On a Specific Method for Characterizing Ion Exchange Membranes to Assess Their Functionality in Salinity Gradient Power Generation Through Reverse Electrodialysis, Including the Effect of Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Ion Conductive Membranes
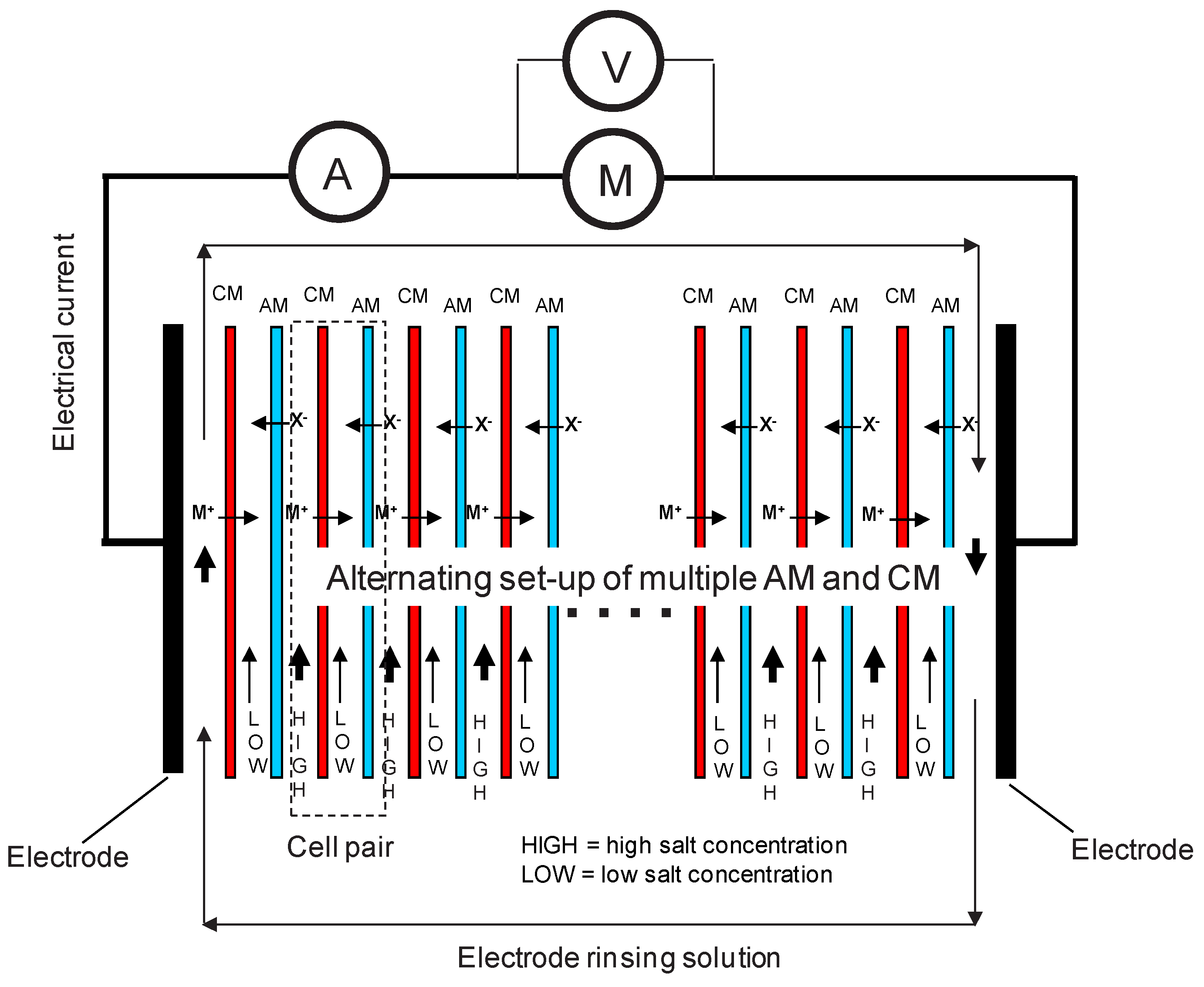
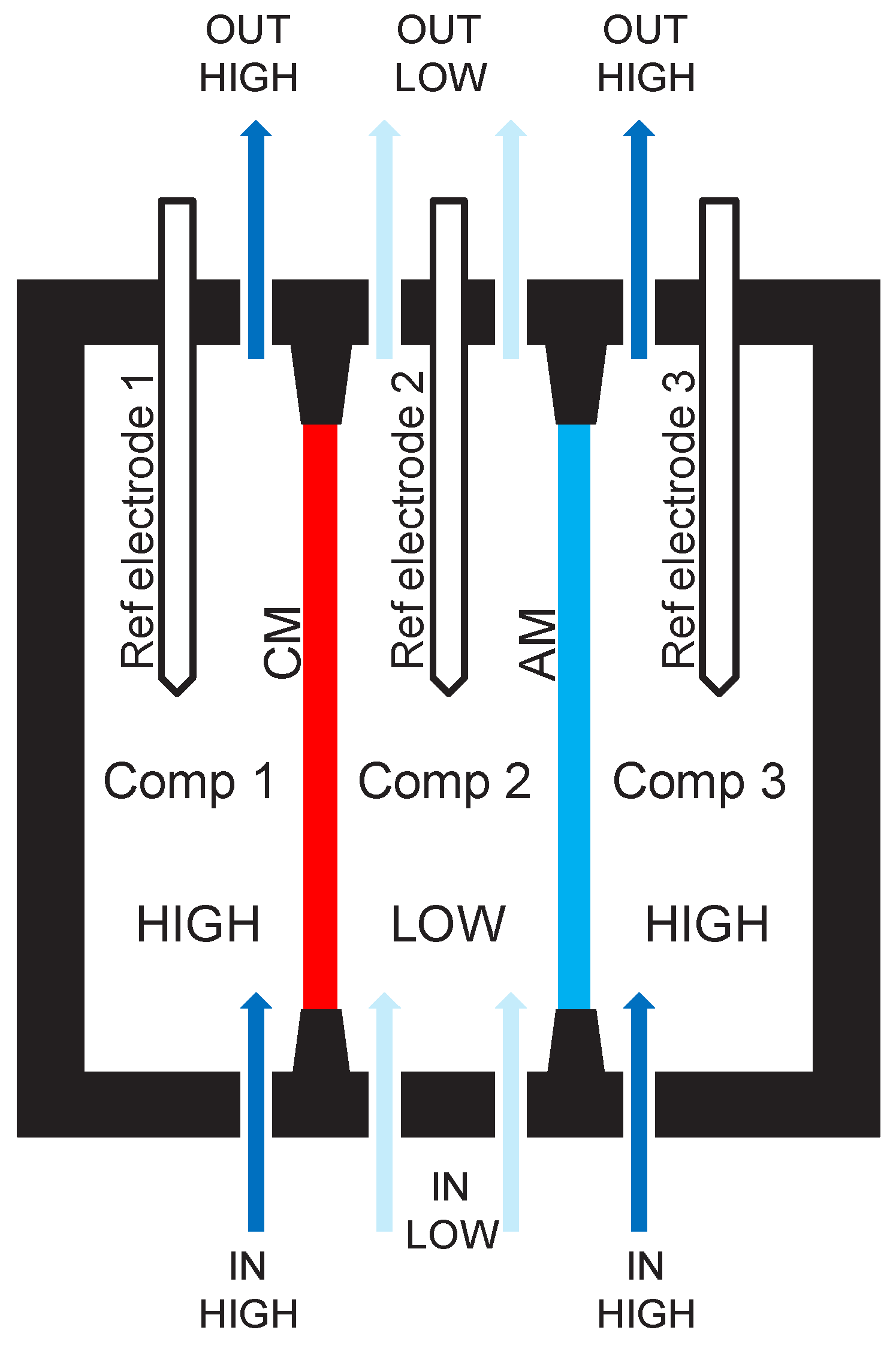
2.2. Pragmatic Principle of a Three-Compartment Set-Up and Data Acquisition
- -
- Compartment 1 is separated from compartment 2 by a cation exchange membrane (CM).
- -
- Compartment 2 is separated from compartment 3 by an anion exchange membrane (AM).
2.3. Data Modeling and Processing
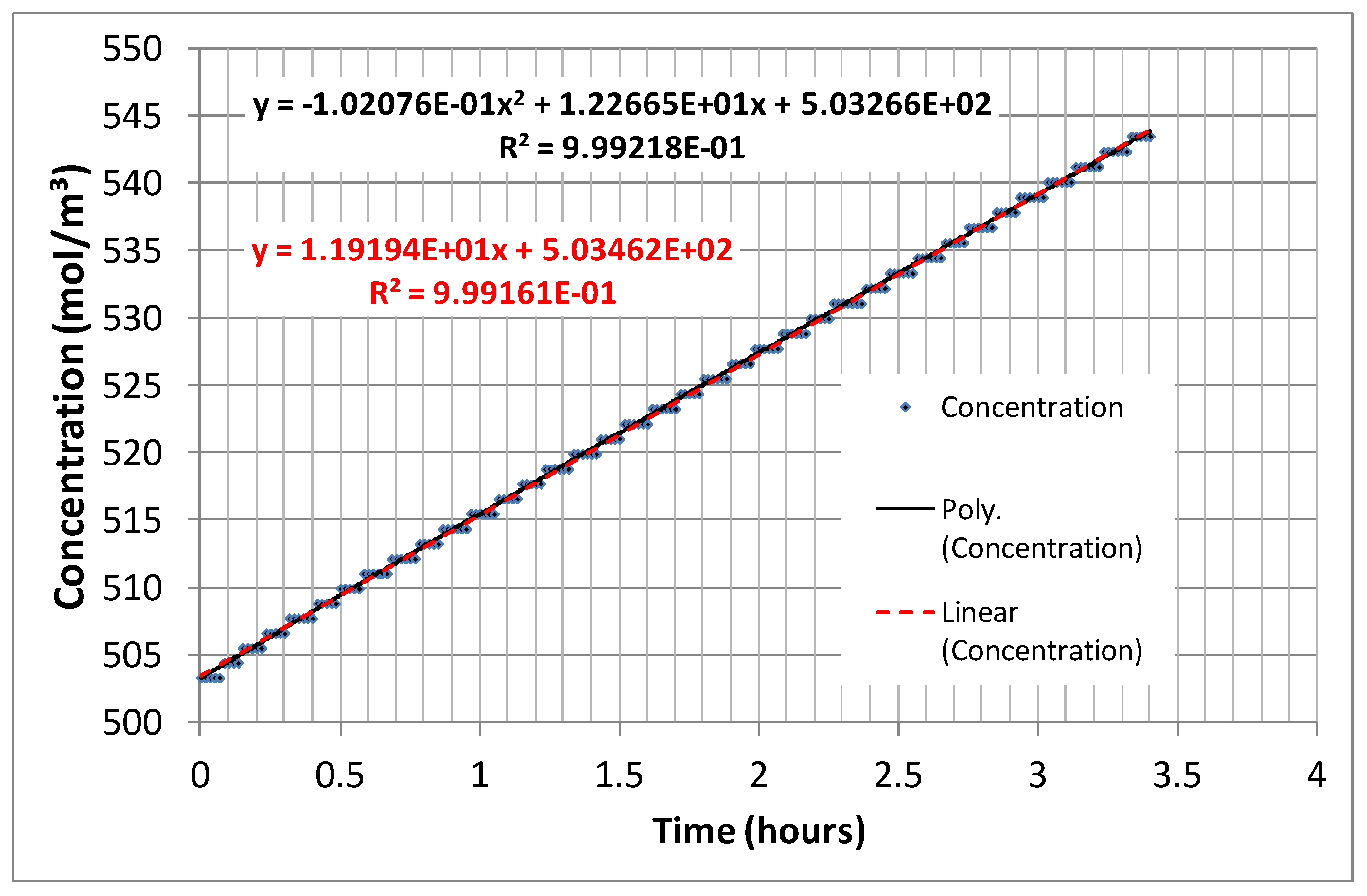
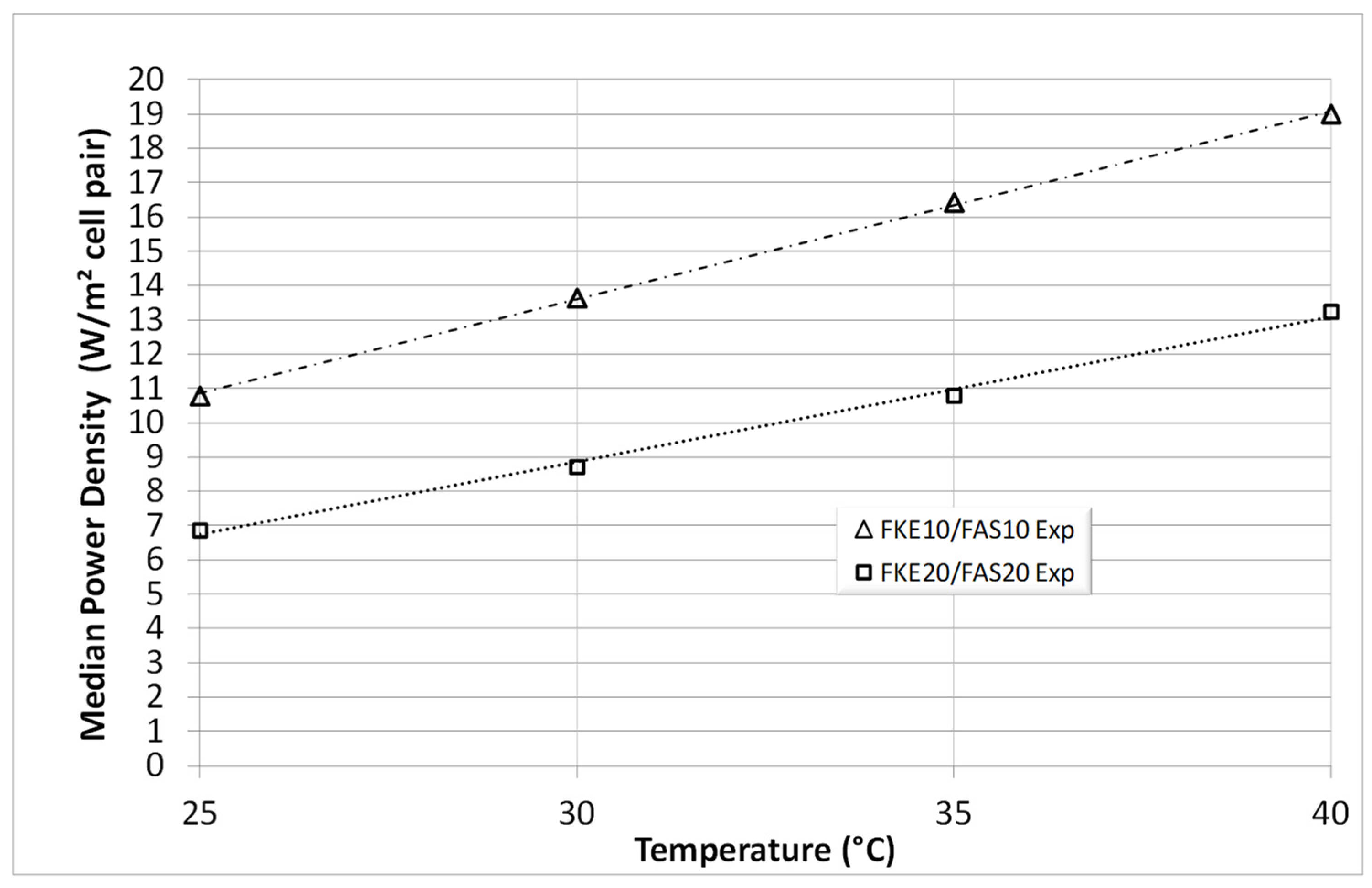
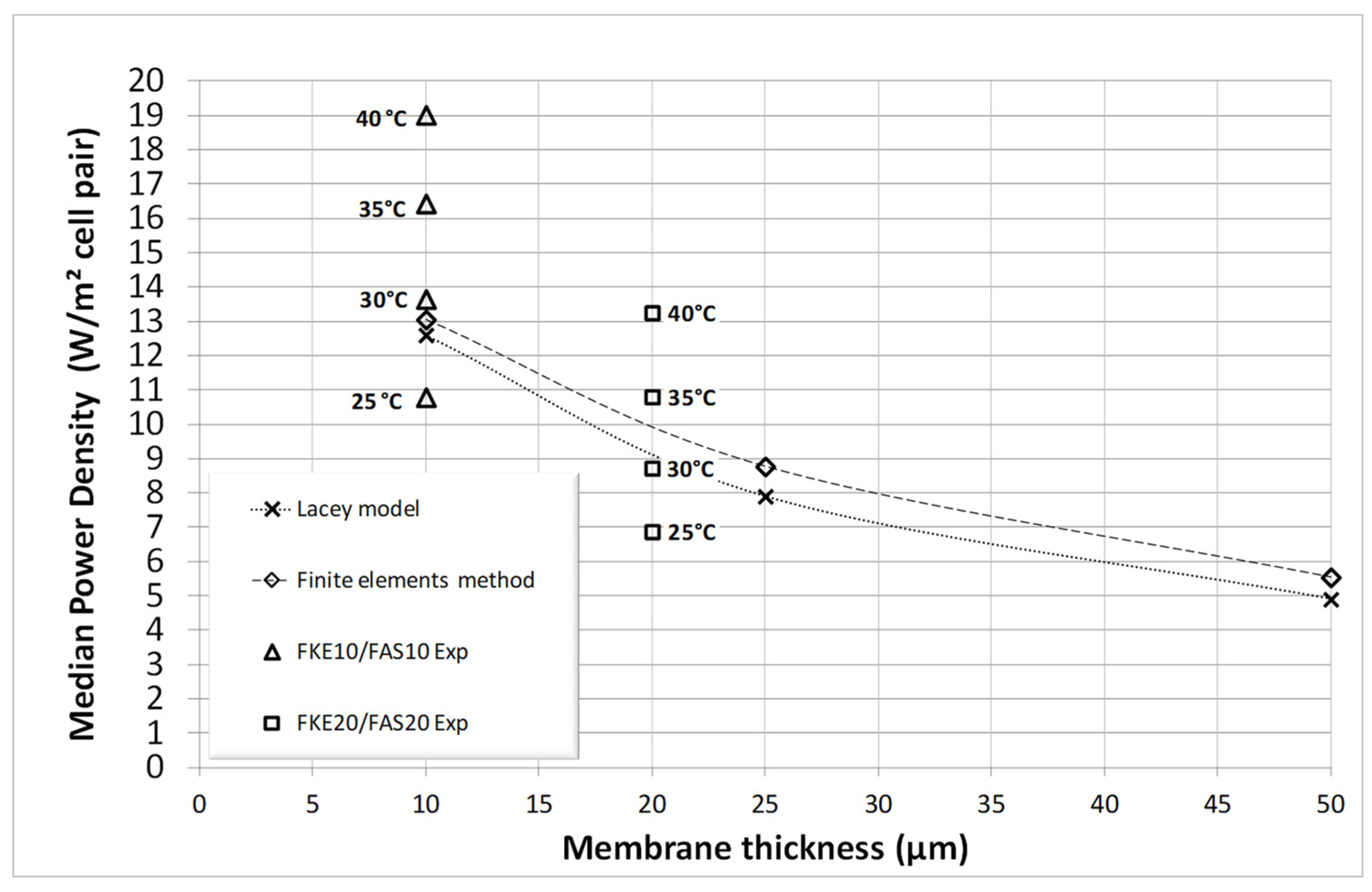
3. Results and Discussion
- -
- Ion flux, which corresponds to current density when multiplied by the Faraday constant.
- -
- The voltage across the membranes, used in Equation (3).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattle, R.E. Production of electric power by mixing fresh and salt water in the hydroelectric pile. Nature 1954, 174, 660. Available online: https://www.nature.com/articles/174660a0 (accessed on 28 November 2024). [CrossRef]
- Weinstein, J.N.; Leitz, F.B. Electric power from differences in salinity: The dialytic battery. Science 1976, 191, 557–559. Available online: https://www.science.org/doi/10.1126/science.191.4227.557 (accessed on 28 November 2024). [CrossRef]
- Wick, G.L.; Isaacs, J.D. Salt Domes: Is there more energy available from their salt than from their oil? Science 1978, 199, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Lacey, R.E. Energy by reverse electrodialysis. Ocean Eng. 1980, 7, 1–47. [Google Scholar] [CrossRef]
- Loeb, S. Large-scale power production by pressure-retarded osmosis, using river water and sea water passing through spiral modules. Desalination 2002, 143, 115–122. [Google Scholar] [CrossRef]
- Brauns, E. Combination of a Desalination Plant and a Salinity Gradient Power Reverse Electrodialysis Plant and Use Thereof. U.S. Patent PCT/BE2006/000078, 19 July 2006. Available online: https://patents.google.com/patent/US8323491B2/en (accessed on 28 November 2024).
- Brauns, E. Towards a worldwide sustainable and simultaneous large scale production of renewable energy and potable water through salinity gradient power by combining reversed electrodialysis and solar power? Desalination 2008, 219, 312–323. [Google Scholar] [CrossRef]
- Brauns, E. An alternative hybrid concept combining seawater desalination, solar energy and reverse electrodialysis for a sustainable production of sweet water and electrical energy. Desalin. Water Treat. 2010, 13, 53–62. [Google Scholar] [CrossRef]
- Post, J.W.; Veerman, J.; Hamelers, H.V.M.; Euverink, G.J.W.; Metz, S.J.; Nymeijer, K.; Buisman, C.J.N. Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis. J. Membr. Sci. 2007, 288, 218–230. [Google Scholar] [CrossRef]
- Post, J.W. Blue Energy, Electricity Production from Salinity Gradients by Reverse Electrodialysis. Ph.D. Thesis, University of Wageningen, Wageningen, The Netherlands, 3 November 2009. Available online: https://www.waddenacademie.nl/fileadmin/inhoud/pdf/06-wadweten/Proefschriften/thesis_jan_Post.pdf (accessed on 28 November 2024).
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Energy recovery from controlled mixing salt and fresh water with a reverse electrodialysis system. Environ. Sci. Technol. 2008, 42, 5785–5790. [Google Scholar] [CrossRef]
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Influence of multivalent ions on power production from mixing salt and fresh water with a reverse electrodialysis system. J. Membr. Sci. 2009, 330, 65–72. [Google Scholar] [CrossRef]
- Brauns, E. Salinity gradient power by reverse electrodialysis: Effect of model parameters on electrical power output. Desalination 2009, 237, 378–391. [Google Scholar] [CrossRef]
- Brauns, E. Finite elements-based 2D theoretical analysis of the effect of IEX membrane thickness and salt solution residence time on the ion transport within a salinity gradient power reverse electrodialysis half cell pair. Desalin. Water Treat. 2013, 51, 6429–6443. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes (Membrane Science and Technology Series Volume 9), 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780444502360. [Google Scholar]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef]
- Tufa, R.A.; Curcio, E.; Brauns, E.; van Baak, W.; Fontananova, E.; Di Profio, G. Membrane Distillation and Reverse Electrodialysis for Near-Zero Liquid Discharge and low energy seawater desalination. J. Membr. Sci. 2015, 496, 325–333. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Pei, Y.; Crittenden, J.C. Unique applications and improvements of reverse electrodialysis: A review and outlook. Appl. Energy 2020, 262, 114482. [Google Scholar] [CrossRef]
- Chae, S.; Kim, H.; Hong, J.G.; Jang, J.; Higa, M.; Pishnamazi, M.; Choi, J.-Y.; Walgama, R.C.; Bae, C.; Kim, I.S.; et al. Clean power generation from salinity gradient using reverse electrodialysis technologies: Recent advances, bottlenecks, and future direction. Chem. Eng. J. 2023, 452, 139482. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Ujike, R.; Higa, M.; Kakihana, Y.; Higa, M. Power Generation Performance of Reverse Electrodialysis (RED) Using Various Ion Exchange Membranes and Power Output Prediction for a Large RED Stack. Membranes 2022, 12, 1141. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Cui, W.-Z.; Ji, Z.-Y.; Tumba, K.; Wang, J.; Fu, L.-J.; Zhang, Z.-X.; Liu, J.; Zhao, Y.-Y.; Zhang, Z.-D.; et al. Deep utilization of salinity gradient energy between concentrated seawater and river water by multi-stage reverse electrodialysis. Desalination 2023, 566, 116900. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z.; Lv, Y.; Zhang, Y.; Xu, S.; Zhu, X. Effects of multivalent ions on hydrogen production from the salinity gradient between desalination concentrated brine and river by reverse electrodialysis. Desalination 2023, 567, 116953. [Google Scholar] [CrossRef]
- Ranade, A.; Singh, K.; Tamburini, A.; Micale, G.; Vermaas, D.A. Feasibility of Producing Electricity, Hydrogen, and Chlorine via Reverse Electrodialysis. Environ. Sci. Technol. 2022, 56, 16062–16072. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Wang, J.; Feng, Z.; Li, X.; Cao, M. Highly conductive anti-fouling anion exchange membranes for power generation by reverse electrodialysis. J. Power Sources 2024, 598, 234176. [Google Scholar] [CrossRef]
- Ma, L.; Gutierrez, L.; Van Vooren, T.; Vanoppen, M.; Kazemabad, M.; Verliefde, A.; Cornelissen, E. Fate of organic micropollutants in reverse electrodialysis: Influence of membrane fouling and channel clogging. Desalination 2021, 512, 115114. [Google Scholar] [CrossRef]

| FKS20-FAS20 | FKE20-FAS20 | |||||
|---|---|---|---|---|---|---|
| t1 | t2 | Mean Value at t1&t2 | t1 | t2 | Mean Value at t1&t2 | |
| Temp. | Dmemb (m2/s) | Dmemb (m2/s) | Dmemb (m2/s) | Dmemb (m2/s) | Dmemb (m2/s) | Dmemb (m2/s) |
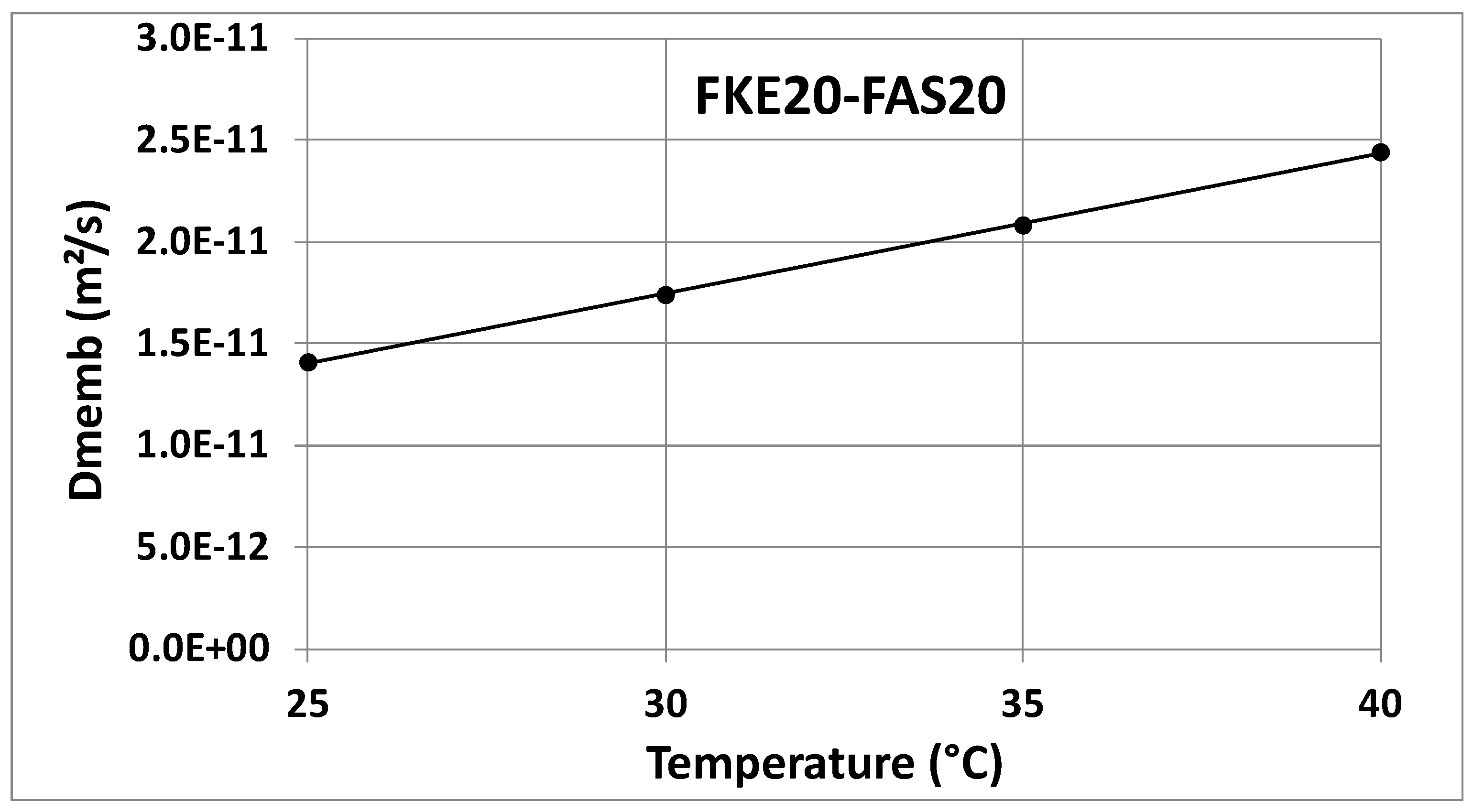
| 25 °C | 1.402 × 10−11 | 1.430 × 10−11 | 1.416 × 10−11 | 1.373 × 10−11 | 1.440 × 10−11 | 1.407 × 10−11 |
| 30 °C | 1.506 × 10−11 | 1.558 × 10−11 | 1.532 × 10−11 | 1.703 × 10−11 | 1.772 × 10−11 | 1.738 × 10−11 |
| 35 °C | 1.980 × 10−11 | 2.077 × 10−11 | 2.028 × 10−11 | 2.042 × 10−11 | 2.118 × 10−11 | 2.080 × 10−11 |
| 40 °C | 2.182 × 10−11 | 2.313 × 10−11 | 2.248 × 10−11 | 2.371 × 10−11 | 2.508 × 10−11 | 2.440 × 10−11 |
| FKS20_FAS20 | A | B | Mean of A&B | C | D | Mean of C&D |
| Power Dens. @0 h | Power Dens. @1 h | Power Dens. | Current Dens. @0 h | Current Dens. @1 h | Current Dens. | |
| (W/m2) | (W/m2) | (W/m2) | (A/m2) | (A/m2) | (A/m2) | |
| 25 °C | 7.38 | 7.32 | 7.35 | 163.5 | 164.4 | 164.0 |
| 30 °C | 7.93 | 8.22 | 8.08 | 173.9 | 179.9 | 176.9 |
| 35 °C | 10.62 | 10.78 | 10.70 | 228.5 | 235.7 | 232.1 |
| 40 °C | 11.83 | 12.13 | 11.98 | 251.2 | 261.8 | 256.5 |
| FKE_FAS | A | B | Mean of A&B | C | D | Mean of C&D |
| Power Dens. @0 h | Power Dens. @1 h | Power Dens. | Current Dens. @0 h | Current Dens. @1 h | Current Dens. | |
| (W/m2) | (W/m2) | (W/m2) | (A/m2) | (A/m2) | (A/m2) | |
| 25 °C | 6.79 | 6.94 | 6.87 | 156.0 | 161.6 | 158.8 |
| 30 °C | 8.65 | 8.78 | 8.72 | 194.5 | 199.7 | 197.1 |
| 35 °C | 10.73 | 10.86 | 10.80 | 235.2 | 240.9 | 238.1 |
| 40 °C | 13.13 | 13.37 | 13.25 | 280.3 | 290.9 | 285.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauns, E.; Helsen, J. On a Specific Method for Characterizing Ion Exchange Membranes to Assess Their Functionality in Salinity Gradient Power Generation Through Reverse Electrodialysis, Including the Effect of Temperature. Membranes 2024, 14, 255. https://doi.org/10.3390/membranes14120255
Brauns E, Helsen J. On a Specific Method for Characterizing Ion Exchange Membranes to Assess Their Functionality in Salinity Gradient Power Generation Through Reverse Electrodialysis, Including the Effect of Temperature. Membranes. 2024; 14(12):255. https://doi.org/10.3390/membranes14120255
Chicago/Turabian StyleBrauns, Etienne, and Joost Helsen. 2024. "On a Specific Method for Characterizing Ion Exchange Membranes to Assess Their Functionality in Salinity Gradient Power Generation Through Reverse Electrodialysis, Including the Effect of Temperature" Membranes 14, no. 12: 255. https://doi.org/10.3390/membranes14120255
APA StyleBrauns, E., & Helsen, J. (2024). On a Specific Method for Characterizing Ion Exchange Membranes to Assess Their Functionality in Salinity Gradient Power Generation Through Reverse Electrodialysis, Including the Effect of Temperature. Membranes, 14(12), 255. https://doi.org/10.3390/membranes14120255






