Wang, S.; Liu, X.; Song, Y.; Deng, Y. Polysialic Acid Self-assembled Nanocomplexes for Neutrophil-Based Immunotherapy to Suppress Lung Metastasis of Breast Cancer. AAPS PharmSciTech. 2022, 23, 109. [Google Scholar] [CrossRef]
- Sui, D.; Liang, K.; Gui, Y.; Du, Z.; Xin, D.; Yu, G.; Zhai, W.; Liu, X.; Song, Y.; Deng, Y. Optimization design of sialic acid derivatives enhances the performance of liposomes for modulating immunosuppressive tumor microenvironments. Life Sci. 2022, 310, 121081. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, S.; Liu, Y.; Luo, X.; Di, D.; Song, Y.; Liu, X.; Deng, Y. Polysialic acid and pluronic F127 mixed polymeric micelles of docetaxel as new approach for enhanced antitumor efficacy. Drug Dev. Ind. Pharm. 2017, 43, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.P.; Tran, P.; Yoo, S.-Y.; Tangchang, W.; Lee, S.; Lee, J.-Y.; Son, H.-Y.; Park, J.-S. Sialic acid-decorated liposomes enhance the anti-cancer efficacy of docetaxel in tumor-associated macrophages. Biomater. Adv. 2023, 154, 213606. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, D.; Li, P.; Wang, D.; Mu, H.; Niu, H.; Duan, J. Novel pH-sensitive polysialic acid based polymeric micelles for triggered intracellular release of hydrophobic drug. Carbohydr. Polym. 2016, 139, 75–81. [Google Scholar] [CrossRef]
- Thwala, L.N.; Beloqui, A.; Csaba, N.S.; González-Touceda, D.; Tovar, S.; Dieguez, C.; Alonso, M.J.; Préat, V. The interaction of protamine nanocapsules with the intestinal epithelium: A mechanistic approach. J. Control. Release 2016, 243, 109–120. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Zhang, A.; Tan, X.; Li, S.; Luo, L.; Wang, S.; Wei, G.; Zhang, Z.; Huo, J. Combination of lipopolysaccharide and polygalacturonic acid exerts antitumor activity and augments anti-PD-L1 immunotherapy. Int. J. Biol. Macromol. 2024, 281, 136390. [Google Scholar] [CrossRef]
- Song, J.; Hua, Y.; Pan, C.; Cui, L.; Fan, X.; Lu, M.; Zhang, Z. Effects of oral administration and intravenous injection of polygalacturonic acid on the immunomodulation and gut microbiota in UC mice. Int. J. Biol. Macromol. 2022, 217, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tao, Y.; Lai, C.; Huang, C.; Ling, Z.; Yong, Q. A method for quantitative characterization of incomplete degradation products of polygalacturonic acid. Int. J. Biol. Macromol. 2021, 188, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kótai, L.; Lázár, K.; Kiss, L.F.; Szentmihályi, K. Reaction of Partially Methylated Polygalacturonic Acid with Iron (III) Chloride and Characterization of a New Mixed Chloride—Polygalacturonate Complex. Molecules 2024, 29, 890. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Pectin and polygalacturonic acid-coated liposomes as novel delivery system for nisin: Preparation, characterization and release behavior. Food Hydrocoll. 2017, 70, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, Z.; Xu, M.-Z.; Zeng, Z.; Huang, J.-P.; Guan, Y.-Q. Natural plant-derived polygalacturonic acid-oleanolic acid assemblies as oral-delivered nanomedicine for insulin resistance treatment. Chem. Eng. J. 2020, 390, 124630. [Google Scholar] [CrossRef]
- Karim, N.; Shishir, M.R.I.; Li, Y.; Zineb, O.Y.; Mo, J.; Tangpong, J.; Chen, W. Pelargonidin-3-O-glucoside encapsulated pectin-chitosan-nanoliposomes recovers palmitic acid-induced hepatocytes injury. Antioxidants 2022, 11, 623. [Google Scholar] [CrossRef]
- Sapoń, K.; Mańka, R.; Janas, T.; Janas, T. The role of lipid rafts in vesicle formation. J. Cell Sci. 2023, 136, jcs260887. [Google Scholar] [CrossRef]
- Cerasuolo, M.; Di Meo, I.; Auriemma, M.C.; Paolisso, G.; Papa, M.; Rizzo, M.R. Exploring the Dynamic Changes of Brain Lipids, Lipid Rafts, and Lipid Droplets in Aging and Alzheimer’s Disease. Biomolecules 2024, 14, 1362. [Google Scholar] [CrossRef]
- Kania, E.; Pajak, B.; Gajkowska, B.; Orzechowski, A. Lipid rafts in Alzheimer’s disease. Postepy Biochem. 2012, 58, 209–216. [Google Scholar]
- Mańka, R.; Sapoń, K.; Zaziąbło, J.; Janas, T.; Czogalla, A.; Janas, T. The role of RNA structural motifs in RNA-lipid raft interaction. Sci. Rep. 2025, 15, 6777. [Google Scholar] [CrossRef] [PubMed]
- Cremesti, A.E.; Goni, F.M.; Kolesnick, R. Role of sphingomyelinase and ceramide in modulating rafts: Do biophysical properties determine biologic outcome? FEBS Lett. 2002, 531, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Noghero, A.; Perino, A.; Seano, G.; Saglio, E.; Sasso, G.L.; Veglio, F.; Primo, L.; Hirsch, E.; Bussolino, F.; Morello, F. Liver X Receptor Activation Reduces Angiogenesis by Impairing Lipid Raft Localization and Signaling of Vascular Endothelial Growth Factor Receptor-2. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yao, J.; Kim, T.-W.; Tall, A.R. Expression of liver X receptor target genes decreases cellular amyloid β peptide secretion. J. Biol. Chem. 2003, 278, 27688–27694. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, J.; Kim, T.-W.; Tall, A.R. Stimulation of liver X receptor has potent anti-HIV effects in a humanized mouse model of HIV infection. J. Pharmacol. Exp. Ther. 2015, 354, 376–383. [Google Scholar]
- Calkin, A.C.; Tontonoz, P. Liver X Receptor Signaling Pathways and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1513–1518. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Toluidine blue staining for cell and tissue biology applications. Acta Histochem. 2019, 121, 101–112. [Google Scholar] [CrossRef]
- Vandamme, M.-P.I.; Tiglias, J.; Nemat, N.; Preston, B.N. Determination of the charge content at the surface of cells using a colloid titration technique. Anal. Biochem. 1994, 223, 62–70. [Google Scholar] [CrossRef]
- Nowotarski, K.; Sapoń, K.; Kowalska, M.; Janas, T.; Janas, T. Membrane potential-dependent binding of polysialic acid to lipid monolayers and bilayers. Cell. Mol. Biol. Lett. 2013, 18, 579–594. [Google Scholar] [CrossRef]
- Sapoń, K.; Maziarz, D.; Janas, T.; Sikorski, A.F.; Janas, T. Cholera toxin subunit B for sensitive and rapid determination of exosomes by gel filtration. Membranes 2020, 10, 172. [Google Scholar] [CrossRef]
- Janas, T.; Sapoń, K.; Janas, T. Selection of bifunctional RNAs with specificity for arginine and lipid membranes. FEBS Lett. 2024, 598, 1061–1079. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.F.; Fedorov, A.; Prieto, M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef]
- Petruzielo, R.S.; Heberle, F.A.; Drazba, P.; Katsaras, J.; Feigenson, G.W. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 1302–1313. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A.; Bagatolli, L.A.; Brown, R.E.; Marsh, D.; Prieto, M.; Thewalt, J.L. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Yarus, M. Visualization of membrane RNAs. RNA 2003, 9, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Sapoń, K.; Janas, T.; Sikorski, A.F.; Janas, T. Polysialic acid chains exhibit enhanced affinity for ordered regions of membranes. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 245–255. [Google Scholar] [CrossRef]
- Nguyen, S.; Alund, S.J.; Hiorth, M.; Kjøniksen, A.-L.; Smistad, G. Studies on pectin coating of liposomes for drug delivery. Colloids Surf. B Biointerfaces 2011, 88, 664–673. [Google Scholar] [CrossRef]
- Sakane, H.; Yamamoto, H.; Matsumoto, S.; Sato, A.; Kikuchi, A. Localization of glypican-4 in different membrane microdomains is involved in the regulation of Wnt signaling. J. Cell Sci. 2012, 125, 449–460. [Google Scholar] [CrossRef]
- Podyma-Inoue, K.A.; Hara-Yokoyama, M.; Shinomura, T.; Kimura, T.; Yanagishita, M. Syndecans reside in sphingomyelin-enriched low-density fractions of the plasma membrane isolated from a parathyroid cell line. PLoS ONE 2012, 7, e32351. [Google Scholar] [CrossRef]
- Arpicco, S.; Lerda, C.; Pozza, E.D.; Costanzo, C.; Tsapis, N.; Stella, B.; Donadelli, M.; Dando, I.; Fattal, E.; Cattel, L.; et al. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur. J. Pharm. Biopharm. 2013, 85, 373–380. [Google Scholar] [CrossRef]
- Bagari, R.; Bansal, D.; Gulbake, A.; Jain, A.; Soni, V.; Jain, S.K. Chondroitin sulfate functionalized liposomes for solid tumor targeting. J. Drug Target. 2011, 19, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Moldovan, L.; Moisei, M.; Trif, M. Liposomal formulation of chondroitin sulfate enhances its antioxidant and anti-inflammatory potential in L929 fibroblast cell line. J. Liposome Res. 2013, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wada, K.; Shimba, K.; Miyamoto, Y.; Yagi, T. Formation of Agarose-supported Liposomes by Polymer-assisted Method toward Biosensor. IEEJ Trans. Electron. Inf. Syst. 2021, 141, 646–653. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; de Oliveira, A.C.; Souza, P.R.; Martins, A.F. Polysaccharide-based polyelectrolyte multilayers fabricated via layer-by-layer approach: From preparation to applications. Prog. Org. Coat. 2024, 196, 108720. [Google Scholar] [CrossRef]
- Gu, X.; Xu, L.; Yuan, H.; Li, C.; Zhao, J.; Li, S.; Yu, D. Sophorolipid-toluidine blue conjugates for improved antibacterial photodynamic therapy through high accumulation. RSC Adv. 2023, 13, 11782–11793. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Rout, L.; Mohanty, N.; Satpathy, A.; Satapathy, B.S.; Rath, S.; Gopinath, D. Exploring the photosensitizing potential of Nanoliposome Loaded Improved Toluidine Blue O (NLITBO) Against Streptococcus mutans: An in-vitro feasibility study. PLoS ONE 2024, 19, e0312521. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Chen, Y.; Zhao, J.; Xu, H.; Weng, J.; Yu, F.; Xiong, A.; Udduttula, A.; Wang, D.; et al. An injectable liposome-anchored teriparatide incorporated gallic acid-grafted gelatin hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 3159. [Google Scholar] [CrossRef]
- Ghirmai, S.; Bülow, L.; Sakai, H. In vivo evaluation of electron mediators for the reduction of methemoglobin encapsulated in liposomes using electron energies produced by red blood cell glycolysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1364–1372. [Google Scholar] [CrossRef]
- Kuntoji, G.; Kousar, N.; Gaddimath, S.; Koodlur Sannegowda, L. Macromolecule–nanoparticle-based hybrid materials for biosensor applications. Biosensors 2024, 14, 277. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gębczyńska, S.; Gdowska, J.; Mikos, A.; Gawrońska, I.; Janas, T.; Czogalla, A.; Janas, T. Toluidine Blue for the Determination of Binding of Anionic Polysaccharides to Lipid Raft Domains by Absorption Spectroscopy. Membranes 2025, 15, 139. https://doi.org/10.3390/membranes15050139
Gębczyńska S, Gdowska J, Mikos A, Gawrońska I, Janas T, Czogalla A, Janas T. Toluidine Blue for the Determination of Binding of Anionic Polysaccharides to Lipid Raft Domains by Absorption Spectroscopy. Membranes. 2025; 15(5):139. https://doi.org/10.3390/membranes15050139
Chicago/Turabian StyleGębczyńska, Sandra, Julia Gdowska, Agata Mikos, Iga Gawrońska, Teresa Janas, Aleksander Czogalla, and Tadeusz Janas. 2025. "Toluidine Blue for the Determination of Binding of Anionic Polysaccharides to Lipid Raft Domains by Absorption Spectroscopy" Membranes 15, no. 5: 139. https://doi.org/10.3390/membranes15050139
APA StyleGębczyńska, S., Gdowska, J., Mikos, A., Gawrońska, I., Janas, T., Czogalla, A., & Janas, T. (2025). Toluidine Blue for the Determination of Binding of Anionic Polysaccharides to Lipid Raft Domains by Absorption Spectroscopy. Membranes, 15(5), 139. https://doi.org/10.3390/membranes15050139






