CO2 and O2 Separation Dual-Phase Membranes for Diesel Heavy-Duty Vehicles Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Eutectic Mixtures
2.2. Dual-Phase Membrane Synthesis
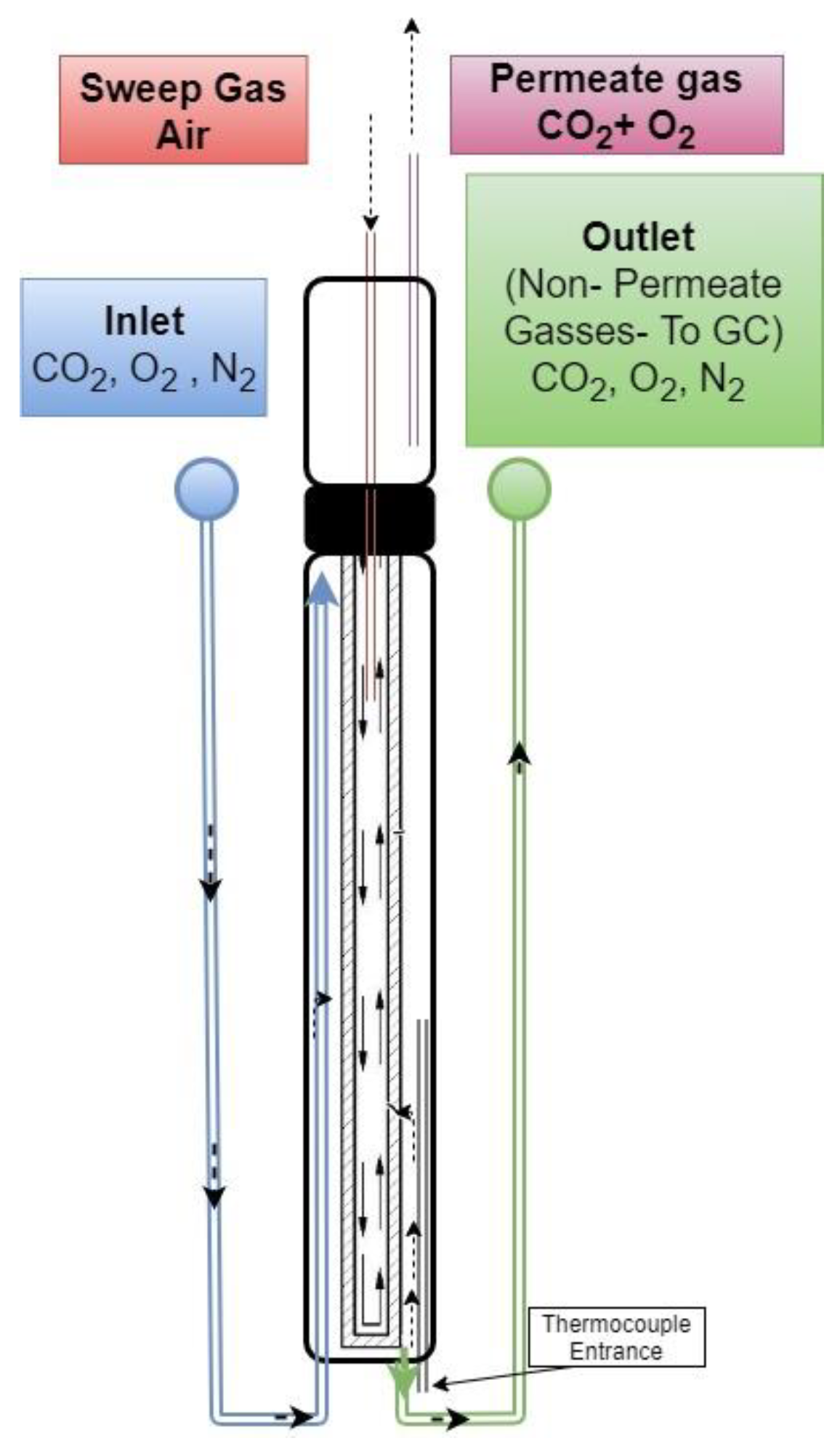
2.3. CO2–O2 Permeation Through the Membrane: Process Simulation and Analysis
2.4. Dual-Phase Membranes for CO2 and O2 Separation Mechanism
3. Results and Discussion
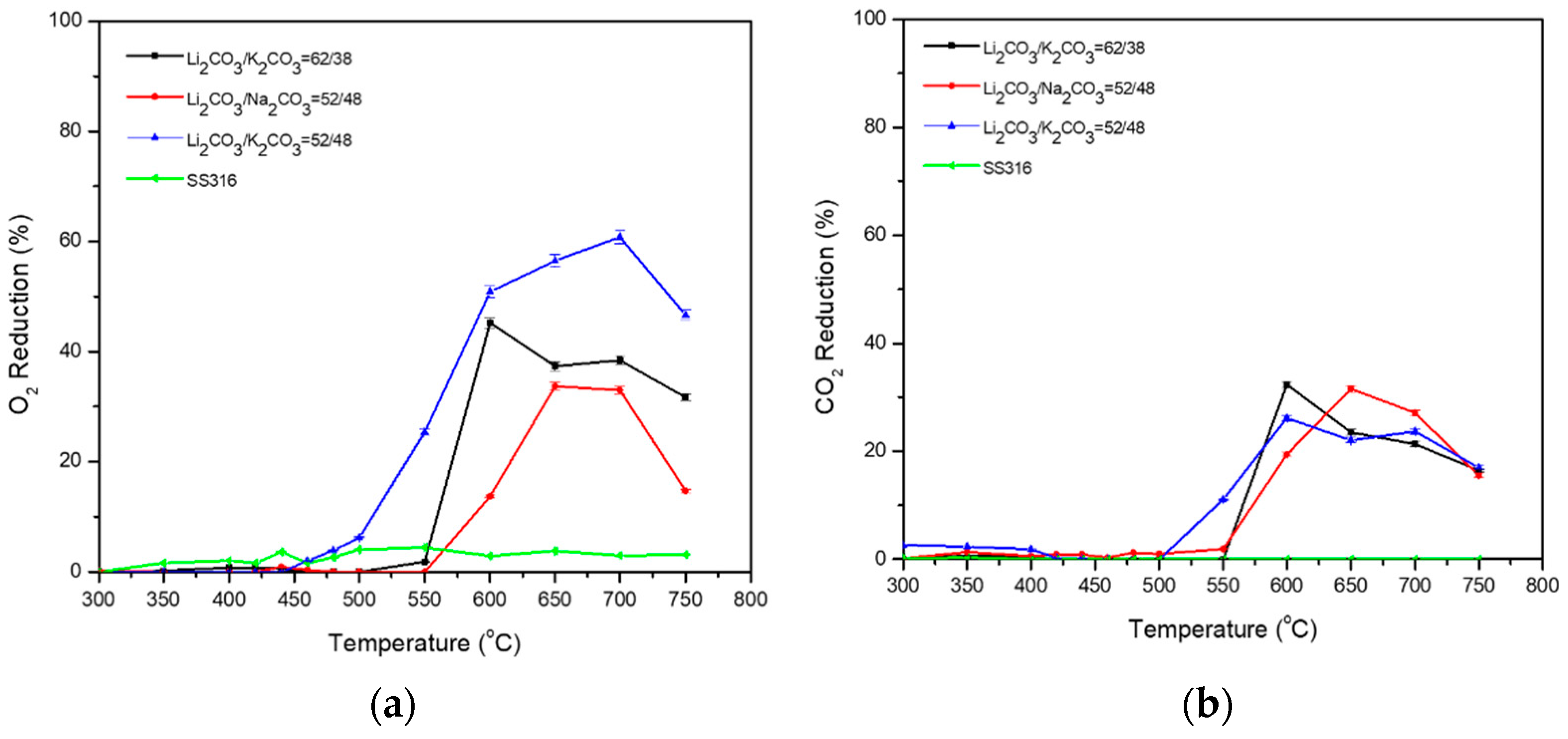
3.1. Binary Carbonate Mixtures
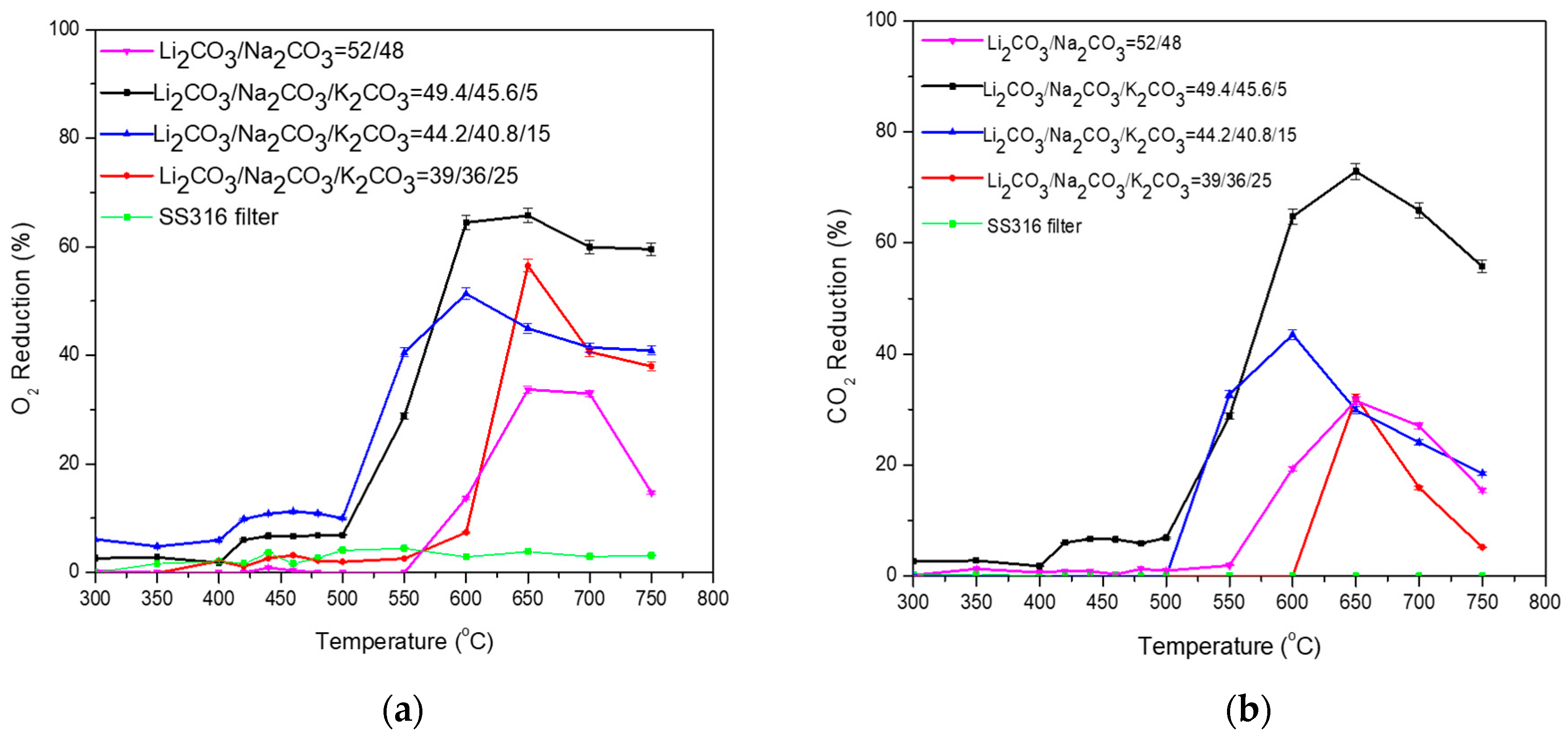
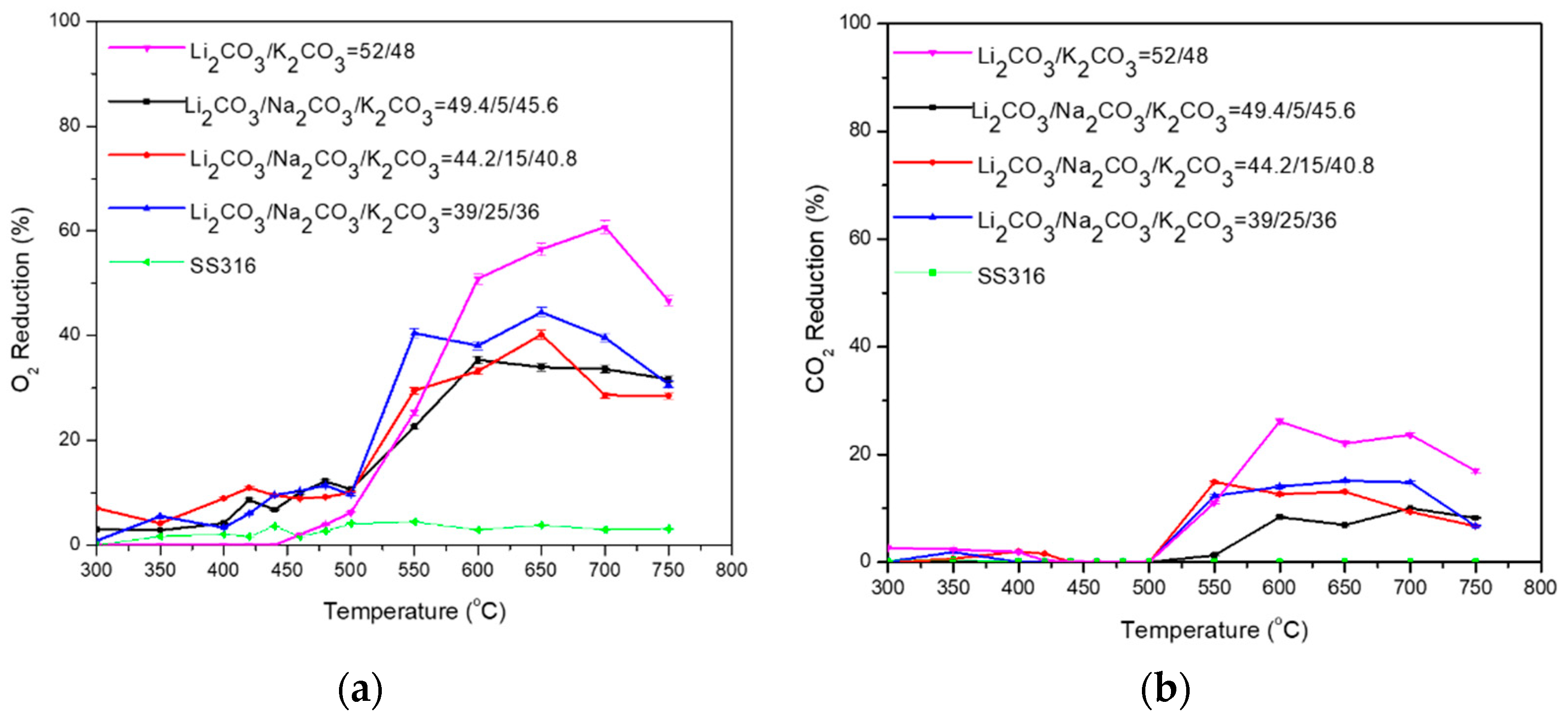
3.2. Ternary Carbonate Mixtures
3.3. O2/CO2 Flux Ratio
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Definition | Symbol | Unit |
|---|---|---|
| Concentration | C | mol L−1 |
| External surface | A | m2 |
| Factor lambda | λ | ratio |
| Flux | J | cm3 min−1 |
| Molar volume | Vm | cm3 mol−1 |
| Partial pressure | p | kPa |
| Permeation rate | r | mol s−1 m−2 |
References
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The Pollutant Emissions from Diesel-Engine Vehicles and Exhaust Aftertreatment Systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Betsi-Argyropoulou, I.I.; Moschovi, A.M.; Polyzou, E.; Yakoumis, I. Towards Ammonia Free Retrofitting of Heavy-Duty Vehicles to Meet Euro VI Standards. Veh. Automot. Eng. 2021, 206–221. [Google Scholar] [CrossRef]
- Matsumoto, S. Catalytic Reduction of Nitrogen Oxides in Automotive Exhaust Containing Excess Oxygen by NOx Storage-Reduction Catalyst. CATTECH 2000, 4, 102–109. [Google Scholar] [CrossRef]
- Dieselnet.com. Available online: https://dieselnet.com/standards/eu/hd.php#intro (accessed on 30 July 2024).
- Yamada, H. PN Emissions from Heavy-Duty Diesel Engine with Periodic Regenerating DPF. SAE Int. J. Engines 2013, 6, 1178–1189. [Google Scholar] [CrossRef]
- Maiboom, A.; Tauzia, X. Experimental Study of an Automotive Diesel Engine Running with Stoichiometric Combustion. 2012. Available online: https://saemobilus.sae.org/papers/experimental-study-automotive-diesel-engine-running-stoichiometric-combustion-2012-01-0699 (accessed on 9 December 2024).
- Ghany, F.A.; Wahono, B.; Praptijanto, A.; Putrasari, Y.; Dimyani, A.; Nur, A.; Suherman; Pratama, M.; Wardana, M.K.A. Study on the Effect of High-Concentration Oxygen Enrichment on Engine Performance and Exhaust Emissions Using Diesel Fuel and Palm Biodiesel Substitute Fuel. Energies 2024, 17, 244–259. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, X.; Bi, Y.; Yan, J.; Liu, S.; Peng, Y. Experimental Study on NOx Reduction of Diesel Engine by EGR Coupled with SCR. ACS Omega 2024, 9, 8308–8319. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Masaki, N.; Ueno, H.; Akagawa, H. Development of Urea-SCR System for Heavy-Duty Commercial Vehicles. J. Fuels Lubr. 2005, 114, 678–685. [Google Scholar] [CrossRef]
- Okubo, M.; Kuwahara, T. Chapter 4—Operation Examples of Emission Control Systems. In New Technologies for Emission Control in Marine Diesel Engines; Okubo, M., Kuwahara, T., Eds.; Elsevier: Oxford, UK; Cambridge, MA, USA, 2020; pp. 145–210. [Google Scholar]
- Suarez-Bertoa, R.; Zardini, A.A.; Astorga, C. Ammonia Exhaust Emissions from Spark Ignition Vehicles over the New European Driving Cycle. Atmos. Environ. 2014, 97, 43–53. [Google Scholar] [CrossRef]
- Battye, W.; Aneja, V.P.; Roelle, P.A. Evaluation and Improvement of Ammonia Emissions Inventories. Atmos. Environ. 2003, 37, 3873–3883. [Google Scholar] [CrossRef]
- Yakoumis, I.; Souentie, S. Device and Process for the Treatment of Engine Flue Gases with High Oxygen Excess. EP 3 542 887 A8 25 September 2019. [Google Scholar]
- Yakoumis, I. Copper and Noble Metal Polymetallic Catalysts for Engine Exhaust Gas Treatment. EP 3 569 309 A1 20 November 2019. [Google Scholar]
- Boriboonsomsin, K.; Durbin, T.; Scora, G.; Johnson, K.; Sandez, D.; Vu, A.; Jiang, Y.; Burnette, A.; Yoon, S.; Collins, J.; et al. Real-World Exhaust Temperature and Engine Load Distributions of on-Road Heavy-Duty Diesel Vehicles in Various Vocations. Data Brief 2018, 18, 1520–1543. [Google Scholar] [CrossRef]
- Chung, S.; Park, J.; Li, D.; Ida, J.-I.; Kumakiri, I.; Lin, J. Dual-Phase Metal−Carbonate Membrane for High-Temperature Carbon Dioxide Separation. Ind. Eng. Chem. Res. 2005, 44, 7999–8006. [Google Scholar] [CrossRef]
- Afzal, S.; Khan, A. Recent Advances in Molten-Carbonate Membranes for Carbon Dioxide Separation: Focus on Material Selection, Geometry, and Surface Modification. Sci. World J. 2021, 1876875–1876896. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Shi, X.; Wu, H.; Ma, Y.; Hu, X.; Chen, T.; Chen, T. Progress and Perspectives in the Development of Inorganic-Carbonate Dual-Phase Membrane for CO2 Separation. Processes 2024, 12, 240–274. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, S.; Du, Z.; Zhao, H. A layered perovskite La2Ni1−xSbxO4+δ-molten carbonate dual-phase membrane for CO2 separation. Sep. Purif. Technol. 2024, 337, 126418–126425. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, J.; Gong, Y.; Han, M.; Wang, S.; Huang, K. Fast Electrochemical CO2 Transport through a Dense Metal-Carbonate Membrane: A New Mechanistic Insight. J. Membr. Sci. 2014, 468, 373–379. [Google Scholar] [CrossRef]
- Frangini, S.; Scaccia, S. The Dissolution of Oxygen in La2O3-Added 52/48 mol % Li/Na Molten Carbonate Determined by Gas Solubility Measurements. J. Electrochem. Soc. 2005, 152, A2155–A2158. [Google Scholar] [CrossRef]
- Spedding, P.L.; Mills, R. Trace-Ion Diffusion in Molten Alkali Carbonates. J. Electroch. Soc. 1965, 112, 594–600. [Google Scholar] [CrossRef]
- Appleby, A.J.; Van Drunen, C. Solubilities of Oxygen and Carbon Monoxide in Carbonate Melts. J. Electrochem. Soc. 1980, 127, 1655–1659. [Google Scholar] [CrossRef]
- Davé, B.B.; White, R.E.; Srinivasan, S.; Appleby, A.J. Electrode Kinetics of Oxygen Reduction in Lithium Carbonate Melt: Use of Impedance Analysis and Cyclic Voltammetric Techniques to Determine the Effects of Partial Pressure of Oxygen. J. Electrochem. Soc. 1991, 138, 673–678. [Google Scholar] [CrossRef]
- Xing, W.; Li, Z.; Peters, T.; Fontaine, M.-L.; McCann, M.; Evans, A.; Norby, T.; Bredesen, R. Improved CO2 flux by dissolution of oxide ions into the molten carbonate phase of dual-phase CO2 separation membranes. Sep. Purif. 2019, 212, 723–727. [Google Scholar] [CrossRef]
- Mutch, G.A.; Qu, L.; Triantafyllou, G.; Xing, W.; Fontaine, M.-L.; Metcalfe, I.S. Supported Molten-salt Membranes for Carbon Dioxide Permeation. J. Mater. Chem. A 2019, 7, 12951–12974. [Google Scholar] [CrossRef]
- Ho, C.Y.; Chu, T.K. Electrical Resistivity and Thermal Conductivity of Nine Selected AISI Stainless Steels. Solid State Commun. 1977, 18, 5–7. [Google Scholar]
- Malzbender, J. Mechanical Aspects of Ceramic Membranes Materials. Ceram. Int. 2016, 42, 7899–7911. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, S.; Pang, B.; Zhu, X.; Yang, W. Effect of molten carbonate composition on CO2 permeation mechanism. J. Membr. Soc. 2022, 645, 120210–120218. [Google Scholar] [CrossRef]
- Kojima, T.; Miyazaki, Y.; Nomura, K.; Tanimoto, K. Density, Surface Tension, and Electrical Conductivity of Ternary Molten System Li2CO3-Na2CO3-K2CO3 and Methods for Their Estimation. J. Electr. Soc. 2008, 155, F150–F156. [Google Scholar] [CrossRef]
- Mohamedi, M.; Hisamitsu, Y.; Ono, Y.; Itoh, T.; Uchida, I. Effect of Molten Carbonate Composition on Oxygen Reduction Under Pressurized Conditions: Cyclic Voltammetry and Equivalent Circuit Analysis. J. Appl. Chem. 2000, 30, 1397–1404. [Google Scholar]
- Nolan, D.P. Classified Area Pump Installations. In Fire Pump Arrangements at Industrial Facilities, 3rd ed.; Nolan, D.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 161–167. [Google Scholar] [CrossRef]
- Frangini, S.; Loreti, S. The role of alkaline-earth additives on the molten carbonate corrosion of 316L stainless steel. Corrosion 2007, 49, 3969–3987. [Google Scholar] [CrossRef]
- Olivares, R.I.; Chen, C.; Wright, S. The Thermal Stability of Molten Lithium-Sodium-Potassium Carbonate and the Influence of Additives on the Melting Point. J. Sol. Energy Eng. 2012, 134, 041002–041010. [Google Scholar] [CrossRef]
- Chen, C.; Tran, T.; Olivares, R.; Wright, S.; Sun, S. Coupled Experimental Study and Thermodynamic Modeling of Melting Point and Thermal Stability of Li2CO3-Na2CO3-K2CO3 Based Salts. J. Solar Energy Engin. 2014, 136, 031017–031024. [Google Scholar] [CrossRef]
- Freestone, N.P. Simple Solutions—Chemistry Counting Moles. 2016. Available online: https://benthambooks.com/book/9781681081007/ (accessed on 9 December 2024).
- Jiang, Y.; Sun, Y.; Bruno, F.; Li, S. Thermal Stability of Na2CO3-Li2CO3 as a High Temperature Phase Change Material for Thermal Energy Storage. Thermochim. Acta 2017, 650, 88–94. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, L.; Zong, B.; Zhang, Y.; Wu, C. Li/Na/K Carbonate Solar Salts for Enhanced and Integrated Carbon Capture and utilization via reverse water gas shift (ICCU-RWGS). Appl. Cat. B Environ. Energy 2025, 361, 124623–124636. [Google Scholar] [CrossRef]
- Tong, J.; Lei, X.; Fang, J.; Huang, K. Remarkable O2 Permeation through a Mixed Conducting Carbon Capture Membrane Functionalized by Atomic Layer Deposition. J. Mater. Chem. A 2016, 5, 1828–1837. [Google Scholar] [CrossRef]
- Xing, W.; Peters, T.; Fontaine, M.-L.; Evans, A.; Henriksen, P.P.; Norby, T.; Bredesen, R. Steam-promoted CO2 flux in dual-phase CO2 separation membranes. J. Membr. Sci. 2015, 482, 115–119. [Google Scholar] [CrossRef]
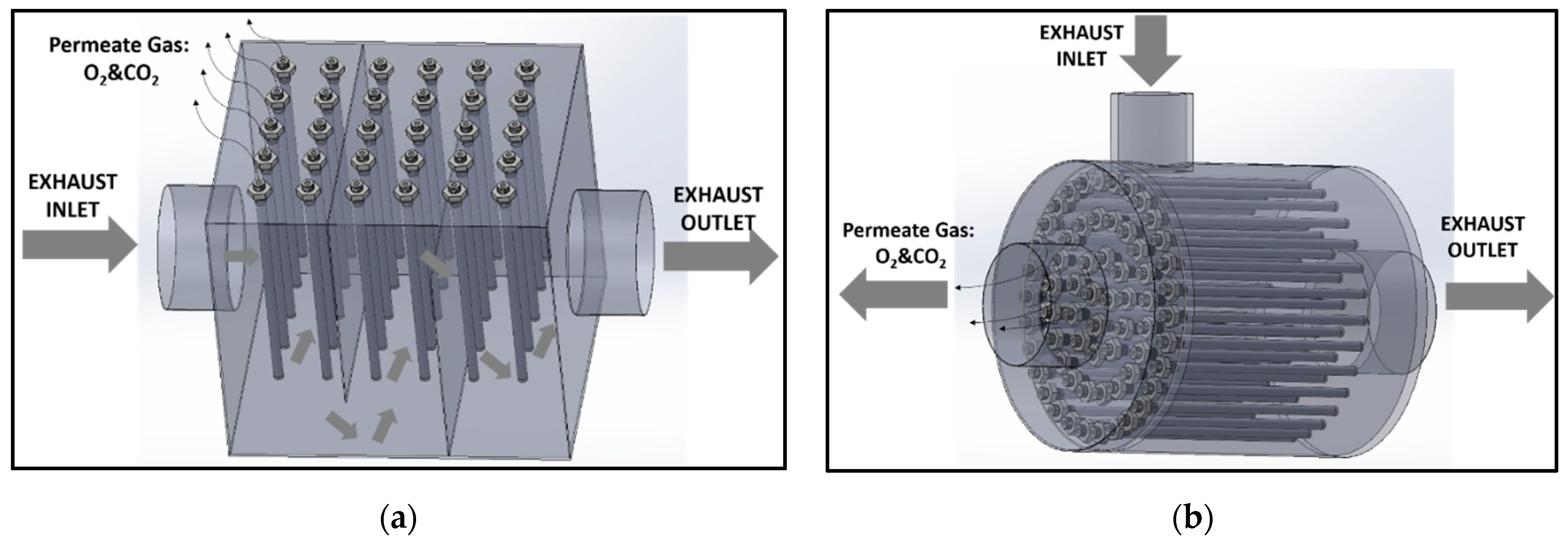

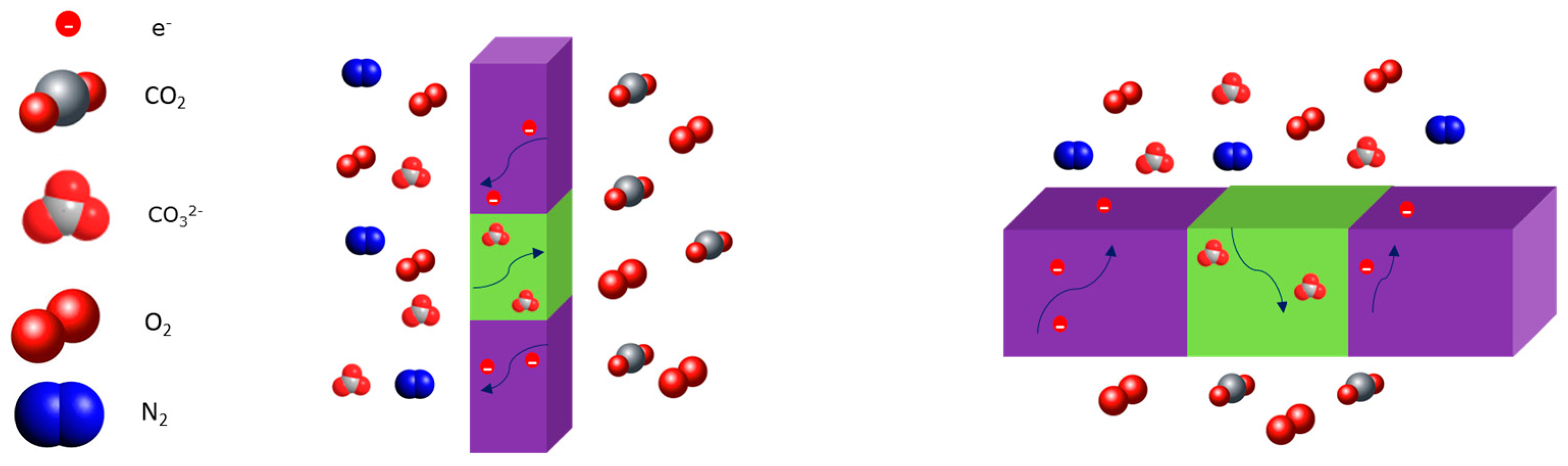
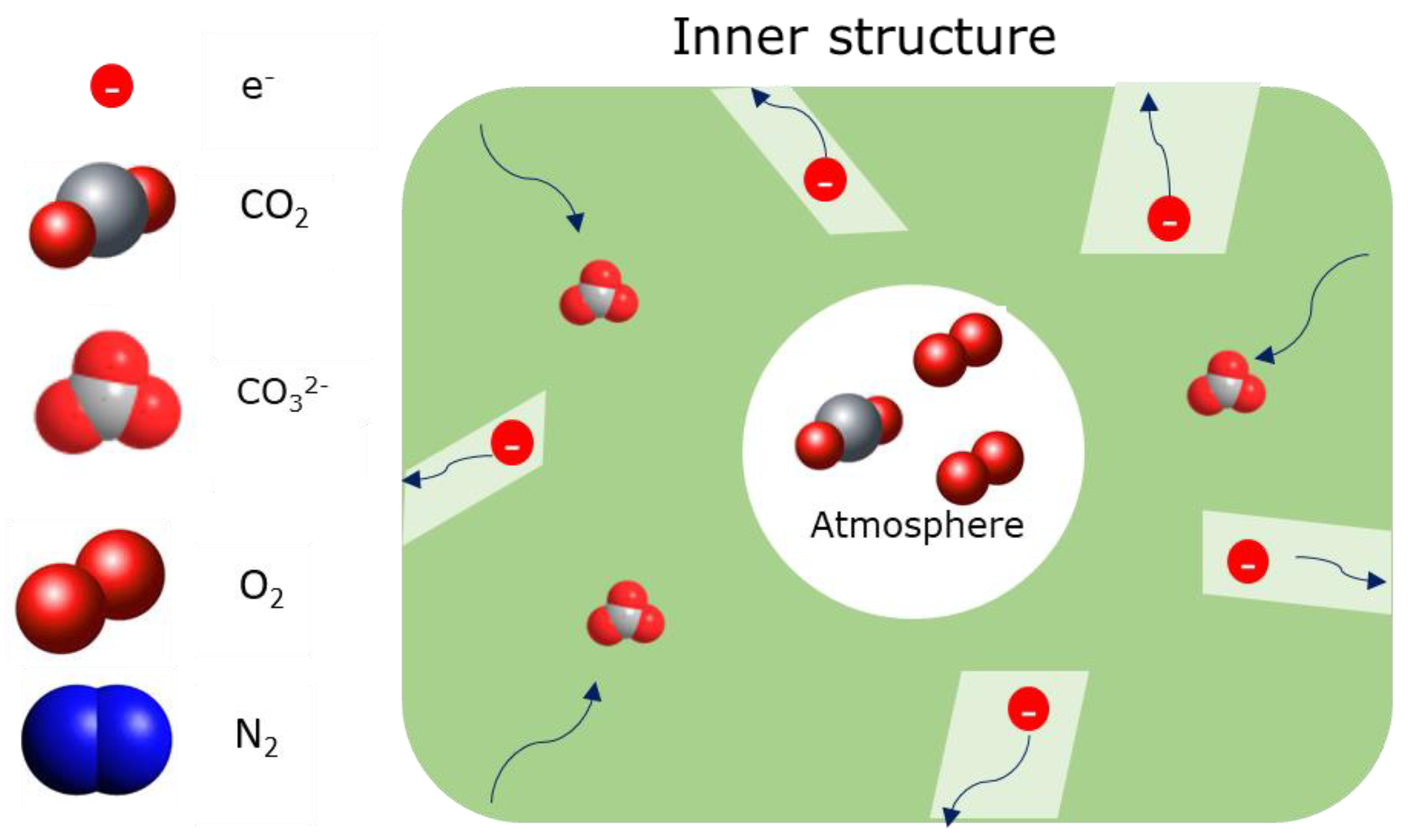
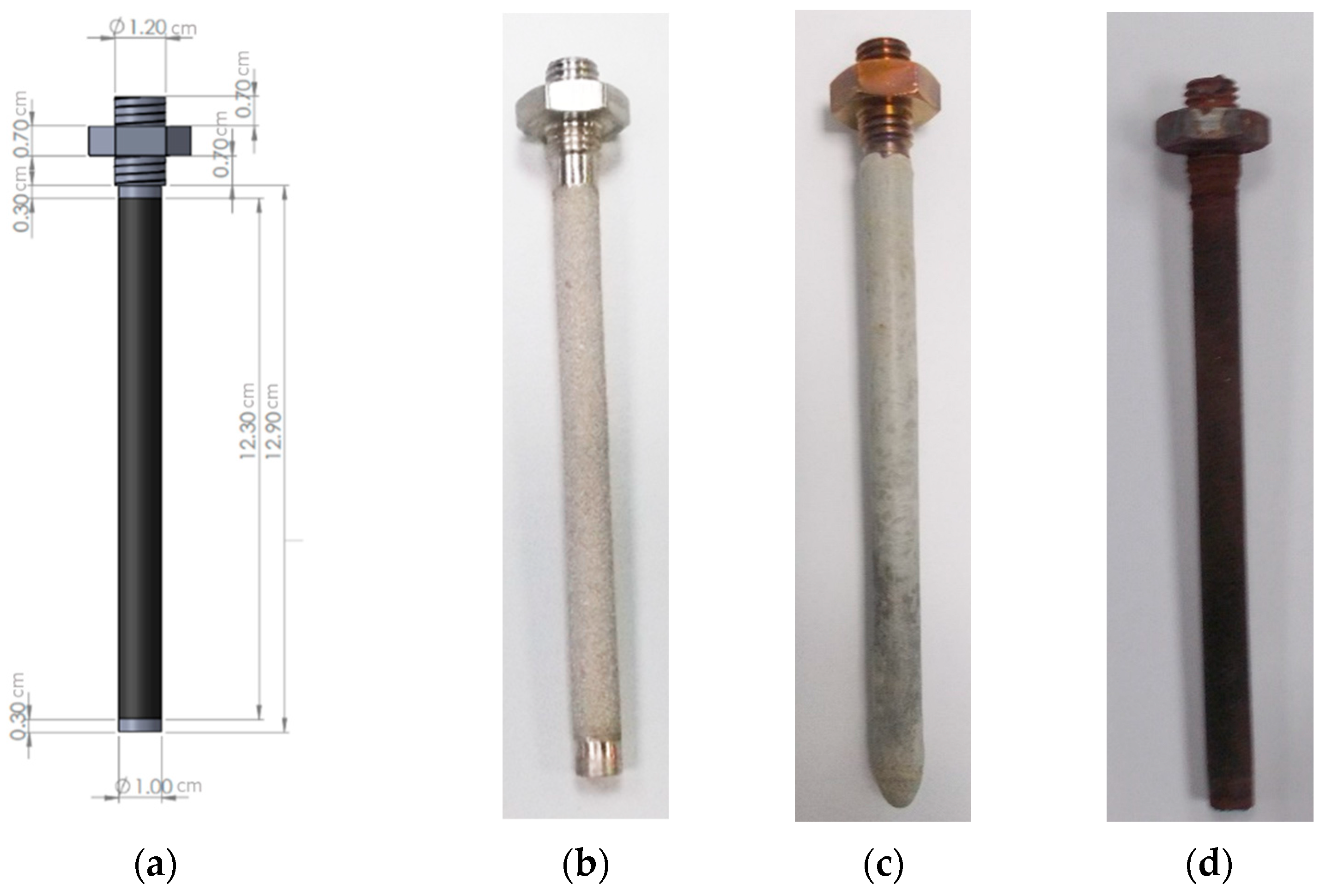


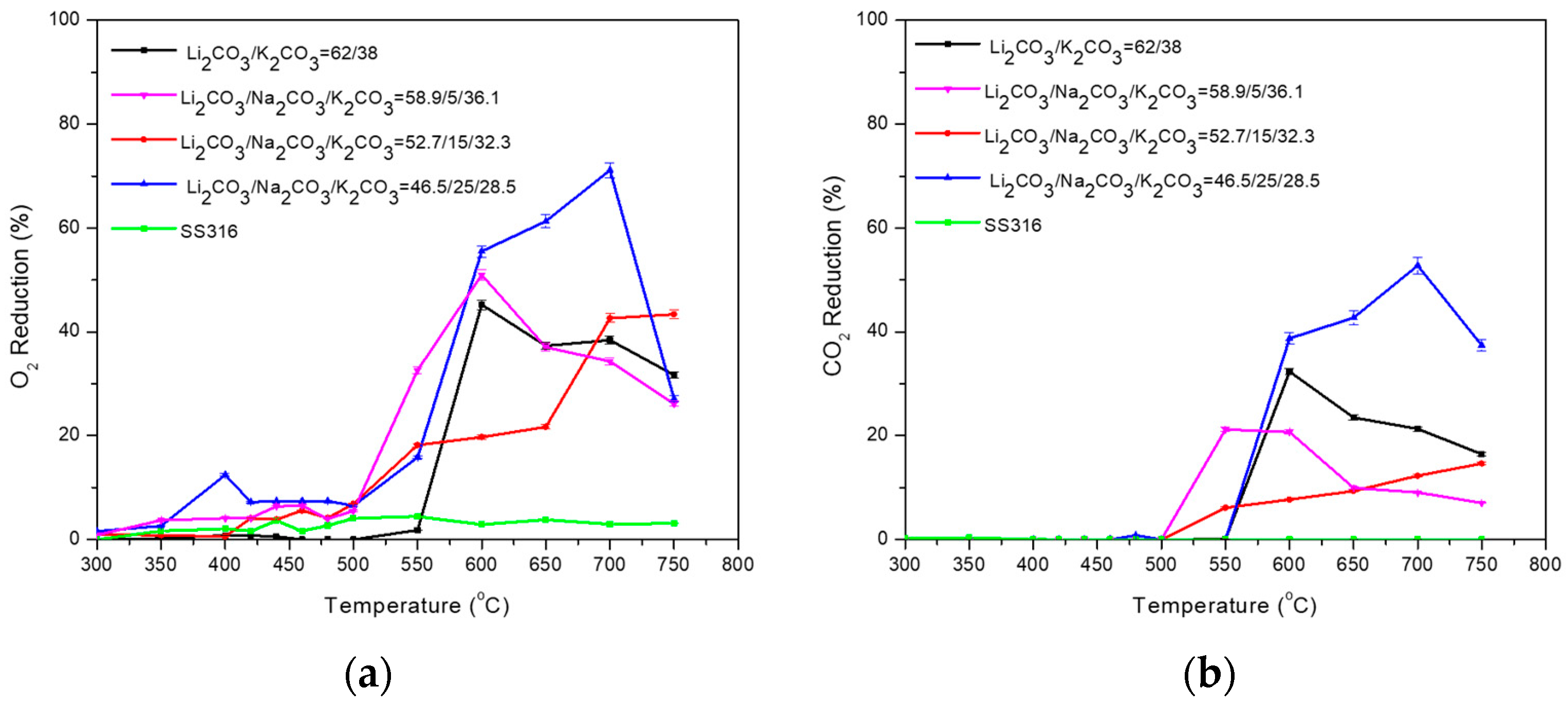
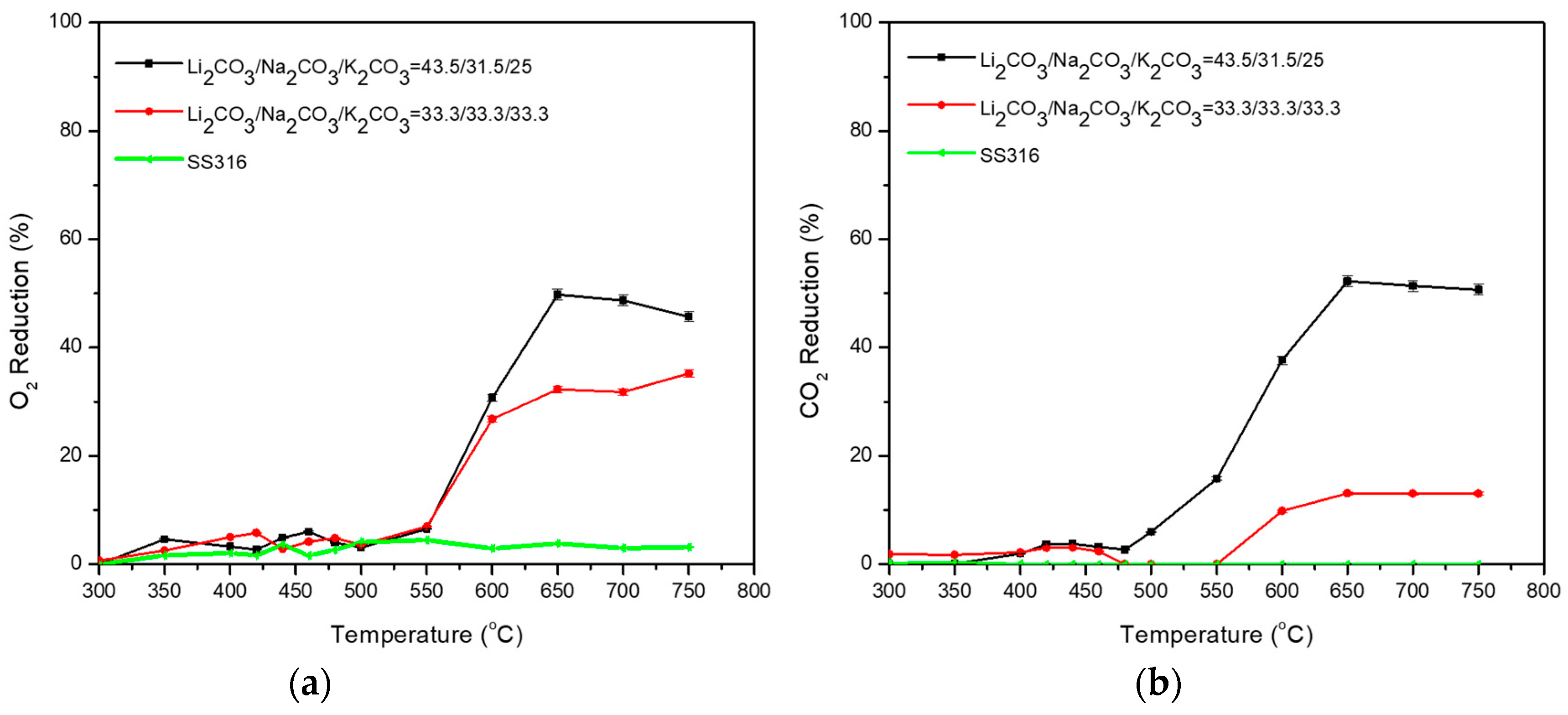
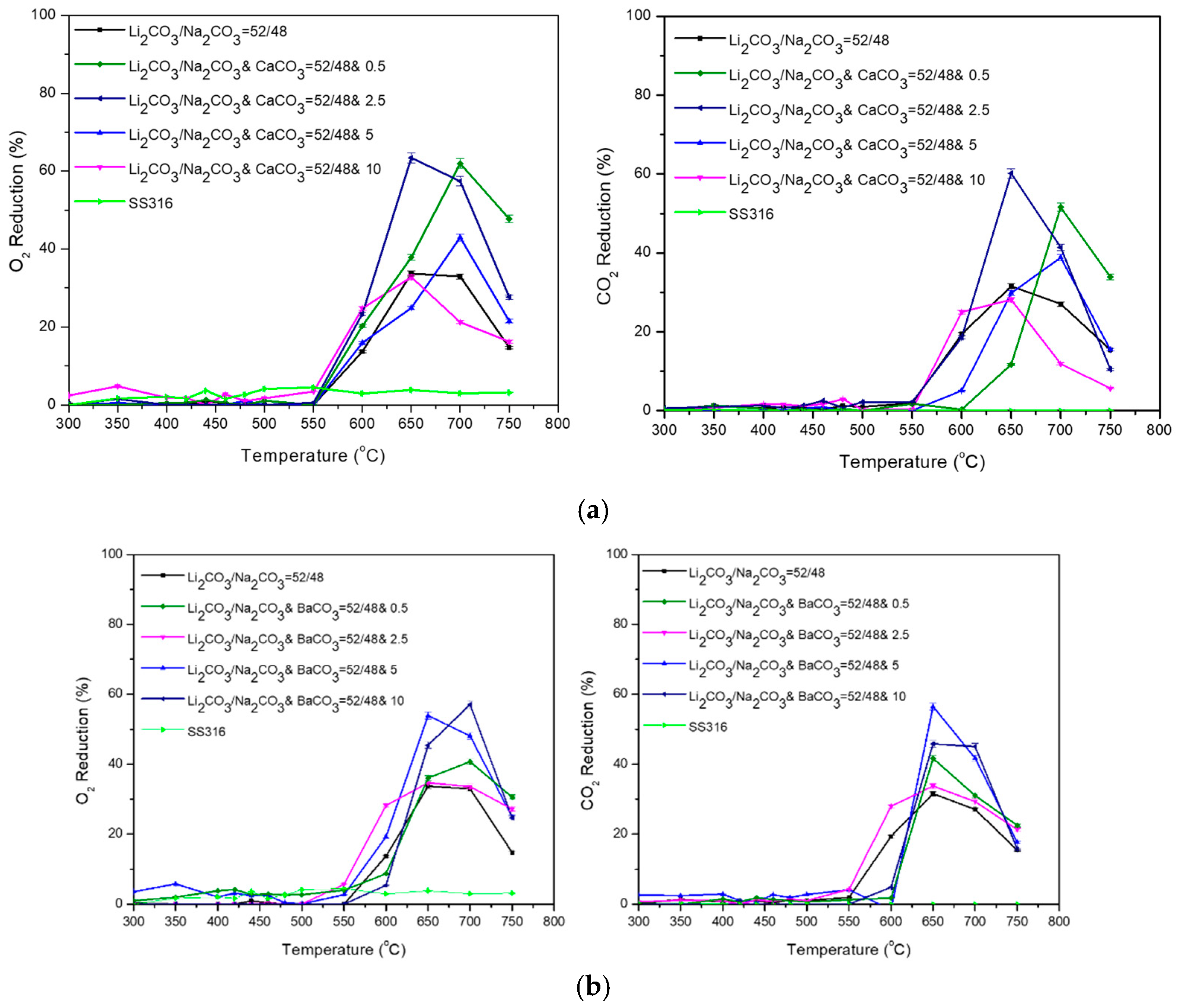
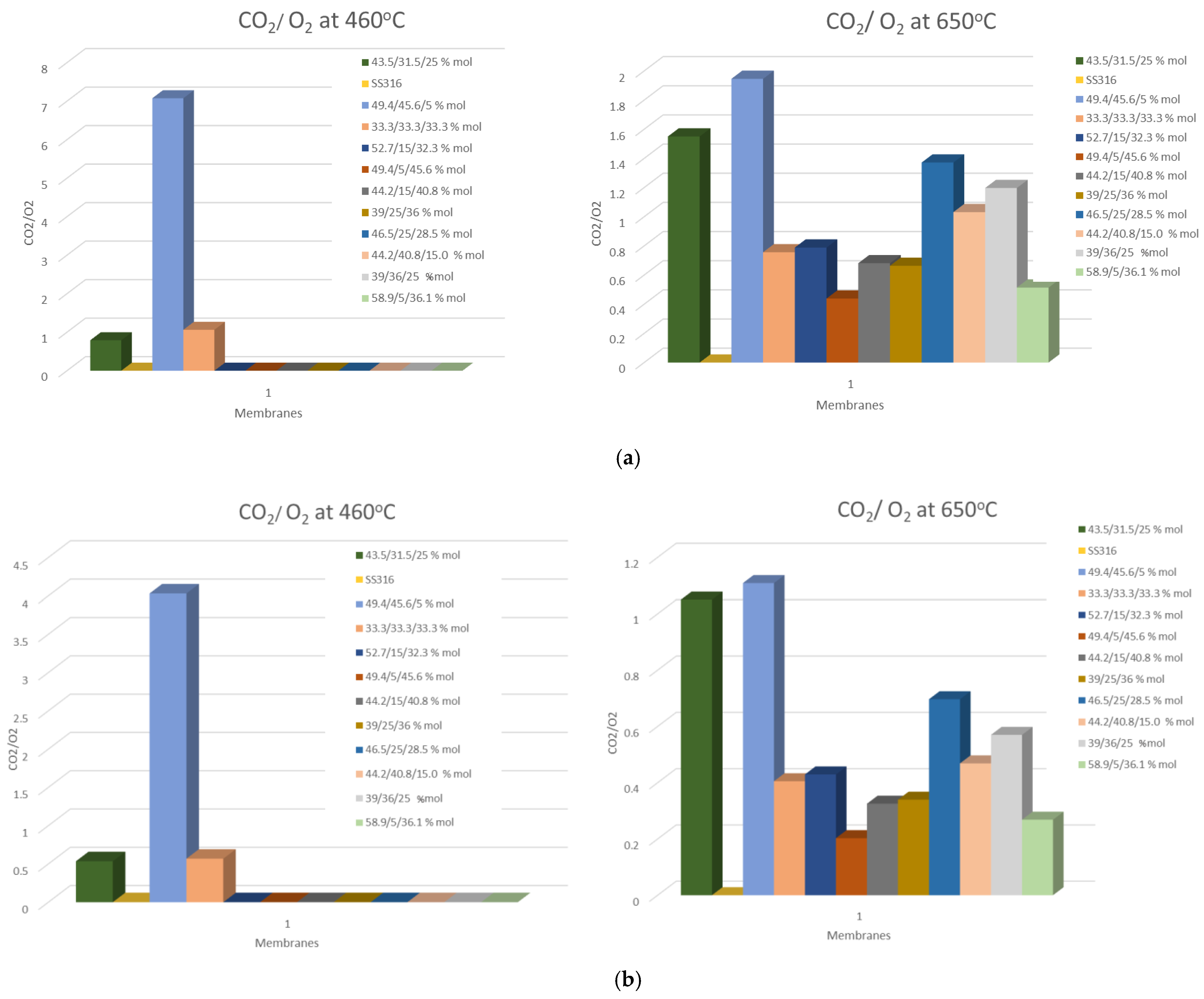
| Carbonate Mixture | Melt Composition (%mol) | Experimental Temperature Window of Phase Change from Solid to Liquid (°C) |
|---|---|---|
| Li2CO3/Na2CO3 | 52/48 | 500–550 |
| Li2CO3/K2CO3 | 52/48 | 480–520 |
| Li2CO3/K2CO3 | 62/38 | 500–530 |
| Carbonate Mixture | Melt Composition (%mol) | Experimental Temperature Window of Phase Change from Solid to Liquid (°C) |
|---|---|---|
| Li2CO3/Na2CO3 | 52/48 | 500–550 |
| Li2CO3/Na2CO3/K2CO3 | 49.4/45.6/5 | 500–530 |
| Li2CO3/Na2CO3/K2CO3 | 44.2/40.8/15 | 500–530 |
| Li2CO3/Na2CO3/K2CO3 | 39/36/25 | 450–520 |
| Carbonate Mixture | Melt Composition (%mol) | Experimental Temperature Window of Phase Change from Solid to Liquid (°C) |
|---|---|---|
| Li2CO3/K2CO3 | 52/48 | 480–520 |
| Li2CO3/Na2CO3/K2CO3 | 49.4/5/45.6 | 480–530 |
| Li2CO3/Na2CO3/K2CO3 | 44.2/15/40.8 | 470–520 |
| Li2CO3/Na2CO3/K2CO3 | 39/25/36 | 450–500 |
| Carbonate Mixture | Melt Composition (%mol) | Experimental Temperature Window of Phase Change from Solid to Liquid (°C) |
|---|---|---|
| Li2CO3/K2CO3 | 62/38 | 500–530 |
| Li2CO3/Na2CO3/K2CO3 | 58.9/5/36.1 | 500–550 |
| Li2CO3/Na2CO3/K2CO3 | 52.7/15/32.3 | 480–530 |
| Li2CO3/Na2CO3/K2CO3 | 46.5/25/28.5 | 470–520 |
| Carbonate Mixture | Melt Composition (%mol) | Experimental Temperature Window of Phase Change from Solid to Liquid (°C) |
|---|---|---|
| Li2CO3/Na2CO3/K2CO3 | 43.5/31.5/25 | 430–500 |
| Li2CO3/Na2CO3/K2CO3 | 33.3/33.3/33.3 | 500–550 |
| Carbonate Mixture | Melt Composition (%mol) |
|---|---|
| Li2CO3/Na2CO3/CaCO3 | 52/48/0.5 |
| Li2CO3/Na2CO3/CaCO3 | 52/48/2.5 |
| Li2CO3/Na2CO3/CaCO3 | 52/48/5 |
| Li2CO3/Na2CO3/CaCO3 | 52/48/10 |
| Li2CO3/Na2CO3/BaCO3 | 52/48/0.5 |
| Li2CO3/Na2CO3/BaCO3 | 52/48/2.5 |
| Li2CO3/Na2CO3/BaCO3 | 52/48/5 |
| Li2CO3/Na2CO3/BaCO3 | 52/48/10 |
| Carbonate Mixture | Melt Composition (%mol) | 500 °C | 550 °C | 600 °C | 650 °C |
|---|---|---|---|---|---|
| O2 Permeation Rate (mol·s−1·m−2) | |||||
| Li2CO3/Na2CO3 | 52/48 | −0.012 | −0.005 | 0.059 | 0.146 |
| Li2CO3/K2CO3 | 52/48 | 0.024 | 0.099 | 0.198 | 0.220 |
| Li2CO3/K2CO3 | 62/38 | −0.001 | 0.009 | 0.212 | 0.175 |
| Carbonate Mixture | Melt Composition (%mol) | 500 °C | 550 °C | 600 °C | 650 °C |
|---|---|---|---|---|---|
| O2 Permeation Rate (mol·s−1·m−2) | |||||
| Li2CO3/Na2CO3 | 52/48 | −0.012 | −0.005 | 0.059 | 0.146 |
| Li2CO3/Na2CO3/K2CO3 | 49.4/45.6/5 | 0.034 | 0.142 | 0.317 | 0.324 |
| Li2CO3/Na2CO3/K2CO3 | 44.2/40.8/15 | 0.061 | 0.201 | 0.254 | 0.217 |
| Li2CO3/Na2CO3/K2CO3 | 39/36/25 | 0.008 | 0.011 | 0.031 | 0.239 |
| Carbonates Mixture | Melt Composition (%mol) | 500 °C | 550 °C | 600 °C | 650 °C |
|---|---|---|---|---|---|
| O2 Permeation Rate (mol·s−1·m−2) | |||||
| Li2CO3/K2CO3 | 52/48 | 0.024 | 0.099 | 0.198 | 0.220 |
| Li2CO3/Na2CO3/K2CO3 | 49.4/5/45.6 | 0.051 | 0.108 | 0.168 | 0.162 |
| Li2CO3/Na2CO3/K2CO3 | 44.2/15/40.8 | 0.049 | 0.143 | 0.161 | 0.194 |
| Li2CO3/Na2CO3/K2CO3 | 39/25/36 | 0.041 | 0.171 | 0.161 | 0.188 |
| Carbonate Mixture | Melt Composition (%mol) | 500 °C | 550 °C | 600 °C | 650 °C |
|---|---|---|---|---|---|
| O2 Permeation Rate (mol·s−1·m−2) | |||||
| Li2CO3/K2CO3 | 62/38 | −0.001 | 0.009 | 0.212 | 0.175 |
| Li2CO3/Na2CO3/K2CO3 | 58.9/5/36.1 | 0.024 | 0.144 | 0.225 | 0.163 |
| Li2CO3/Na2CO3/K2CO3 | 52.7/15/32.3 | 0.032 | 0.084 | 0.092 | 0.101 |
| Li2CO3/Na2CO3/K2CO3 | 46.5/25/28.5 | 0.028 | 0.069 | 0.242 | 0.267 |
| Carbonate Mixture | Melt Composition (%mol) | 500 °C | 550 °C | 600 °C | 650 °C |
|---|---|---|---|---|---|
| O2 Permeation Rate (mol·s−1·m−2) | |||||
| Li2CO3/Na2CO3/K2CO3 | 43.5/31.5/25 | 0.014 | 0.029 | 0.138 | 0.224 |
| Li2CO3/Na2CO3/K2CO3 | 33.3/33.3/33.3 | 0.018 | 0.035 | 0.133 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagoraiou, E.; Cappai, L.; Moschovi, A.M.; Mulas, G.; Yakoumis, I. CO2 and O2 Separation Dual-Phase Membranes for Diesel Heavy-Duty Vehicles Applications. Membranes 2025, 15, 49. https://doi.org/10.3390/membranes15020049
Zagoraiou E, Cappai L, Moschovi AM, Mulas G, Yakoumis I. CO2 and O2 Separation Dual-Phase Membranes for Diesel Heavy-Duty Vehicles Applications. Membranes. 2025; 15(2):49. https://doi.org/10.3390/membranes15020049
Chicago/Turabian StyleZagoraiou, Eirini, Luca Cappai, Anastasia Maria Moschovi, Gabriele Mulas, and Iakovos Yakoumis. 2025. "CO2 and O2 Separation Dual-Phase Membranes for Diesel Heavy-Duty Vehicles Applications" Membranes 15, no. 2: 49. https://doi.org/10.3390/membranes15020049
APA StyleZagoraiou, E., Cappai, L., Moschovi, A. M., Mulas, G., & Yakoumis, I. (2025). CO2 and O2 Separation Dual-Phase Membranes for Diesel Heavy-Duty Vehicles Applications. Membranes, 15(2), 49. https://doi.org/10.3390/membranes15020049







