Membrane Technology for Valuable Resource Recovery from Palm Oil Mill Effluent (POME): A Review
Abstract
1. Introduction
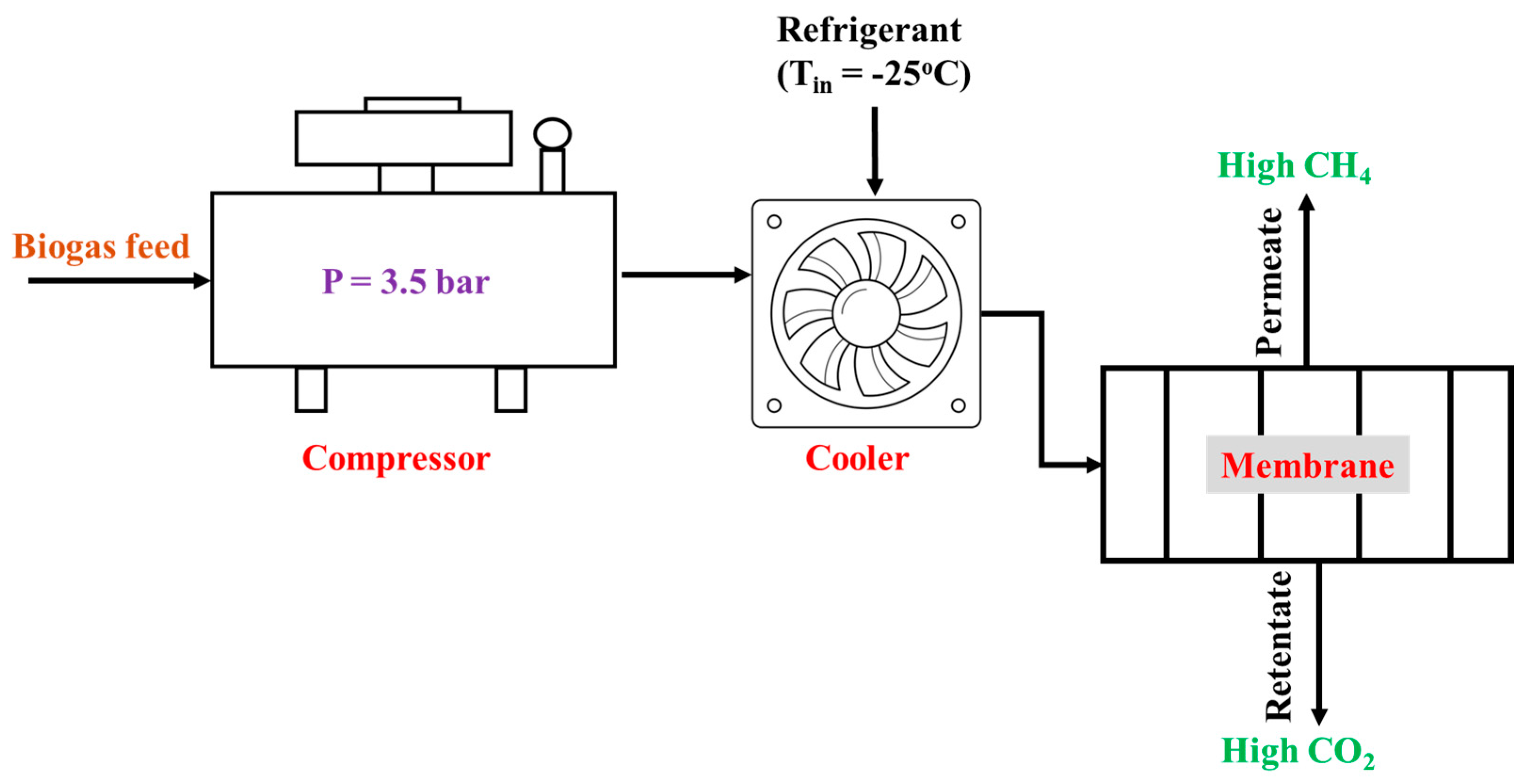
2. Purification of Biogas from POME
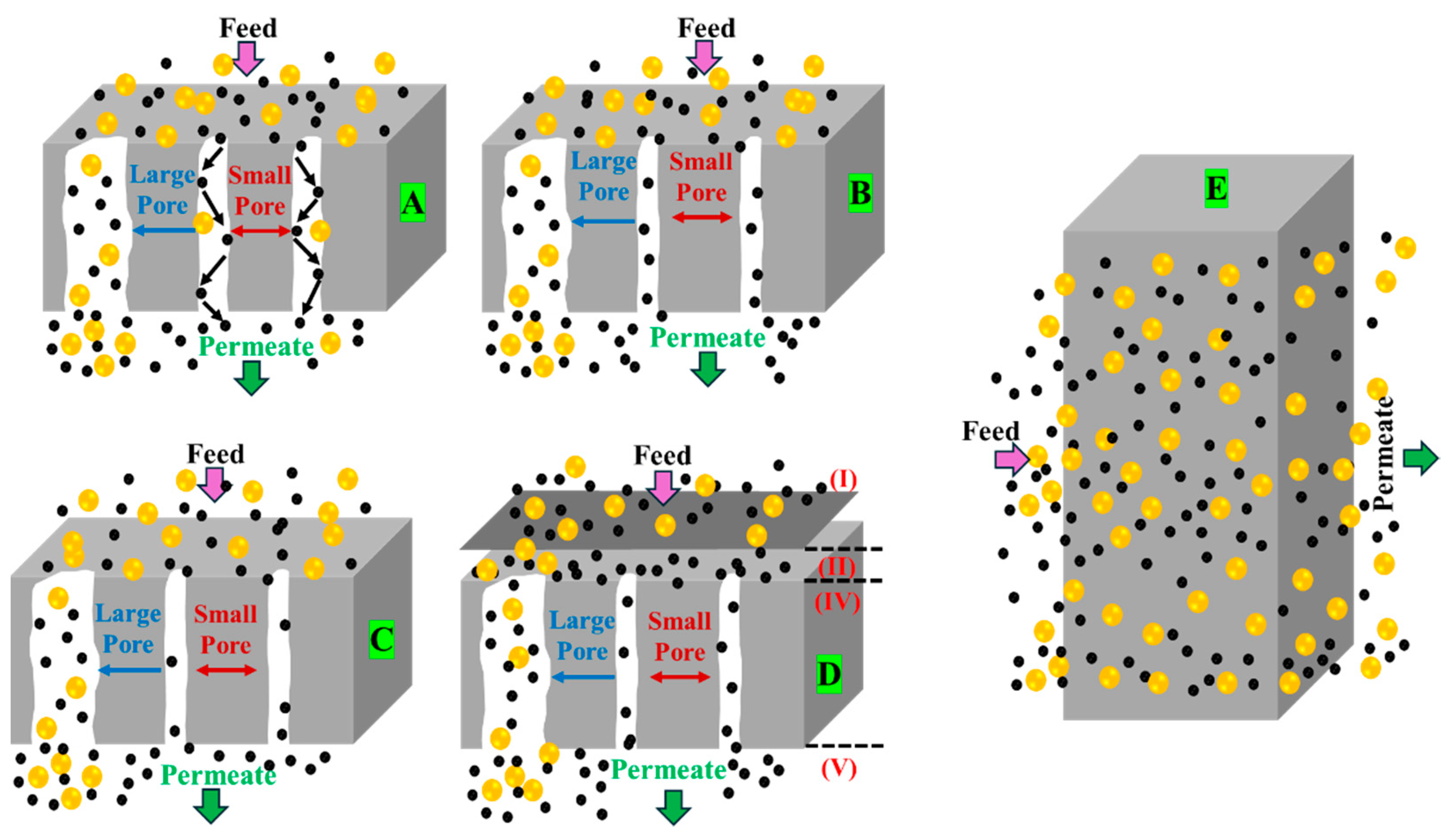
2.1. Biogas Purification Mechanism Using Membranes
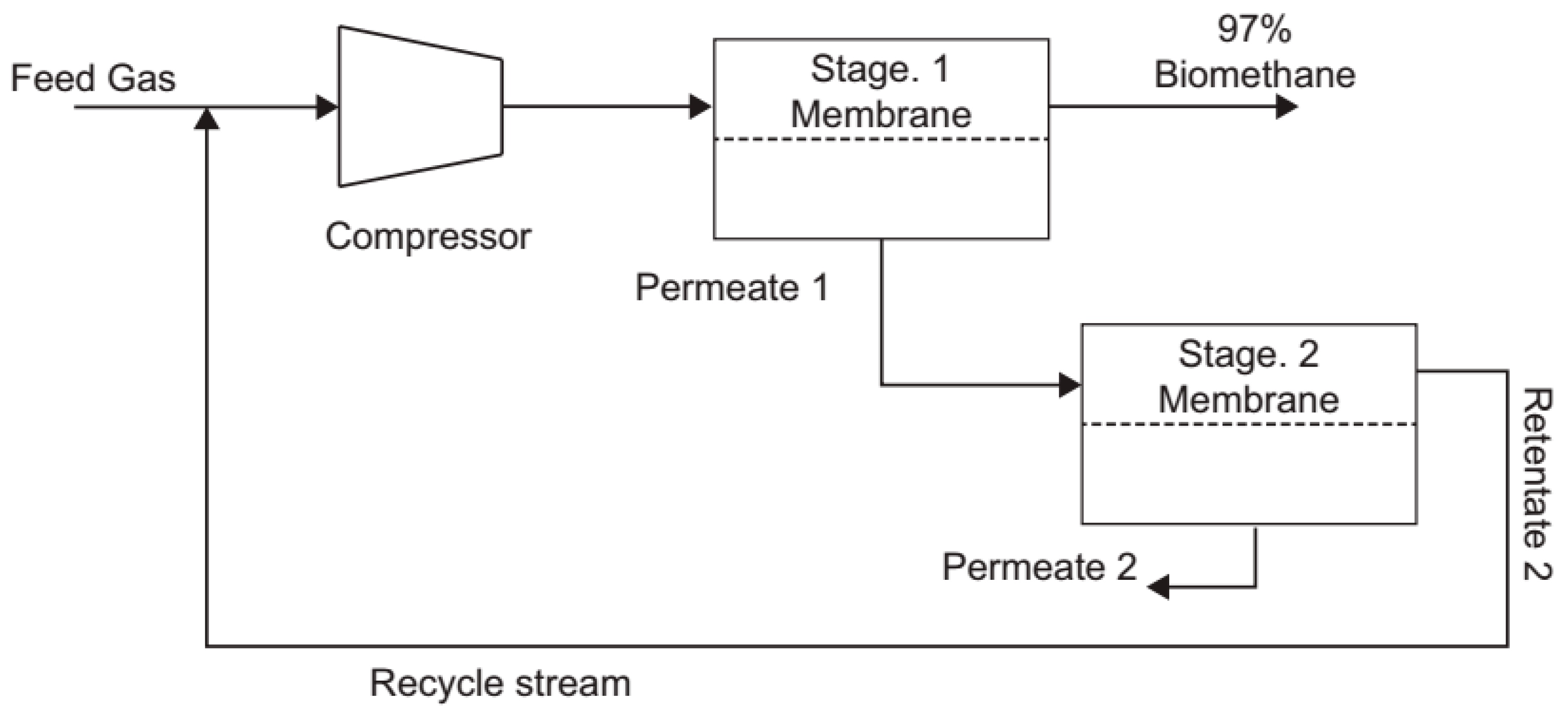
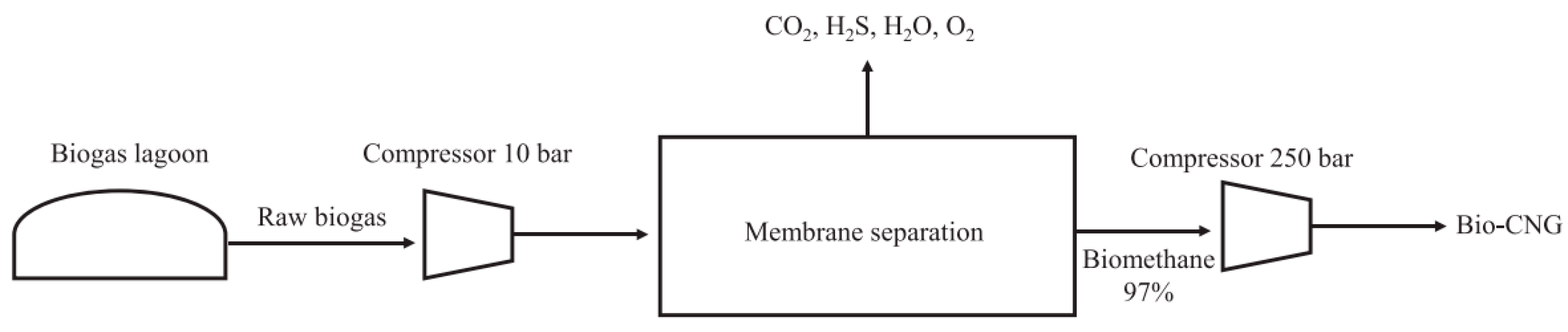
2.2. Study on the Use of Membranes for Biogas Purification from POME
3. Hybrid Membrane Systems Enhance POME Treatment Efficiency and Biogas Production
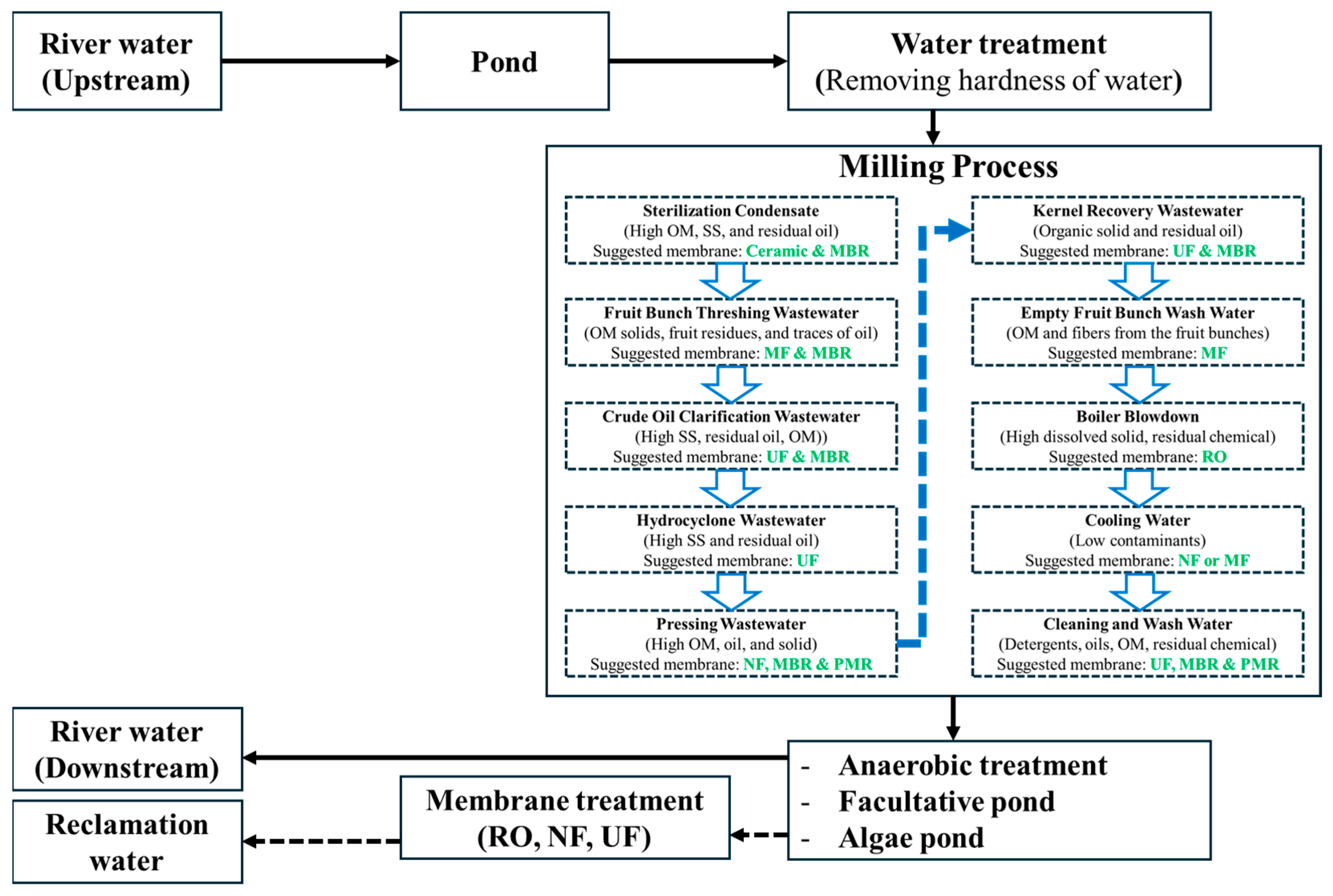
4. Membrane Technology for Water Reclamation from POME
| POME | Pretreatment | Type of Membrane/Operation Parameters/Operation Mode | Performance Efficiency | Cleaning Frequency (Hours) | Reuse Application | Refs. |
|---|---|---|---|---|---|---|
| POME: COD = 50,000 mg/L, TDS = 20,500 mg/L, turbidity = 11,000 NTU, and oil and grease = 4000 mg/L | Coagulation and flocculation | UF/pressure 2 bar and RO/pressure 45 bar/pilot plant | COD = 88 mg/L, TDS = 130 mg/L, turbidity, NTU = 0.02. Color, odor, turbidity, and oil and grease were completely removed at a pH of 6.63 | 24 | Drinking water set by USEPA | [85] |
| Aerobic digester POME with dilution: COD = 152 mg/L, TDS = 394.68 mg/L, and turbidity = 18.1 NTU | No applications | NF (NF270) with a water permeability coefficient of 21.18 L/m2/h, and RO (XLE and BW30) with water permeability coefficients of 14.15 L/m2/h and 6.54 L/m2/h, respectively/bench scale | NF270: COD = 8 mg/L, TDS = 24 mg/L. XLE: COD = 5 mg/L, TDS = 21.1 mg/L BW30: COD = 5 mg/L, TDS = 21.5 mg/L | 6 | Boiler feed water set by USEPA | [84] |
| POME: COD = 43,155 mg/L, SS = 18,975 mg/L, oil and grease = 3172 mg/L | Natural coagulant, latex adsorption and activated carbon | UF (ceramic and PVDF)/pilot plant | Ceramic membrane: COD, SS, and oil and grease = 5.767, 0.27, and 1.22 mg/L, respectively. PVDF: COD = 258 mg/L, SS and oil and grease are completely removed. | No mention | Drinking water set by USEPA | [118] |
| BPOME: COD = 1387 mg/L, TDS = 970 mg/L, turbidity NTU = 840 | PAC adsorption | MF/0.1 µm, 40 kPa/bench scale | COD = 92 mg/L, TDS = 760 mg/L, turbidity NTU = 5 | 1 | Irrigation water | [119] |
| BPOME: COD = 1387 mg/L, TDS = 970 mg/L, and turbidity NTU = 840 | PAC adsorption | UF (polyethersulfone material), effective filtration area: 0.1 m2/bench scale | COD = 4 mg/L, TDS = 380 mg/L, and turbidity NTU < 1 | 1 | Boiler feed water set by USEPA | [120] |
| POME: BOD = 2700 mg/L turbidity NTU = 8124 SS = 5709 mg/L | Coagulation and flocculation | UF/5 bar and RO/30 bar/pilot plant | UF: BOD = 390 mg/L, turbidity NTU = 1.08, SS = 505.74 mg/L RO: BOD = 30 mg/L, turbidity NTU = 0.05, SS = 198.17 mg/L | 1 | WHO water reuse standard | [121] |
| POME: COD = 26,107 mg/L, BOD = 15,800 mg/L, turbidity NTU = 10,563 | Coagulation, sedimentation, and adsorption | UF (0.5–1 µm, 0–7 bar) and TFC-type tubular RO (0–60 bar)/pilot plant | COD = 314 mg/L; BOD = 91 mg/L; yurbidity NTU = 0.81 | No mention | Boiler feed water | [107] |
| POME: Oil and grease = 1077–7582 mg/L | Biological treatment | UF (2 bar) and RO (13 bar)/pilot scale | Completely remove oil and grease | No mention | Boiler feed water | [108] |
| POME: COD = 75,000 mg/L, BOD = 27,000 mg/L, SS = 50,000 mg/L | Biological treatment | UF (2 bar) and RO (13 bar)/pilot scale | The effluents COD, BOD, and SS were not detected. | No mention | Boiler feed water | [102] |
| POME at the point before discharging to river COD = 170.8 mg/L, BOD = 97 mg/L, SS = 43 mg/L, turbidity NTU = 17.6 | No application | UF (PEC and RC)/bench scale | COD = 48 mg/L, BOD = 21.8 mg/L, SS < 25 mg/L, turbidity NTU = 0.72 | No mention | Recycling of mill follows WHO water reuse standards. | [122] |
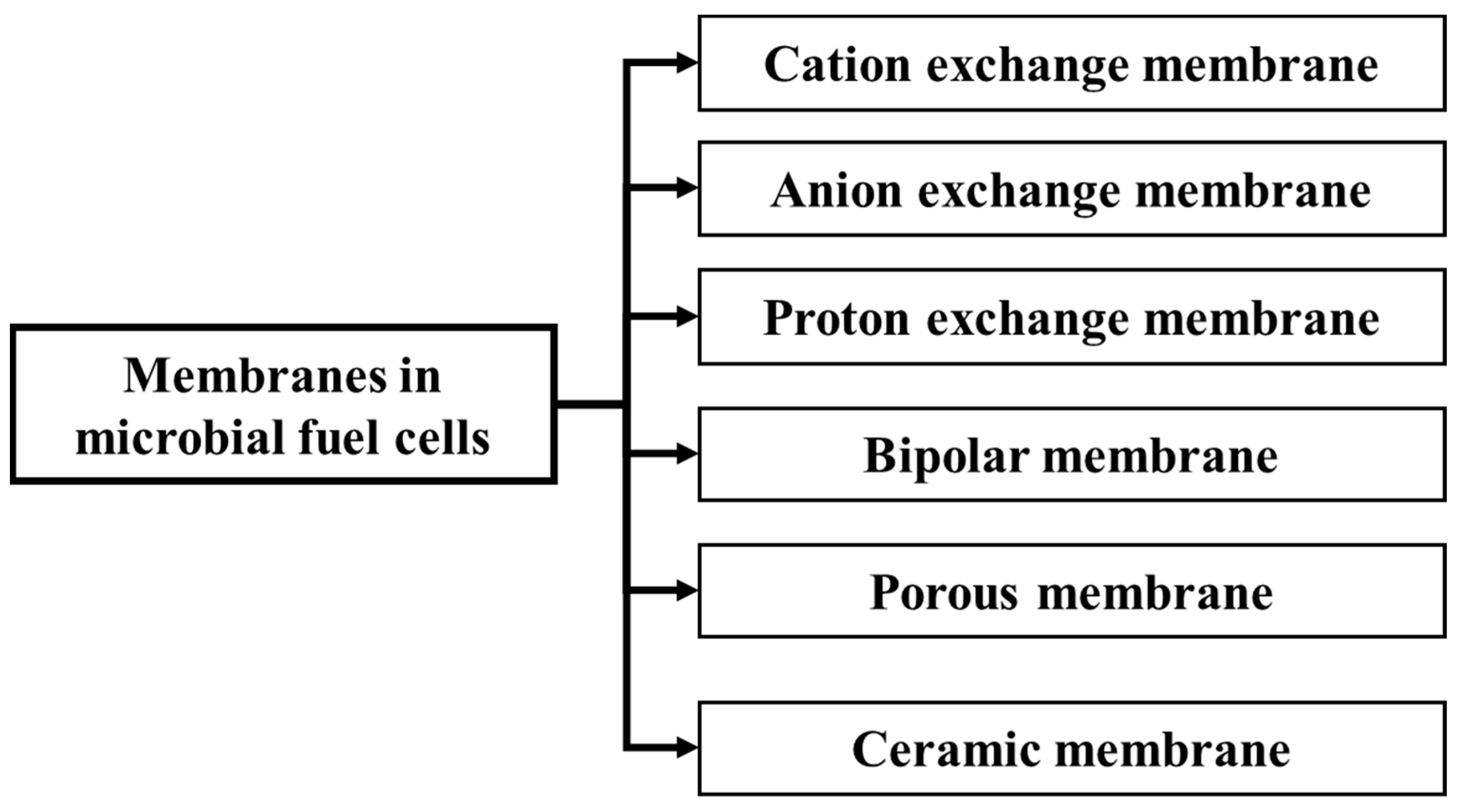
5. Membrane Technology in MFCs for Electricity Recovery from POME
| Type of POME | Type of MFCs | Anode Material | Cathode Material | Membrane | Power Density/Volumetric Power Density/Voltage | Refs |
|---|---|---|---|---|---|---|
| POME + anaerobic sludge (COD = 34,180 mg/L) | Single-chamber air cathode | Carbon brush | PACF | PEM | 1667 mWm−3 | [142] |
| POME + anaerobic sludge (COD = 60,600 mg/L) | Two-compartment MFC | PACF | PACF | PEM | 45 mWm−2/304 mWm−3 | [140] |
| POME (COD = 589 mg/L) | Two-compartment MFC | Carbon paper | Carbon paper | PEM | 0.444 V | [147] |
| POME (COD = 68,360 mg/L) | Air cathode, single chamber | Carbon brush | PACF | PEM | 1236 mWm−3 | [141] |
| POME (COD = 98,000 mg/L) | Two-compartment MFC | Carbon rod | Carbon rod | CEM | 3.21 mWm−2 | [148] |
| POME from anaerobic tank (COD = 1000 mg/L) | Tubular configuration with an air cathode | Carbon brush + PDDMAC and PTFE | Carbon cloth with activate carbon | AEM | 180 mWm−2 | [144] |
| POME (COD = 66,133 mg/L) | Air cathode double-chambered MFC | Pretreated carbon cloth | Pretreated carbon cloth | CEM | 38.38 mWm−2 | [146] |
| Diluted POME (COD = 1000 mg/L) | Two cylindrical compartments | PACF | PACF | PEM | 22 mWm−2 | [145] |
| POME (COD = 49,590 mg/L) | Two-compartment MFC | Carbon electrode | Carbon electrode | CEM | 625 mV | [149] |
6. Sustainability of POME Valuable Resources, Challenging, and Membrane Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Low, S.S.; Bong, K.X.; Mubashir, M.; Cheng, C.K.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Munawaroh, H.S.H.; Show, P.L. Microalgae cultivation in palm oil mill effluent (POME) treatment and biofuel production. Sustainability 2021, 13, 3247. [Google Scholar] [CrossRef]
- Hadiyanto, M.C.; Soetrisnanto, D.; Christwardhana, M. Phytoremediations of palm oil mill effluent (POME) by using aquatic plants and microalgae for biomass production. J. Environ. Sci. Technol. 2013, 6, 79–90. [Google Scholar]
- Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm oil mill effluent treatment processes—A review. Processes 2021, 9, 739. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Abdul Wahid, M. Pollutant in palm oil production process. J. Air Waste Manag. Assoc. 2015, 65, 773–781. [Google Scholar] [CrossRef]
- Ahmad, A.; Ghufran, R.; Wahid, Z.A. Role of calcium oxide in sludge granulation and methanogenesis for the treatment of palm oil mill effluent using UASB reactor. J. Hazard. Mater. 2011, 198, 40–48. [Google Scholar] [CrossRef]
- Abdurahman, N.; Rosli, Y.; Azhari, N. Development of a membrane anaerobic system (MAS) for palm oil mill effluent (POME) treatment. Desalination 2011, 266, 208–212. [Google Scholar] [CrossRef]
- Hayawin, Z.N.; Ibrahim, M.; Kamarudin, H.; Norfaizah, J.; Ropandi, M.; Astimar, A.; Abd-Aziz, S. Production of a bioadsorbent from oil palm kernel shell, and application for pollutants and colour removal in palm oil mill effluent final discharge. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 022045. [Google Scholar]
- Karno, R.; Arisoesilaningsih, E.; Mustafa, I.; Siswanto, D. Physicochemical Properties of Wastewater from Palm Oil Mill Secondary Effluent (POMSE) for Water Evaluation Quality. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2024; p. 01039. [Google Scholar]
- Poh, P.E.; Yong, W.-J.; Chong, M.F. Palm oil mill effluent (POME) characteristic in high crop season and the applicability of high-rate anaerobic bioreactors for the treatment of POME. Ind. Eng. Chem. Res. 2010, 49, 11732–11740. [Google Scholar] [CrossRef]
- Nasrin, A.; Raman, A.A.A.; Sukiran, M.A.; Bukhari, N.A.; Buthiyappan, A.; Subramaniam, V.; AZIZ, A.A.; Loh, S. Renewable energy and greenhouse gases emission reduction potential of biogas from palm oil mill effluent. J. Oil Palm Res. 2024, 36, 456–468. [Google Scholar]
- Foong, S.Z.; Chong, M.F.; Ng, D.K. Strategies to promote biogas generation and utilisation from palm oil mill effluent. Process Integr. Optim. Sustain. 2021, 5, 175–191. [Google Scholar] [CrossRef]
- Azmi, N.S.; Yunos, K.F.M. Wastewater treatment of palm oil mill effluent (POME) by ultrafiltration membrane separation technique coupled with adsorption treatment as pre-treatment. Agric. Agric. Sci. Procedia 2014, 2, 257–264. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P.; Sopian, K. Production of biogas and performance evaluation of existing treatment processes in palm oil mill effluent (POME). Renew. Sustain. Energy Rev. 2015, 42, 1260–1278. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Rezania, S.; Khademi, T.; Kumar, A. Palm oil mill effluent as an environmental pollutant. Palm Oil 2018, 13, 13–28. [Google Scholar]
- Nmaduka, N.; Obioma, N.; Victor, A.; Chukwudi, O.; Juliet, O. Impact of palm oil mill effluent (POME) contamination on soil enzyme activities and physicochemical properties. Res. J. Environ. Toxicol. 2018, 12, 34–41. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Hamzah, S.; Harun, M.H.C.; Ali, A.; Rasit, N.; Awang, M.; Rahman, W.R.W.A.; Azmi, A.A.A.R.; Habib, A.A.; Zahid, M.S.A. Integration of copperas and calcium hydroxide as a chemical coagulant and coagulant aid for efficient treatment of palm oil mill effluent. Chemosphere 2021, 281, 130873. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Ahmad, A.; Rahim, M.H.A.; Sakinah, M. Biohydrogen production from palm oil mill effluent using immobilized Clostridium butyricum EB6 in polyethylene glycol. Process Biochem. 2013, 48, 294–298. [Google Scholar] [CrossRef]
- Shakirah, H.; Zulilah, Z.; Aniyyah, M.; Nabihah, A.; Radhiah, G. Extraction of polyhydroxyakanoate (PHA) from palm oil mill effluent (POME) using chemical solvent extraction. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; p. 012015. [Google Scholar]
- Singh, L.; Siddiqui, M.F.; Ahmad, A.; Rahim, M.H.A.; Sakinah, M.; Wahid, Z.A. Application of polyethylene glycol immobilized Clostridium sp. LS2 for continuous hydrogen production from palm oil mill effluent in upflow anaerobic sludge blanket reactor. Biochem. Eng. J. 2013, 70, 158–165. [Google Scholar] [CrossRef]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Kolade Amosa, M.; Saedi Jami, M.; Aremu Muyibi, S.; Alkhatib, R.; Noraini Jimat, D. Zero liquid discharge and water conservation through water reclamation & reuse of Biotreated Palm Oil Mill Effluent: A review. Int. J. Acad. Res. 2013, 5, 170–183. [Google Scholar]
- Wulandari, W.; Senda, S. Analysis of biogas production from palm oil mill effluent at different feed flow rates in biogas plant Sei Pagar Riau. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; p. 012006. [Google Scholar]
- Harsono, S.S.; Grundmann, P.; Soebronto, S. Anaerobic treatment of palm oil mill effluents: Potential contribution to net energy yield and reduction of greenhouse gas emissions from biodiesel production. J. Clean. Prod. 2014, 64, 619–627. [Google Scholar] [CrossRef]
- Matinja, A.I.; Zain, N.A.M.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA-alginate-sulfate beads. Renew. Energy 2019, 135, 1178–1185. [Google Scholar] [CrossRef]
- Suwanno, S.; Rakkan, T.; Yunu, T.; Paichid, N.; Kimtun, P.; Prasertsan, P.; Sangkharak, K. The production of biodiesel using residual oil from palm oil mill effluent and crude lipase from oil palm fruit as an alternative substrate and catalyst. Fuel 2017, 195, 82–87. [Google Scholar] [CrossRef]
- Rakkan, T.; Suwanno, S.; Paichid, N.; Yunu, T.; Klomklao, S.; Sangkharak, K. Optimized synthesis method for transesterification of residual oil from palm oil mill effluent and lipase from Pacific white shrimp (Litopenaeus vannamei) hepatopancreas to environmentally friendly biodiesel. Fuel 2017, 209, 309–314. [Google Scholar] [CrossRef]
- Mahdi, M.Z.; Titisari, Y.N.; Hadiyanto, H.; Christwardana, M. Evaluation Of Spirulina, Nannochloropsis, and Chlorella Micro-algae Growth in Palm Oil Mill Effluent (POME) Medium with Variation of Medium Types and Time Adding Nutrient. J. Bioresour. Environ. Sci. 2022, 1, 27–32. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Djarot, I.N.; Boelen, P.; Hadiyanto; Heeres, H. J. Co-cultivation of microalgae growing on palm oil mill effluent under outdoor condition for lipid production. Environ. Pollut. Bioavailab. 2022, 34, 537–548. [Google Scholar] [CrossRef]
- Imam, S.S.; Sani, S.; Mujahid, M.; Adnan, R. Valuable resources recovery from palm oil mill effluent (POME): A short review on sustainable wealth reclamation. Waste Manag. Bull. 2025, 3, 1–16. [Google Scholar] [CrossRef]
- Hanafiah, M.M.; Ali, M.Y.M.; Aziz, N.I.H.A.; John, A. Biogas production from agrowaste and effluents. Acta Chem. Malays. (ACMY) 2017, 1, 13–15. [Google Scholar] [CrossRef]
- Kusrini, E.; Lukita, M.; Gozan, M.; Susanto, B.H.; Widodo, T.W.; Nasution, D.A.; Wu, S.; Rahman, A.; Siregar, Y.D.I. Biogas from palm oil mill effluent: Characterization and removal of CO2 using modified clinoptilolite zeolites in a fixed-bed column. Int. J. Technol. 2016, 7, 625–634. [Google Scholar] [CrossRef]
- Domrongpokkaphan, V.; Phalakornkule, C.; Khemkhao, M. In-situ methane enrichment of biogas from anaerobic digestion of palm oil mill effluent by addition of zero valent iron (ZVI). Int. J. Hydrogen Energy 2021, 46, 30976–30987. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kumar, J.; Vu, M.T.; Mohammed, J.A.; Pathak, N.; Commault, A.S.; Sutherland, D.; Zdarta, J.; Tyagi, V.K.; Nghiem, L.D. Biomethane production from anaerobic co-digestion at wastewater treatment plants: A critical review on development and innovations in biogas upgrading techniques. Sci. Total Environ. 2021, 765, 142753. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Jahim, J.M.; Abdul, P.M. Pretreatment conditions of palm oil mill effluent (POME) for thermophilic biohydrogen production by mixed culture. Int. J. Hydrogen Energy 2017, 42, 27512–27522. [Google Scholar] [CrossRef]
- Naha, A.; Debroy, R.; Sharma, D.; Shah, M.P.; Nath, S. Microbial fuel cell: A state-of-the-art and revolutionizing technology for efficient energy recovery. Clean. Circ. Bioeconomy 2023, 5, 100050. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, S.Y.; Lee, K.B.; Nam, S.C. Simultaneous removal of CO2 and H2S from biogas by blending amine absorbents: A performance comparison study. Energy Fuels 2020, 34, 1992–2000. [Google Scholar] [CrossRef]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Devaisy, S.; Kandasamy, J.; Nguyen, T.V.; Ratnaweera, H.; Vigneswaran, S. Membranes in Water Reclamation: Treatment, Reuse and Concentrate Management. Membranes 2023, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Rahimalimamaghani, A.; Ramezani, R.; Tanaka, D.A.P.; Gallucci, F. Carbon molecular sieve membranes for selective CO2/CH4 and CO2/N2 separation: Experimental study, optimal process design, and economic analysis. Ind. Eng. Chem. Res. 2023, 62, 19116–19132. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Xi, Y. PVDF-modified Nafion membrane for improved performance of MFC. Membranes 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- del Rosario Rodero, M.; Muñoz, R.; González-Sánchez, A.; Ruiz, H.A.; Quijano, G. Membrane materials for biogas purification and upgrading: Fundamentals, recent advances and challenges. J. Environ. Chem. Eng. 2024, 12, 114106. [Google Scholar] [CrossRef]
- Spillman, R.W. Economics of gas separation membranes. Chem. Eng. Prog. 1989, 85, 41–62. [Google Scholar]
- Yampolskii, Y. Polymeric gas separation membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Gallucci, F. Modeling of membrane reactors. In Current Trends and Future Developments on (Bio) Membranes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 315–335. [Google Scholar]
- Gitis, V.; Rothenberg, G. Ceramic Membranes: New Opportunities and Practical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Garcia-Fayos, J.; Serra, J.M.; Luiten-Olieman, M.W.; Meulenberg, W.A. Gas separation ceramic membranes. In Advanced Ceramics for Energy Conversion and Storage; Elsevier: Amsterdam, The Netherlands, 2020; pp. 321–385. [Google Scholar]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Ferrari, M.-C. Polymer membranes for sustainable gas separation. In Sustainable Nanoscale Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 265–296. [Google Scholar]
- De Haart, L.; Spiller, M. Fuel Cells–Solid Oxide Fuel Cells|Gas Distribution. In Encyclopedia of Electrochemical Power Sources, 1st ed.; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sato, S.; Nagai, K. Synthetic polymer-based membranes for acidic gas removal. In Synthetic Polymeric Membranes for Advanced Water Treatment, Gas Separation, and Energy Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–190. [Google Scholar]
- Seo, S.; Kim, T. Gas transport mechanisms through gas-permeable membranes in microfluidics: A perspective. Biomicrofluidics 2023, 17, 061301. [Google Scholar] [CrossRef]
- Haider, S.; Lindbråthen, A.; Hägg, M.-B. Techno-economical evaluation of membrane based biogas upgrading system: A comparison between polymeric membrane and carbon membrane technology. Green Energy Environ. 2016, 1, 222–234. [Google Scholar] [CrossRef]
- Aguilloso, G.; Arpia, K.; Khan, M.; Sapico, Z.A.; Lopez, E.C.R. Recent Advances in Membrane Technologies for Biogas Upgrading. Eng. Proc. 2024, 67, 57. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Izah, S.C. A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renew. Sustain. Energy Rev. 2017, 70, 242–253. [Google Scholar] [CrossRef]
- Bachmann, N.; la Cour Jansen, J.; Bochmann, G.; Montpart, N. Sustainable Biogas Production in Municipal Wastewater Treatment Plants; IEA Bioenergy: Massongex, Switzerland, 2015; Volume 20. [Google Scholar]
- Rashid, M.; Shakib, N.; Rahman, T. Biogas production from POME by optimum level of inputs. Smart Grid Renew. Energy 2019, 10, 203–212. [Google Scholar] [CrossRef]
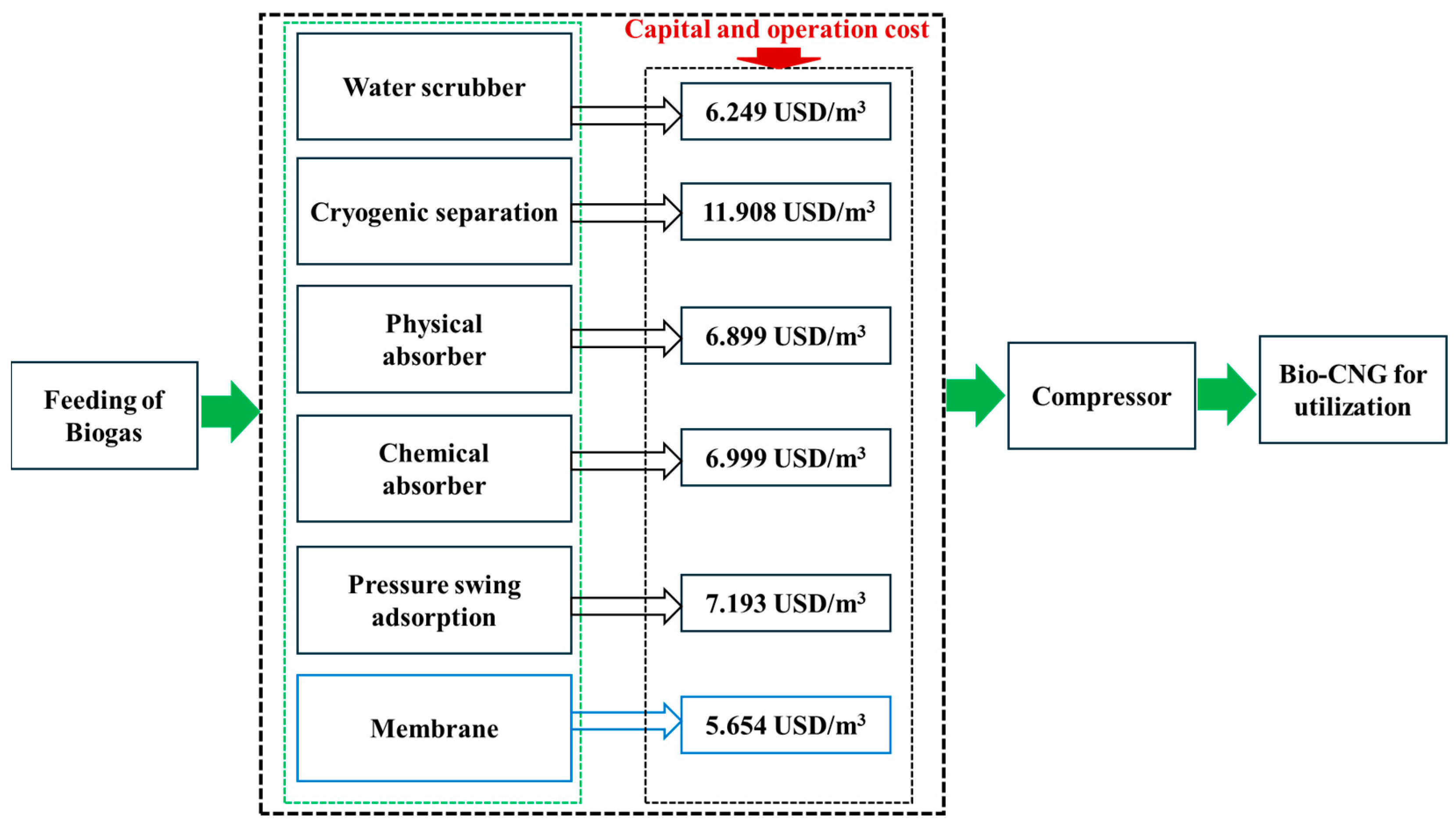
- Febijanto, I.; Hermawan, E.; Adiarso, A.; Mustafa, A.; Rahardjo, P.; Wijono, R.A.; Sudjadi, U. Techno-enviro-economic assessment of bio-CNG derived from Palm Oil Mill Effluent (POME) for public transportation in Pekanbaru City. Renew. Energy Focus 2024, 49, 100569. [Google Scholar] [CrossRef]
- Ng, D.K.; Wong, S.L.; Andiappan, V.; Ng, L.Y. Mathematical optimisation for sustainable bio-methane (Bio-CH4) production from palm oil mill effluent (POME). Energy 2023, 265, 126211. [Google Scholar] [CrossRef]
- Mohtar, A.; Ho, W.S.; Idris, A.M.; Hashim, H.; Lim, J.S.; Liew, P.Y.; Teck, G.L.H.; Ho, C.S. Palm Oil Mill Effluent (POME) biogas techno-economic analysis for utilisation as bio compressed natural gas. Chem. Eng. Trans. 2018, 63, 265–270. [Google Scholar]
- Nasrin, A.; Raman, A.; Loh, S.; Sukiran, M.; Bukhari, N.; Aziz, A.; Saad, M.; Kamarudin, M.; Buthiyappan, A. Characteristics and techno-economic potential of bio-compressed natural gas (Bio-CNG) from palm oil mill effluent (POME). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 022060. [Google Scholar]
- Park, Y.G. Study for the bio-CNG recovery of methane gas in the anaerobic co-digestion using Malaysian POME (palm oil mill effluent). Biotechnol. Bioprocess Eng. 2021, 26, 435–446. [Google Scholar] [CrossRef]
- Norddahl, B.; Roda-Serrat, M.C.; Errico, M.; Christensen, K.V. Membrane-based technology for methane separation from biogas. In Emerging Technologies and Biological Systems for Biogas Upgrading; Elsevier: Amsterdam, The Netherlands, 2021; pp. 117–157. [Google Scholar]
- Wahyudhie, S.N.; Lusman, S.R.; Widyanto, A.R.; Widiastuti, N.; Gunawan, T.; Fansuri, H.; Kusumawati, Y.; Akhlus, S.; Hamzah, A.; Pramata, A.D. Performance of polysulfone membrane for biogas upgrading. AIP Conf. Proc. 2024, 3071, 020010. [Google Scholar]
- Junaidi, M.U.M.; Leo, C.; Ahmad, A. Biogas upgrading system: Techno-economic analysis of membrane separation process implementation. Int. J. Appl. Eng. Res. 2016, 11, 973–4562. [Google Scholar]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H.; Hayder, G.; Norasyikin, I. A hybrid ultrasonic membrane anaerobic system (UMAS) development for palm oil mill effluent (POME) treatment. Processes 2023, 11, 2477. [Google Scholar] [CrossRef]
- Abdurahman, N.; Rosli, Y.; Azhari, N.; Bari, H.A. The potential of ultrasonic membrane anaerobic systems in treating slaughterhouse wastewater. J. Water Reuse Desalin. 2015, 5, 293–300. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chai, X.; Fujii, N. Ultrasound enhanced cross-flow membrane filtration. Sep. Purif. Technol. 1999, 17, 31–40. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Noor, M. Treatment of palm oil mill effluent (POME) with the membrane anaerobic system (MAS). Water Sci. Technol. 1999, 39, 159–163. [Google Scholar] [CrossRef]
- Nour, A.H.; Nour, A.H.; Pahang-UMP, P. Production of Biogas and Performance Evaluation of Ultrasonic Membrane Anaerobic System (UMAS) for Palm Oil Mill Effluent Treatment (POME); INTECH: Shah Alam, Malaysia, 2017; pp. 229–243. [Google Scholar]
- Bokhary, A.; Leitch, M.; Liao, B. Effect of organic loading rates on the membrane performance of a thermophilic submerged anaerobic membrane bioreactor for primary sludge treatment from a pulp and paper mill. J. Environ. Chem. Eng. 2022, 10, 107523. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Che Man, H.; Isma Idris, A.; Faezah Yunos, K.; Zainal Abidin, Z. Treatment of palm oil mill effluent using membrane bioreactor: Novel processes and their major drawbacks. Water 2018, 10, 1165. [Google Scholar] [CrossRef]
- Ziegler, A.S.; McIlroy, S.J.; Larsen, P.; Albertsen, M.; Hansen, A.A.; Heinen, N.; Nielsen, P.H. Dynamics of the fouling layer microbial community in a membrane bioreactor. PLoS ONE 2016, 11, e0158811. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, A.; Ujang, Z.; Salim, M.; Olsson, G. The effect of mixed liquor suspended solids (MLSS) on biofouling in a hybrid membrane bioreactor for the treatment of high concentration organic wastewater. Water Sci. Technol. 2011, 63, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Irvan, I.; Trisakti, B.; Sidabutar, R.; Lubis, A.; Cahyani, S.; Zusri, A.; Daimon, H. Combination of CSTR and membrane process in treating palm oil mill effluent (POME). AIP Conf. Proc. 2020, 2197, 110003. [Google Scholar]
- Ng, C.A.; Wong, L.Y.; Chai, H.Y.; Bashir, M.J.; Ho, C.-D.; Nisar, H.; Lo, P.K. Investigation on the performance of hybrid anaerobic membrane bioreactors for fouling control and biogas production in palm oil mill effluent treatment. Water Sci. Technol. 2017, 76, 1389–1398. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Lee, D.-J.; Tung, K.-L.; Jin, P.; Wang, J.; Wu, Y. Challenges in biogas production from anaerobic membrane bioreactors. Renew. Energy 2016, 98, 120–134. [Google Scholar] [CrossRef]
- Adriani, L.; Trisakti, B.; Irvan, I.; Sidabutar, R.; Manurung, J.; Passa, R.; Simbolon, D.; Barasa, J. Design and fabrication of UASB-HCPB reactor assisted UF membrane for POME treatment to produce biogas. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; p. 012036. [Google Scholar]
- Othman, M.R.; Hassan, M.A.; Shirai, Y.; Baharuddin, A.S.; Ali, A.A.M.; Idris, J. Treatment of effluents from palm oil mill process to achieve river water quality for reuse as recycled water in a zero emission system. J. Clean. Prod. 2014, 67, 58–61. [Google Scholar] [CrossRef]
- Samavati, Z.; Goh, P.S.; Ismail, A.F.; Lau, W.J.; Samavati, A.; Ng, B.C.; Abdullah, M.S. Advancements in membrane technology for efficient POME treatment: A comprehensive review and future perspectives. J. Environ. Sci. 2025, 155, 730–761. [Google Scholar] [CrossRef]
- Ghani, M.S.H.; Haan, T.Y.; Lun, A.W.; Mohammad, A.W.; Ngteni, R.; Yusof, K.M.M. Fouling assessment of tertiary palm oil mill effluent (POME) membrane treatment for water reclamation. J. Water Reuse Desalin. 2018, 8, 412–423. [Google Scholar] [CrossRef]
- Ahmad, A.; Chong, M.; Bhatia, S.; Ismail, S. Drinking water reclamation from palm oil mill effluent (POME) using membrane technology. Desalination 2006, 191, 35–44. [Google Scholar] [CrossRef]
- Falahi, O.A.A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ewadh, H.M.; Kurniawan, S.B.; Imron, M.F. Occurrence of pharmaceuticals and personal care products in domestic wastewater, available treatment technologies, and potential treatment using constructed wetland: A review. Process Saf. Environ. Prot. 2022, 168, 1067–1088. [Google Scholar] [CrossRef]
- Wang, Y.; Serventi, L. Sustainability of dairy and soy processing: A review on wastewater recycling. J. Clean. Prod. 2019, 237, 117821. [Google Scholar] [CrossRef]
- Ng, A.N.; Kim, A.S. A mini-review of modeling studies on membrane bioreactor (MBR) treatment for municipal wastewaters. Desalination 2007, 212, 261–281. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Yu, H.; Zou, D. Pressure–driven membrane filtration technology for terminal control of organic DBPs: A review. Sci. Total Environ. 2023, 904, 166751. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Gao, X.; Ma, Z.; Wang, X.; Wei, Y.; Gao, C. A review of graphene-based separation membrane: Materials, characteristics, preparation and applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and chemical cleaning of microfiltration membranes: A mini-review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Goh, P.S.; Ismail, A.F.; Abdullah, M.S. A comprehensive review of recent advances in nanofiltration membranes for heavy metal removal from wastewater. Chem. Eng. Res. Des. 2023, 189, 530–571. [Google Scholar] [CrossRef]
- Roth, C.D.; Poh, S.C.; Vuong, D.X. Customization and multistage nanofiltration applications for potable water, treatment, and reuse. In Nanotechnology Applications for Clean Water; Elsevier: Amsterdam, The Netherlands, 2009; pp. 107–114. [Google Scholar]
- Matin, A.; Laoui, T.; Falath, W.; Farooque, M. Fouling control in reverse osmosis for water desalination & reuse: Current practices & emerging environment-friendly technologies. Sci. Total Environ. 2021, 765, 142721. [Google Scholar]
- Nafi, A.W.; Taseidifar, M. Removal of hazardous ions from aqueous solutions: Current methods, with a focus on green ion flotation. J. Environ. Manag. 2022, 319, 115666. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, S.S.; Takriff, M.S.; AL-Rajabi, M.M.; Abdul, P.M.; Gunny, A.A.N.; Silvamany, H.; Jahim, J.M. Water reclamation from palm oil mill effluent (POME): Recent technologies, by-product recovery, and challenges. J. Water Process Eng. 2023, 52, 103488. [Google Scholar] [CrossRef]
- Ahmad, A.; Chong, M.; Bhatia, S. A comparative study on the membrane based palm oil mill effluent (POME) treatment plant. J. Hazard. Mater. 2009, 171, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Zhang, Y.; Zhang, Z. An integrated method for palm oil mill effluent (POME) treatment for achieving zero liquid discharge—A pilot study. J. Clean. Prod. 2015, 95, 148–155. [Google Scholar] [CrossRef]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in MF and UF membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Al Aani, S.; Wright, C.J.; Hilal, N. Investigation of UF membranes fouling and potentials as pre-treatment step in desalination and surface water applications. Desalination 2018, 432, 115–127. [Google Scholar] [CrossRef]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, J.; Wang, Z.; Yang, Z.; Yang, W.; Yin, Z. Defective MOFs-based electrocatalytic self-cleaning membrane for wastewater reclamation: Enhanced antibiotics removal, membrane fouling control and mechanisms. Water Res. 2022, 220, 118635. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination 2003, 157, 87–95. [Google Scholar] [CrossRef]
- Wang, J.; Mahmood, Q.; Qiu, J.-P.; Li, Y.-S.; Chang, Y.-S.; Chi, L.-N.; Li, X.-D. Zero discharge performance of an industrial pilot-scale plant treating palm oil mill effluent. BioMed Res. Int. 2015, 2015, 617861. [Google Scholar] [CrossRef]
- Damayanti, A.; Ujang, Z.; Salim, M. The influenced of PAC, zeolite, and Moringa oleifera as biofouling reducer (BFR) on hybrid membrane bioreactor of palm oil mill effluent (POME). Bioresour. Technol. 2011, 102, 4341–4346. [Google Scholar] [CrossRef]
- Yuniarto, A.; Ujang, Z.; Noor, Z.Z. Performance of bio-fouling reducers in aerobic submerged membrane bioreactor for palm oil mill effluent treatment. J. Teknol. 2008, 2008, 555–566. [Google Scholar]
- Wang, J.; Liu, X. Forward osmosis technology for water treatment: Recent advances and future perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Ibrar, I.; Altaee, A.; Zhou, J.L.; Naji, O.; Khanafer, D. Challenges and potentials of forward osmosis process in the treatment of wastewater. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1339–1383. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.; Kemperman, A.J. Forward osmosis: A critical review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Osman, N.A.; Ujang, F.A.; Roslan, A.M.; Ibrahim, M.F.; Hassan, M.A. The effect of palm oil mill effluent final discharge on the characteristics of Pennisetum purpureum. Sci. Rep. 2020, 10, 6613. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward osmosis application in manufacturing industries: A short review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef]
- Korenak, J.; Basu, S.; Balakrishnan, M.; Hélix-Nielsen, C.; Petrinic, I. Forward osmosis in wastewater treatment processes. Acta Chim. Slov. 2017, 64, 83–94. [Google Scholar] [CrossRef]
- Arifin, H.; Choong, T.S.; Rong, C.K.; Ahmadun, F.l.A.-R.; Abdullah, L.C. Forward Osmosis: Temperature effects by using pome as feed solution. ASEAN J. Chem. Eng. 2015, 15, 31–40. [Google Scholar] [CrossRef]
- Ahmad, A.; Idris, I.; Chan, C.; Ismail, S. Reclamation from palm oil mill effluent using an integrated zero discharge membrane-based process. Pol. J. Chem. Technol. 2015, 17, 49–55. [Google Scholar] [CrossRef]
- Amosa, M.K.; Jami, M.S.; Alkhatib, M.a.F.R.; Majozi, T. Studies on pore blocking mechanism and technical feasibility of a hybrid PAC-MF process for reclamation of irrigation water from biotreated POME. Sep. Sci. Technol. 2016, 51, 2047–2061. [Google Scholar] [CrossRef]
- Amosa, M.K.; Jami, M.S.; Alkhatib, M.a.F.R.; Majozi, T. Technical feasibility study of a low-cost hybrid PAC-UF system for wastewater reclamation and reuse: A focus on feedwater production for low-pressure boilers. Environ. Sci. Pollut. Res. 2016, 23, 22554–22567. [Google Scholar] [CrossRef]
- Azmi, N.S.; Yunos, K.F.M.; Baharuddin, A.S.; Dom, Z.M. The Effect of Operating Parameters on Ultrafiltration and Reverse Osmosis of Palm Oil Mill Effluent for Reclamation and Reuse of Water. BioResources 2013, 8, 76–87. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Yunos, K.F.M.; Naim, M.N.M.; Baharuddin, A.S. Performances of Sandwich Membrane in Reclamation of Water from Final Discharged POME. In Proceedings of the International Conference on Environmental Sustainability and Resource Security, Kuala Lumpur, Malaysia, 5–6 November 2019; pp. 315–321. [Google Scholar]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Feng, C.; Sharma, S.C.D.; Yu, C.-P. Microbial fuel cells for wastewater treatment. In Biotechnologies and Biomimetics for Civil Engineering; Springer: Cham, Switzerland, 2015; pp. 411–437. [Google Scholar]
- Radeef, A.Y.; Najim, A.A. Microbial fuel cell: The renewable and sustainable magical system for wastewater treatment and bioenergy recovery. Energy 360 2024, 1, 100001. [Google Scholar] [CrossRef]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef]
- Ho, Q.N.; Mitsuoka, K.; Yoshida, N. Microbial fuel cell in long-term operation and providing electricity for intermittent aeration to remove contaminants from sewage. Environ. Res. 2024, 259, 119503. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Daud, S.M.; Daud, W.R.W.; Bakar, M.H.A.; Kim, B.H.; Somalu, M.R.; Jahim, J.M.; Muchtar, A.; Ghasemi, M. A comparison of long-term fouling performance by zirconia ceramic filter and cation exchange in microbial fuel cells. Int. Biodeterior. Biodegrad. 2019, 136, 63–70. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and important properties of a membrane with its recent advancement in a microbial fuel cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Jalili, P.; Ala, A.; Nazari, P.; Jalili, B.; Ganji, D.D. A comprehensive review of microbial fuel cells considering materials, methods, structures, and microorganisms. Heliyon 2024, 10, e25439. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Zuo, Y.; Regan, J.M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.; De Los Ríos, A.P.; Salar-García, M.; Ortiz-Martínez, V.; Lozano-Blanco, L.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Wu, H.; Fu, Y.; Guo, C.; Li, Y.; Jiang, N.; Yin, C. Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/acetone wastewater. Bioresour. Technol. 2018, 260, 130–134. [Google Scholar] [CrossRef]
- Zhao, Z.-y.; Yan, H.; Zuo, J.; Tian, Y.-x.; Zillante, G. A critical review of factors affecting the wind power generation industry in China. Renew. Sustain. Energy Rev. 2013, 19, 499–508. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial fuel cell construction features and application for sustainable wastewater treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef]
- Mohan, S.V.; Sravan, J.S.; Butti, S.K.; Krishna, K.V.; Modestra, J.A.; Velvizhi, G.; Kumar, A.N.; Varjani, S.; Pandey, A. Microbial electrochemical technology: Emerging and sustainable platform. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–18. [Google Scholar]
- Dharmalingam, S.; Kugarajah, V.; Sugumar, M. Membranes for microbial fuel cells. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–194. [Google Scholar]
- Baranitharan, E.; Khan, M.R.; Prasad, D.; Salihon, J.B. Bioelectricity generation from palm oil mill effluent in microbial fuel cell using polacrylonitrile carbon felt as electrode. Water Air Soil Pollut. 2013, 224, 1–11. [Google Scholar] [CrossRef]
- Islam, M.A.; Rahman, M.; Yousuf, A.; Cheng, C.K.; Wai, W.C. Performance of Klebsiella oxytoca to generate electricity from POME in microbial fuel cell. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; p. 03004. [Google Scholar]
- Islam, M.A.; Karim, A.; Woon, C.W.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. Augmentation of air cathode microbial fuel cell performance using wild type Klebsiella variicola. RSC Adv. 2017, 7, 4798–4805. [Google Scholar] [CrossRef]
- Jong, B.; Liew, P.; Juri, M.L.; Kim, B.; Mohd Dzomir, A.; Leo, K.; Awang, M. Performance and microbial diversity of palm oil mill effluent microbial fuel cell. Lett. Appl. Microbiol. 2011, 53, 660–667. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Tanaka, F.; Yoshida, N.; Jaafar, J.; Zailani, M.Z.; Ahmad, S.N.A. Preliminary evaluation of electricity recovery from palm oil mill effluent by anion exchange microbial fuel cell. Bioelectrochemistry 2024, 160, 108770. [Google Scholar] [CrossRef] [PubMed]
- Baranitharan, E.; Khan, M.R.; Prasad, D.; Teo, W.F.A.; Tan, G.Y.A.; Jose, R. Effect of biofilm formation on the performance of microbial fuel cell for the treatment of palm oil mill effluent. Bioprocess Biosyst. Eng. 2015, 38, 15–24. [Google Scholar] [CrossRef]
- Alkhair, K.B.; Hassan, O.H.; Mohamed, S.A.S.; Andrew, Y.K.C.; Rahman, Z.A.; Kudin, T.I.T.; Ali, A.M.M.; Yahya, M.Z.A.; Zainal, M.H. Comparative study of microbial fuel cell’s performance using three different electrodes. Malays. J. Anal. Sci. 2018, 22, 499–507. [Google Scholar]
- Hisham, N.S.N.; Zain, S.M.; Jusoh, S.; Anuar, N.; Suja, F.; Ismail, A.; Basri, N.E.A. Microbial fuel cells using different types of wastewater for electricity generation and simultaneously removed pollutant. J. Eng. Sci. Technol. 2013, 8, 316–325. [Google Scholar]
- Jalilluddin, A.M.; Chia-Chay, T.; Abdul-Talib, S. Performance of two-chambered microbial fuel cell (MFC) at different pH anode microenvironment using palm oil mill effluent (POME) as substrate. Appl. Mech. Mater. 2015, 773, 511–519. [Google Scholar] [CrossRef]
- Sumairi, M.S.; Rahman, M.A.A.; Raman, S.A.; Azwan, N.S.; Elham, O.S.J. The Effect of Electrode Number in Electricity Generation by Using Different Type of Wastewater via Microbial Fuel Cell (MFC). Available online: https://ir.uitm.edu.my/id/eprint/50214/1/50214.pdf (accessed on 18 February 2025).
- Chin, M.J.; Poh, P.E.; Tey, B.T.; Chan, E.S.; Chin, K.L. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Tsekouras, G.J.; Deligianni, P.M.; Kanellos, F.D.; Kontargyri, V.T.; Kontaxis, P.A.; Manousakis, N.M.; Elias, C.N. Microbial fuel cell for wastewater treatment as power plant in smart grids: Utopia or reality? Front. Energy Res. 2022, 10, 843768. [Google Scholar] [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Aziz, F.; Bilad, M.R.; Alkahtani, M. A crucial review on the challenges and recent gas membrane development for biogas upgrading. J. Environ. Chem. Eng. 2023, 11, 110235. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies–Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Iovane, P.; Nanna, F.; Ding, Y.; Bikson, B.; Molino, A. Experimental test with polymeric membrane for the biogas purification from CO2 and H2S. Fuel 2014, 135, 352–358. [Google Scholar] [CrossRef]
- Peppers, J.; Li, Y.; Xue, J.; Chen, X.; Alaimo, C.; Wong, L.; Young, T.; Green, P.G.; Jenkins, B.; Zhang, R. Performance analysis of membrane separation for upgrading biogas to biomethane at small scale production sites. Biomass Bioenergy 2019, 128, 105314. [Google Scholar] [CrossRef]
- Zito, P.; Brunetti, A.; Barbieri, G. Advanced membrane-based processes for biogas upgrading. In Membrane Engineering in the Circular Economy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 345–373. [Google Scholar]
- Seong, M.S.; Kong, C.I.; Park, B.R.; Lee, Y.; Na, B.K.; Kim, J.H. Optimization of pilot-scale 3-stage membrane process using asymmetric polysulfone hollow fiber membranes for production of high-purity CH4 and CO2 from crude biogas. Chem. Eng. J. 2020, 384, 123342. [Google Scholar] [CrossRef]
- Žák, M.; Bendová, H.; Friess, K.; Bara, J.E.; Izák, P. Single-step purification of raw biogas to biomethane quality by hollow fiber membranes without any pretreatment–An innovation in biogas upgrading. Sep. Purif. Technol. 2018, 203, 36–40. [Google Scholar] [CrossRef]
- Lim, Y.-G.; Bak, C.-U.; Kim, Y.-D. Comprehensive experimental and theoretical insights into the performance of polysulfone hollow-fiber membrane modules in biogas purification process. Chem. Eng. J. 2022, 433, 134616. [Google Scholar] [CrossRef]
- Dolejš, P.; Poštulka, V.; Sedláková, Z.; Jandová, V.; Vejražka, J.; Esposito, E.; Jansen, J.C.; Izák, P. Simultaneous hydrogen sulphide and carbon dioxide removal from biogas by water–swollen reverse osmosis membrane. Sep. Purif. Technol. 2014, 131, 108–116. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Estrada, J.M.; Lebrero, R.; Muñoz, R. A comparative analysis of biogas upgrading technologies: Photosynthetic vs physical/chemical processes. Algal Res. 2017, 25, 237–243. [Google Scholar] [CrossRef]
- Francisco López, A.; Lago Rodríguez, T.; Faraji Abdolmaleki, S.; Galera Martínez, M.; Bello Bugallo, P.M. From Biogas to Biomethane: An In-Depth Review of Upgrading Technologies That Enhance Sustainability and Reduce Greenhouse Gas Emissions. Appl. Sci. 2024, 14, 2342. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Aryanti, P.T.P.; Harsono, B.; Biantoro, M.F.W.; Romariyo, R.; Putri, T.A.; Hakim, A.N.; Setia, G.A.; Saputra, D.I.; Khoiruddin, K. The role of membrane technology in palm oil mill effluent (POME) decontamination: Current trends and future prospects. J. Environ. Manag. 2025, 374, 124094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xiangli, Q.; Lina, C.; Xiangjun, N.; Zhijian, M.; Zhang, Z. Integration of biological method and membrane technology in treating palm oil mill effluent. J. Environ. Sci. 2008, 20, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Duan, C.; Yue, P.; Li, T. Removal characteristics of dissolved organic matter and membrane fouling in ultrafiltration and reverse osmosis membrane combined processes treating the secondary effluent of wastewater treatment plant. Water Sci. Technol. 2021, 83, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Hanafiah, M.M.; Ali, M.; Anjum, M.; Baki, Z.A.; Mekkey, S.D.; Ullah, S.; Khurshid, S.; Ullah, H.; Arshad, U. Review of the performance and energy requirements of metals modified TiO2 materials based photocatalysis for phenolic compounds degradation: A case of agro-industrial effluent. J. Environ. Chem. Eng. 2024, 12, 112766. [Google Scholar] [CrossRef]
- Özgenç, E. Evaluation of the Spreading Dynamics and Interactions of Lead-Carrier Microplastics Affected by Biofilm: A Mini-Review. Water Air Soil Pollut. 2024, 235, 281. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Zainal, B.S.; Jamadon, N.H.; Yaw, T.C.S.; Abdullah, L.C. Filtration analysis and fouling mechanisms of PVDF membrane for POME treatment. J. Water Reuse Desalin. 2020, 10, 187–199. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly (arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Jaleh, B.; Eslamipanah, M.; Nasri, A.; Sheibani, R.; Kulkarni, S.; Shindalkar, S.; Shinde, A.; Gawande, M.B. Advances in Biomass and Nature-Derived Ceramic Membranes and Their Environmental Applications in Wastewater Treatment. Adv. Eng. Mater. 2024, 26, 2400049. [Google Scholar] [CrossRef]
- Koók, L.; Žitka, J.; Szakács, S.; Rózsenberszki, T.; Otmar, M.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Efficiency, operational stability and biofouling of novel sulfomethylated polystyrene-block-poly (ethylene-ran-butylene)-block-polystyrene cation exchange membrane in microbial fuel cells. Bioresour. Technol. 2021, 333, 125153. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.-P.; Luo, H.-W.; Li, W.-W.; Wang, L.-F.; Yu, H.-Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Pandit, S.; Sonawane, J.M.; Gupta, P.K.; Prasad, R.; Chendake, A.D. Effect of membrane biofouling on the performance of microbial electrochemical cells and mitigation strategies. Bioresour. Technol. Rep. 2021, 15, 100822. [Google Scholar] [CrossRef]



| Parameters (a) | Concentration | Standard Discharge Limits (b) | References |
|---|---|---|---|
| pH | 3.4–5.2 | 5.0–9.0 | [11] |
| BOD | 10,250–43,750 | 20 | [11] |
| COD | 15,000–100,000 | NA | [11] |
| Turbidity, NTU | 17,000 | NA | [12] |
| Color, ADMI | >500 | 100 | [13] |
| Total solids | 40,500–75,000 | 200 | [14] |
| Total volatile solids | 9000–72,000 | NA | [15] |
| Grease and oil | 130–18,000 | 5 | [16] |
| Ammonium–nitrogen | 25–35 | NA | [17] |
| Total nitrogen (TN) | 180–1400 | 150 | [18] |
| Total phosphorous (TP) | 95–120 | NA | [19] |
| Alkalinity | 100–150 | NA | [17] |
| Total carbohydrate | 16,200–20,000 | NA | [17] |
| Pectin | 3400 | NA | [3] |
| Lignin | 4700 | NA | [3] |
| Phenol | 5800 | NA | [20] |
| Carotene | 8 | NA | [3] |
| Potassium (K) | 1281–1928 | NA | [13] |
| Magnesium (Mg) | 254–344 | NA | [13] |
| Calcium (Ca) | 276–405 | NA | [13] |
| Boron (B) | 7–8 | NA | [21] |
| Parameters | Units | Input | Retentate | Permeate | |||
|---|---|---|---|---|---|---|---|
| S-MTR | D-MTR | S-MTR | D-MTR | S-MTR | D-MTR | ||
| Mass Flow | kg/h | 1828.31 | 1828.31 | 1184.38 | 1184.38 | 643.41 | 643.41 |
| Pressure | bar | 1.0342 | 13.7 | 1.1031 | 0.6 | 14.8754 | 0.9 |
| CO2 | % | 40 | 9.1 | 90.7871 | 75.3 | 9.2129 | 0.2 |
| CH4 | % | 58.999 | 90.2 | 7.4464 | 3.1 | 90.2486 | 98.9 |
| H2S | ppm | - | 1 | - | 1 | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Q.N.; Lau, W.J.; Jaafar, J.; Othman, M.H.D.; Yoshida, N. Membrane Technology for Valuable Resource Recovery from Palm Oil Mill Effluent (POME): A Review. Membranes 2025, 15, 138. https://doi.org/10.3390/membranes15050138
Ho QN, Lau WJ, Jaafar J, Othman MHD, Yoshida N. Membrane Technology for Valuable Resource Recovery from Palm Oil Mill Effluent (POME): A Review. Membranes. 2025; 15(5):138. https://doi.org/10.3390/membranes15050138
Chicago/Turabian StyleHo, Que Nguyen, Woei Jye Lau, Juhana Jaafar, Mohd Hafiz Dzarfan Othman, and Naoko Yoshida. 2025. "Membrane Technology for Valuable Resource Recovery from Palm Oil Mill Effluent (POME): A Review" Membranes 15, no. 5: 138. https://doi.org/10.3390/membranes15050138
APA StyleHo, Q. N., Lau, W. J., Jaafar, J., Othman, M. H. D., & Yoshida, N. (2025). Membrane Technology for Valuable Resource Recovery from Palm Oil Mill Effluent (POME): A Review. Membranes, 15(5), 138. https://doi.org/10.3390/membranes15050138










