Environment-Oriented Assessment of Hybrid Methods for Separation of N-Propanol–Water Mixtures: Combination of Distillation and Hydrophilic Pervaporation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling of Pervaporation
2.2. Simulation of Hybrid Distillation and Pervaporation Methods
2.2.1. Hybrid Distillation–Hydrophilic Pervaporation Method (D + HPV) for N-Propanol and Water Purification (Configuration “A”)
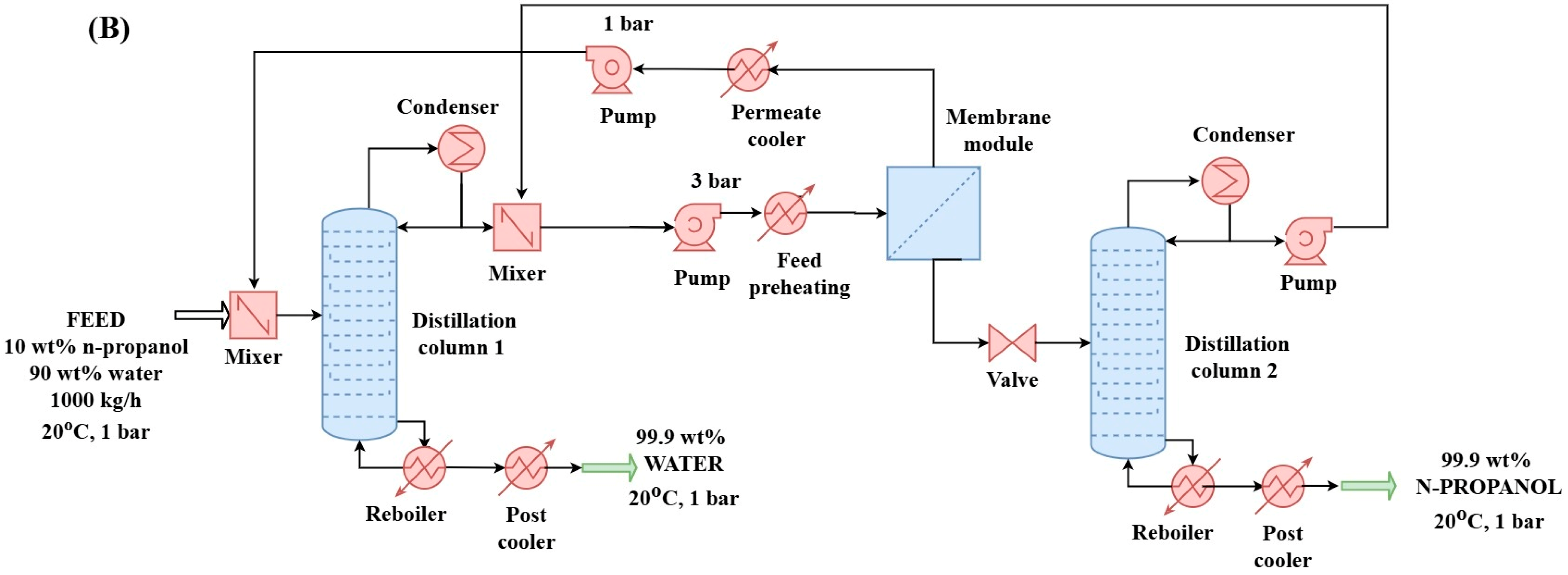
2.2.2. Hybrid Distillation–Hydrophilic Pervaporation–Distillation Method (D + HPV + D) for N-Propanol and Water Purification (Configuration “B”)
2.2.3. Hybrid Distillation–Hydrophilic Pervaporation–Distillation Method with Partial Heat Integration (D + HPV + D + HI) for N-Propanol and Water Purification (Configuration “C”)
2.3. Life Cycle Assessment
2.3.1. Goal and Scope
2.3.2. Inventory Data
2.3.3. Life Cycle Impact Assessment
3. Results and Discussion
3.1. Optimization of the Hybrid Methods for N-Propanol–Water Separation
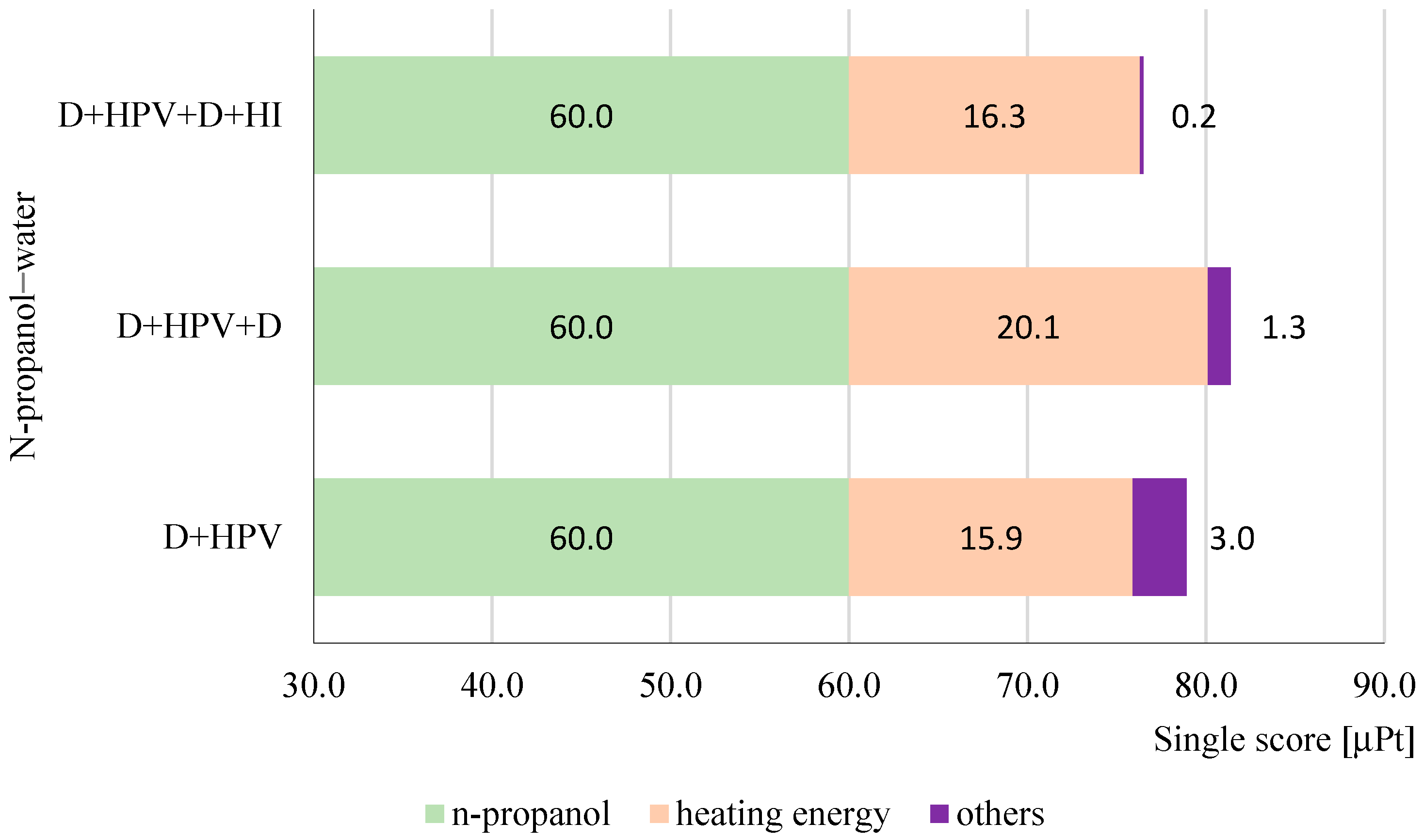
3.2. Life Cycle Assessment of Hybrid Methods for N-Propanol Dehydration in Case Obtaining 1 kg of Products with a 99.9 wt% Purity
3.3. Economic Results of Hybrid Separation Methods for N-Propanol–Water Separation
4. Conclusions
- Process efficiency: The D + HPV + D + HI configuration demonstrated superior energy efficiency, reducing the calculated heat duties of the D + HPV + D by 18.5% through heat integration.
- Environmental sustainability: While the D + HPV method achieved the lowest carbon footprint (0.957 kg CO₂-eq), the D + HPV + D + HI method offered a balanced approach, emitting 0.965 kg CO₂-eq—a difference of just 0.8%—while maintaining high energy efficiency.
- Economic feasibility: The D + HPV + D + HI configuration achieved the lowest total annual cost (148,000 EUR/year), outperforming the other methods by optimizing heat utilization and minimizing membrane area requirements.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Membrane transfer area | ||
| Constant in Equations (1) and (2) | ||
| Distillate product, distillation | ||
| Transport coefficient of component | ||
| Relative transport coefficient of component | ||
| Activation energy of component in Equation (4) for temperature dependence of the transport coefficient | ||
| Feed | ||
| Component number | ||
| Component number | ||
| Partial flux | ||
| N | Number of theoretical stages | [-] |
| Permeate | ||
| Pure i component vapor pressure | ||
| Partial pressure of component on the | ||
| liquid phase membrane side | ||
| Partial pressure of component on the | ||
| vapor phase membrane side | ||
| Pressure on the permeate side | ||
| Heat stream | ||
| Q0 | Permeability of the porous supporting layer | |
| of the membrane | [] | |
| Retentate | ||
| Ʀ | Gas constant | |
| Temperature | ||
| Reference temperature: 293 | ||
| Concentration of component in the feed | ||
| Abbreviations | ||
| CTUe | Comparative toxic unit for ecosystem | |
| CTUh | Comparative toxic unit for human | |
| EF | Environmental footprint | |
| FU | Functional unit | |
| GHG | Greenhouse gas | |
| HI | Heat integration | |
| HPV | Hydrophilic pervaporation | |
| hydr | Hydrophilic | |
| IC | Investment cost [EUR] | |
| LCA | Life cycle assessment | |
| LCI | Life cycle inventory | |
| LCIA | Life cycle inventory assessment | |
| OC | Operating cost [EUR] | |
| OF | Objective function | |
| PAF | Potentially affected fraction | |
| PV | Pervaporation | |
| TAC | Total annual cost [EUR/year] | |
| TIC | Total investment cost [EUR/year] | |
| TOC | Total operation cost [EUR/year] | |
| Greek letters | ||
| Average activity coefficient of component | ||
| Activity coefficient of component in the feed | ||
| δ | Membrane thickness | |
| ∆T | Temperature difference | |
References
- Favre, E. Temperature polarization in pervaporation. Desalination 2003, 154, 129–138. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Salt, Y.; Keleşer, S.; Özkan, S.; Dinçer, S. Pervaporation separation of ethyl acetate–ethanol binary mixtures using polydimethylsiloxane membranes. Chem. Eng. Process. 2005, 44, 375–381. [Google Scholar] [CrossRef]
- Tóth, A.J.; Haáz, E.; Nagy, T.; Tarjáni, A.J.; Fózer, D.; André, A.; Valentínyi, N.; Mizsey, P. Treatment of pharmaceutical process wastewater with hybrid separation method: Distillation and hydrophilic pervaporation. Waste Treat. Recovery 2018, 3, 8–13. [Google Scholar] [CrossRef]
- Valentínyi, N.; Cséfalvay, E.; Mizsey, P. Modelling of pervaporation: Parameter estimation and model development. Chem. Eng. Res. Des. 2013, 91, 174–183. [Google Scholar] [CrossRef]
- Jonquières, A.; Clément, R.; Lochon, P.; Néel, J.; Dresch, M.; Chrétien, B. Industrial state-of-the-art of pervaporation and vapour permeation in the western countries. J. Membr. Sci. 2002, 206, 87–117. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Kruber, B.; Skiborowski, M.; Gao, X. Process intensification by integration of distillation and vapor permeation or pervaporation—An academic and industrial perspective. Results Eng. 2022, 15, 100527. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Kuo, J.F.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Separation of ethanol–water solution by poly(acrylonitrile-co-acrylic acid) membranes. Sep. Purif. Technol. 2005, 41, 39–47. [Google Scholar] [CrossRef]
- Lipnizki, F.; Field, R.W.; Ten, P.-K. Pervaporation-based hybrid process: A review of process design, applications and economics. J. Membr. Sci. 1999, 153, 183–210. [Google Scholar] [CrossRef]
- Konieczny, K.; Bodzek, M.; Panek, D. Removal of volatile compounds from the wastewaters by use of pervaporation. Desalination 2008, 223, 344–348. [Google Scholar] [CrossRef]
- Lipnizki, F.; Hausmanns, S.; Ten, P.-K.; Field, R.W.; Laufenberg, G. Organophilic pervaporation: Prospects and performance. Chem. Eng. J. 1999, 73, 113–129. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K. Sorption and permeation studies of tetrahydrofuran–water mixtures using full interpenetrating network membranes. Sep. Purif. Technol. 2012, 89, 39–50. [Google Scholar] [CrossRef]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Fouda, A.E.; Matsuura, T. A study of pervaporation of aqueous benzyl alcohol solution by polydimethylsiloxane membrane. J. Membr. Sci. 1992, 70, 249–255. [Google Scholar] [CrossRef]
- Kim, H.J.; Nah, S.S.; Min, B.R. A new technique for preparation of PDMS pervaporation membrane for VOC removal. Adv. Environ. Res. 2002, 6, 255–264. [Google Scholar] [CrossRef]
- Fleming, H.L.; Slater, C.S. Pervaporation. In Membrane Handbook; Springer: New York, NY, USA, 1992; Volume 1. [Google Scholar]
- Liu, Q.; Noble, R.D.; Falconer, J.L.; Funke, H.H. Organics/water separation by pervaporation with a zeolite membrane. J. Membr. Sci. 1996, 117, 163–174. [Google Scholar] [CrossRef]
- Mizsey, P.; Szanyi, A.; Raab, A.; Manczinger, J.; Fonyo, Z. Intensification of a Solvent Recovery Technology through the Use of Hybrid Equipment. In Computer Aided Chemical Engineering; Grievink, J., van Schijndel, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 10, pp. 121–126. [Google Scholar] [CrossRef]
- Li, S.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Properties and separation performance of Ge-ZSM-5 membranes. Microporous Mesoporous Mater. 2003, 58, 137–154. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Liao, M.; Guan, H.; Zuo, H.; Ren, G.; Gong, G. High-Performance Flexible Hybrid Silica Membranes with an Ultrasonic Atomization-Assisted Spray-Coated Active Layer on Polymer for Isopropanol Dehydration. Membranes 2024, 14, 154. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Myznikov, D.; Selyutin, A.; Su, R.; Penkova, A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Dmitrenko, M.; Zolotarev, A.; Kuzminova, A.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel Thin Film Nanocomposite Membranes Based on Chitosan Succinate Modified with Fe-BTC for Enhanced Pervaporation Dehydration of Isopropanol. Membranes 2022, 12, 653. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Toth, A.J. N-Propanol Dehydration with Distillation and Pervaporation: Experiments and Modelling. Membranes 2022, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Toutianoush, A.; Krasemann, L.; Tieke, B. Polyelectrolyte multilayer membranes for pervaporation separation of alcohol/water mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2002, 198–200, 881–889. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Bugrov, A.N.; Smirnov, M.A.; Smirnov, A.V.; Lahderanta, E.; Svetlichnyi, V.M.; Toikka, A.M. Effect of Domain Structure of Segmented Poly(urethane-imide) Membranes with Polycaprolactone Soft Blocks on Dehydration of n-Propanol via Pervaporation. Polymers 2018, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Meng, D.; Yao, D.; Liu, X.; Xu, Y.; Zhu, Z.; Wang, Y.; Gao, J. Mechanism Analysis, Economic Optimization, and Environmental Assessment of Hybrid Extractive Distillation–Pervaporation Processes for Dehydration of n-Propanol. ACS Sustain. Chem. Eng. 2020, 8, 4561–4571. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Ma, Z.; Qi, H.; Chen, Z.; Shen, Y.; Yang, J.; Cui, P.; Wang, Y.; Zhu, Z.; et al. Application of energy-saving hybrid distillation-pervaporation process for recycling organics from wastewater based on thermoeconomic and environmental analysis. J. Clean. Prod. 2021, 294, 126297. [Google Scholar] [CrossRef]
- Pla-Franco, J.; Lladosa, E.; Loras, S.; Montón, J.B. Approach to the 1-propanol dehydration using an extractive distillation process with ethylene glycol. Chem. Eng. Process. 2015, 91, 121–129. [Google Scholar] [CrossRef]
- Koczka, K.; Mizsey, P.; Fonyo, Z. Rigorous modelling and optimization of hybrid separation processes based on pervaporation. Cent. Eur. J. Chem. 2007, 5, 1124–1147. [Google Scholar] [CrossRef]
- Szilagyi, B.; Toth, A.J. Improvement of Component Flux Estimating Model for Pervaporation Processes. Membranes 2020, 10, 418. [Google Scholar] [CrossRef]
- Toth, A.J.; Andre, A.; Haaz, E.; Mizsey, P. New horizon for the membrane separation: Combination of organophilic and hydrophilic pervaporations. Sep. Purif. Technol. 2015, 156, 432–443. [Google Scholar] [CrossRef]
- Schaetzel, P.; Vauclair, C.; Nguyen, Q.T.; Bouzerar, R. A simplified solution–diffusion theory in pervaporation: The total solvent volume fraction model. J. Membr. Sci. 2004, 244, 117–127. [Google Scholar] [CrossRef]
- Marriott, J.; Sørensen, E. A general approach to modelling membrane modules. Chem. Eng. Sci. 2003, 58, 4975–4990. [Google Scholar] [CrossRef]
- Rautenbach, R.; Herion, C.; Meyer-Blumentoth, U. Pervaporation Membrane Separation Processes; Huang, R.Y.M., Ed.; Membrane Science and Technology Series; Elsevier: New York, NY, USA, 1991; Volume 1, pp. 181–191. [Google Scholar]
- Mizsey, P.K.K.; Deák, A.; Fonyó, Z. Simulation of pervaporation using the “solution-diffusion” model. J. Hung. Chem. 2005, 7, 239–242. [Google Scholar]
- Kleinekorte, J.; Kleppich, J.; Fleitmann, L.; Beckert, V.; Blodau, L.; Bardow, A. Appropriate Life Cycle Assessment: A Process-Specific, Predictive Impact Assessment Method for Emerging Chemical Processes. ACS Sustain. Chem. Eng. 2023, 11, 9303–9319. [Google Scholar] [CrossRef]
- Hessel, V.; Escribà-Gelonch, M.; Bricout, J.; Tran, N.N.; Anastasopoulou, A.; Ferlin, F.; Valentini, F.; Lanari, D.; Vaccaro, L. Quantitative Sustainability Assessment of Flow Chemistry–From Simple Metrics to Holistic Assessment. ACS Sustain. Chem. Eng. 2021, 9, 9508–9540. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 31 January 2025).
- ISO 14044:2006; Environmental Management—Life Cycle assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14044:ed-1:v1:en (accessed on 31 January 2025).
- Rebitzer, G.; Ekvall, T.; Frischknecht, R.; Hunkeler, D.; Norris, G.; Rydberg, T.; Schmidt, W.P.; Suh, S.; Weidema, B.P.; Pennington, D.W. Life cycle assessment: Part 1: Framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 2004, 30, 701–720. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A.; Bhattacharyya, D. Analysis, Synthesis and Design of Chemical Processes, 4th ed.; Pearson Education: New York, NY, USA, 2012. [Google Scholar]
- González, B.; Ortiz, I. Modelling and simulation of a hybrid process (pervaporation–distillation) for the separation of azeotropic mixtures of alcohol–ether. J. Chem. Technol. Biotechnol. 2002, 77, 29–42. [Google Scholar] [CrossRef]
- Douglas, J.M. Conceptual Design of Chemical Processes; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Do Thi, H.T.; Toth, A.J. Environmental evaluation and comparison of hybrid separation methods based on distillation and pervaporation for dehydration of binary alcohol mixtures with life cycle, PESTLE, and multi-criteria decision analyses. Sep. Purif. Technol. 2024, 348, 127684. [Google Scholar] [CrossRef]
- Wang, K.; Xin, L.; Zhang, Y.; Qi, J.; Zhu, Z.; Wang, Y.; Zhong, L.; Cui, P. Sustainable and efficient process design for wastewater recovery of cyclohexane/isopropyl alcohol azeotrope by extractive distillation based on multi-objective genetic algorithm optimization. Chem. Eng. Res. Des. 2024, 201, 593–602. [Google Scholar] [CrossRef]


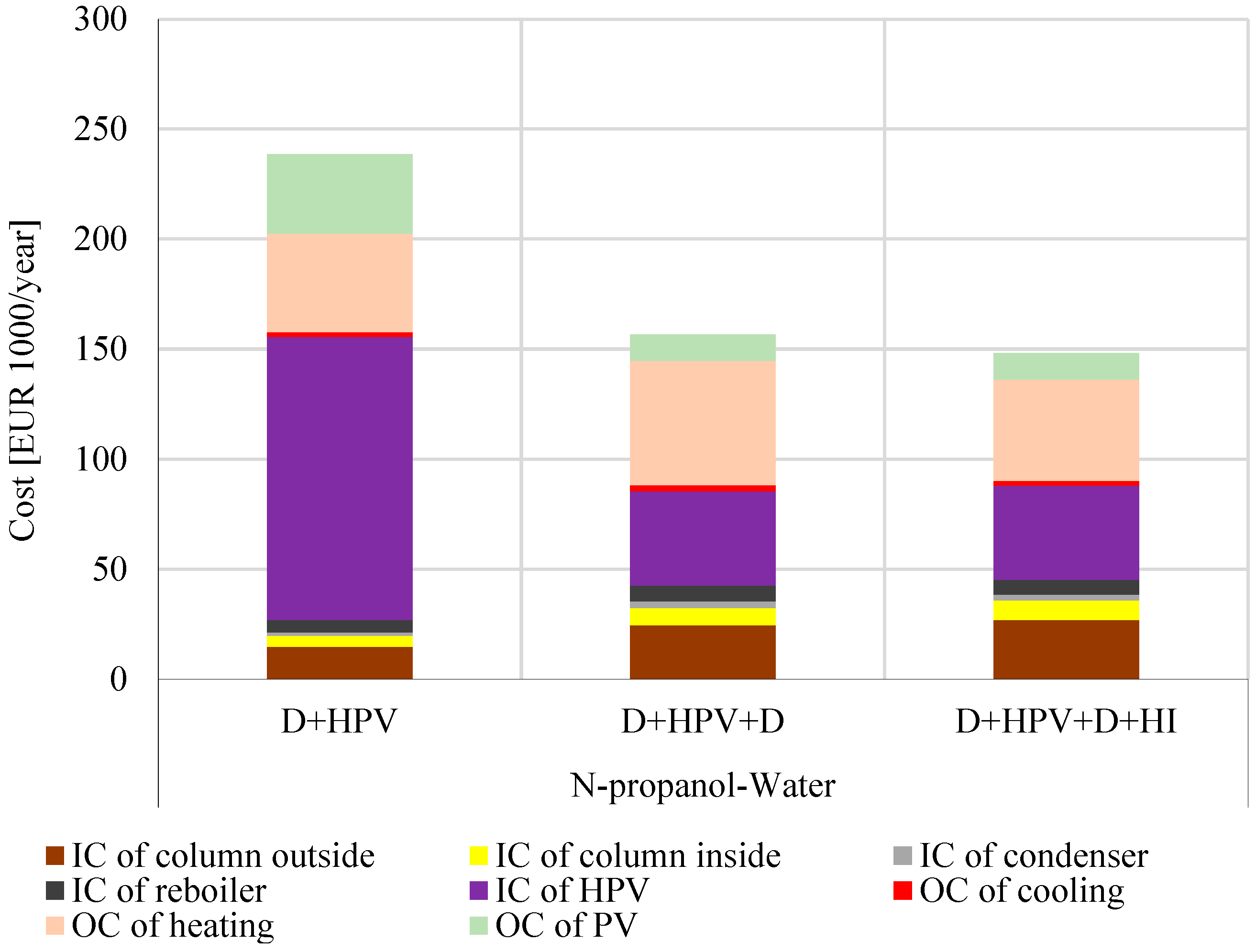
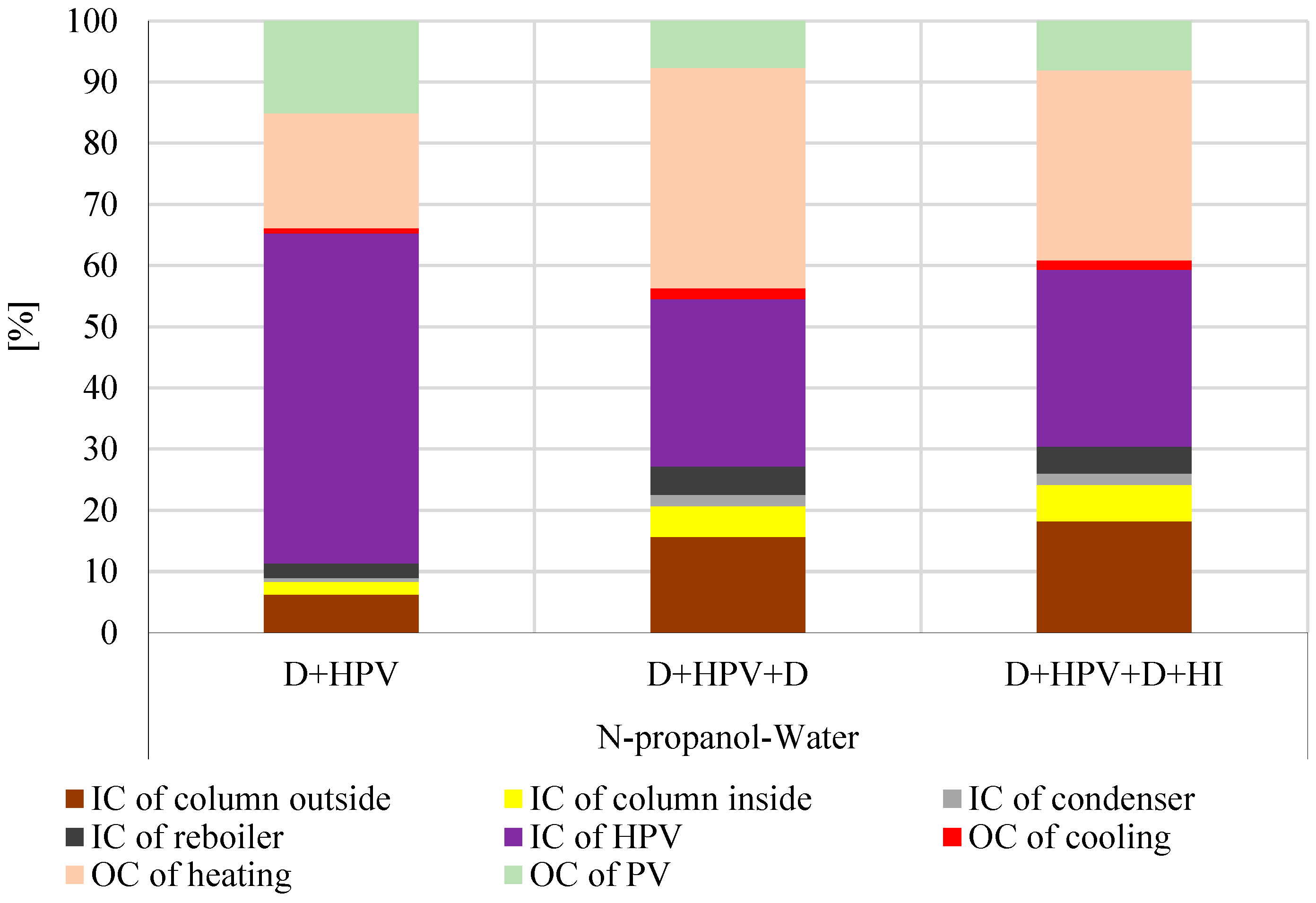
| n-Propanol | Water | |
|---|---|---|
| [kJ/kmol] | 29,966 | 27,707 |
| [kmol/m2h] | 2.32 × 10−3 | 2.40 × 10−5 |
| B [-] | −12.08 | 8.38 |
| OF [-] | 0.125 | 0.123 |
| D + HPV | D + HPV + D | D + HPV + D + HI | ||
|---|---|---|---|---|
| Input | n-propanol [kg] | 1.01 | 1.01 | 1.01 |
| Water [kg] | 9.07 | 9.07 | 9.07 | |
| Heating energy [MJ] | 7.62 | 9.61 | 7.83 | |
| Cooling water [L] | 8.85 | 14.07 | 17.37 | |
| Output | n-propanol [kg] | 1.00 | 1.00 | 1.00 |
| Water [kg] | 9.08 | 9.08 | 9.08 | |
| Cooling water [L] | 8.85 | 14.07 | 17.37 | |
| Parameters | Unit | n-Propanol–Water Binary Mixture | |||
|---|---|---|---|---|---|
| D + HPV | D + HPV + D | D + HPV + D + HI | |||
| First distillation column | Total plates | - | 40 | 40 | 40 |
| Feed plate | - | 20 | 20 | 20 | |
| Reflux ratio | - | 1 | 1 | 1 | |
| Distillate product stream | kg/h | 140.9 | 140.3 | 140.3 | |
| Distillate product temperature | °C | 87.0 | 87.0 | 87.0 | |
| Alcohol of distillate product | wt% | 70.8 | 70.8 | 70.8 | |
| Bottom product stream | kg/h | 900.8 | 900.8 | 900.8 | |
| Bottom product temperature | °C | 99.4 | 99.4 | 99.4 | |
| Alcohol of bottom product | wt% | 0.1 | 0.1 | 0.1 | |
| Reboiler duty | MJ/h | 664.7 | 663.1 | 363.7 | |
| Condenser duty | MJ/h | −330.1 | −328.9 | −328.9 | |
| Parameters | Unit | n-Propanol–Water Binary Mixture | |||
|---|---|---|---|---|---|
| D + HPV | D + HPV + D | D + HPV + D + HI | |||
| Second distillation column | Total plates | - | - | 30 | 30 |
| Feed plate | - | - | 15 | 15 | |
| Reflux ratio | - | - | 3 | 5 | |
| Distillate product stream | kg/h | - | 43.8 | 43.8 | |
| Distillate product temperature | °C | - | 87.0 | 87.0 | |
| Alcohol of distillate product | wt% | - | 70.8 | 70.8 | |
| Bottom product stream | kg/h | - | 99.2 | 99.2 | |
| Bottom product temperature | °C | - | 96.7 | 96.7 | |
| Alcohol of bottom product | wt% | - | 99.9 | 99.9 | |
| Reboiler duty | MJ/h | - | 206.4 | 329.4 | |
| Condenser duty | MJ/h | - | −196.0 | −319.0 | |
| HPV | Number of module units | piece | 10 | 6 | 6 |
| Total membrane area | m2 | 360 | 120 | 120 | |
| Permeate product stream | kg/h | 41.6 | 41.2 | 41.2 | |
| Retentate product stream | kg/h | 99.2 | 143.0 | 143.0 | |
| Retentate product heating | MJ/h | 90.0 | 81.7 | 81.7 | |
| Permeate cooler | MJ/h | −108.8 | −107.8 | −107.8 | |
| Calculated Heat Duties [MJ/h] | D + HPV | D + HPV + D | D + HPV + D + HI | ||||
|---|---|---|---|---|---|---|---|
| Qheating | Qcooling | Qheating | Qcooling | Qheating | Qcooling | ||
| Distillation column 1 | Reboiler | 664.7 | 663.1 | 363.7 | |||
| Condenser | −330.1 | −328.9 | −328.9 | ||||
| Post-cooler | −299.5 | −299.5 | |||||
| Hydrophilic pervaporation | Feed preheating | 1.4 | 1.9 | 1.9 | |||
| Retentate heating | 90.0 | 81.7 | 81.7 | ||||
| Permeate cooler | −108.8 | −107.8 | −107.8 | ||||
| Post-cooler | −17.7 | ||||||
| Distillation column 2 | Reboiler | 206.4 | 329.4 | ||||
| Condenser | −196.0 | −319.0 | |||||
| Post-cooler | −21.0 | −21.0 | |||||
| Total | 756 | −756 | 953 | −953 | 777 | −777 | |
| Calculated Heat Duties [%] | D + HPV | D + HPV + D | D + HPV + D + HI | ||||
|---|---|---|---|---|---|---|---|
| Qheating | Qcooling | Qheating | Qcooling | Qheating | Qcooling | ||
| Distillation column 1 | Reboiler | 87.9 | 69.6 | 46.8 | |||
| Condenser | 43.7 | 34.5 | 42.4 | ||||
| Post-cooler | 39.6 | 31.4 | |||||
| Hydrophilic pervaporation | Feed preheating | 0.2 | 0.2 | 0.2 | |||
| Retentate heating | 11.9 | 8.6 | 10.5 | ||||
| Permeate cooler | 14.4 | 11.3 | 13.9 | ||||
| Post-cooler | 2.3 | ||||||
| Distillation column 2 | Reboiler | 21.7 | 42.4 | ||||
| Condenser | 20.6 | 41.1 | |||||
| Post-cooler | 2.2 | 2.7 | |||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do Thi, H.T.; Toth, A.J. Environment-Oriented Assessment of Hybrid Methods for Separation of N-Propanol–Water Mixtures: Combination of Distillation and Hydrophilic Pervaporation Processes. Membranes 2025, 15, 48. https://doi.org/10.3390/membranes15020048
Do Thi HT, Toth AJ. Environment-Oriented Assessment of Hybrid Methods for Separation of N-Propanol–Water Mixtures: Combination of Distillation and Hydrophilic Pervaporation Processes. Membranes. 2025; 15(2):48. https://doi.org/10.3390/membranes15020048
Chicago/Turabian StyleDo Thi, Huyen Trang, and Andras Jozsef Toth. 2025. "Environment-Oriented Assessment of Hybrid Methods for Separation of N-Propanol–Water Mixtures: Combination of Distillation and Hydrophilic Pervaporation Processes" Membranes 15, no. 2: 48. https://doi.org/10.3390/membranes15020048
APA StyleDo Thi, H. T., & Toth, A. J. (2025). Environment-Oriented Assessment of Hybrid Methods for Separation of N-Propanol–Water Mixtures: Combination of Distillation and Hydrophilic Pervaporation Processes. Membranes, 15(2), 48. https://doi.org/10.3390/membranes15020048







