A Facile Way to Fabricate GO-EDA/Al2O3 Tubular Nanofiltration Membranes with Enhanced Desalination Stability via Fine-Tuning the pH of the Membrane-Forming Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
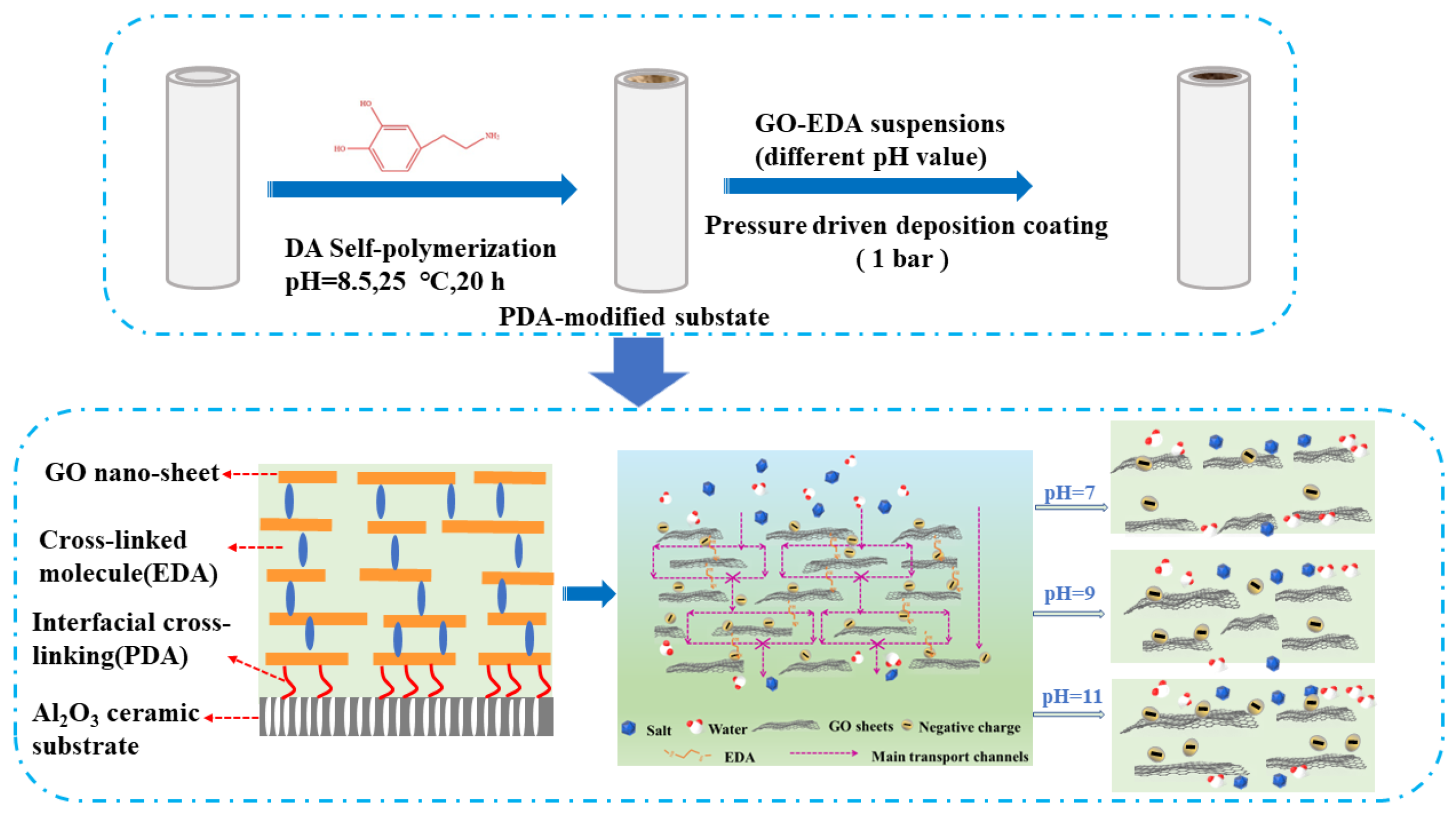
2.2. Preparation of PDA-Modified Tubular Al2O3 Ceramic Membrane
2.3. Preparation of EDA-Crosslinked GO-EDA/Al2O3 Membrane
2.4. Characterizations
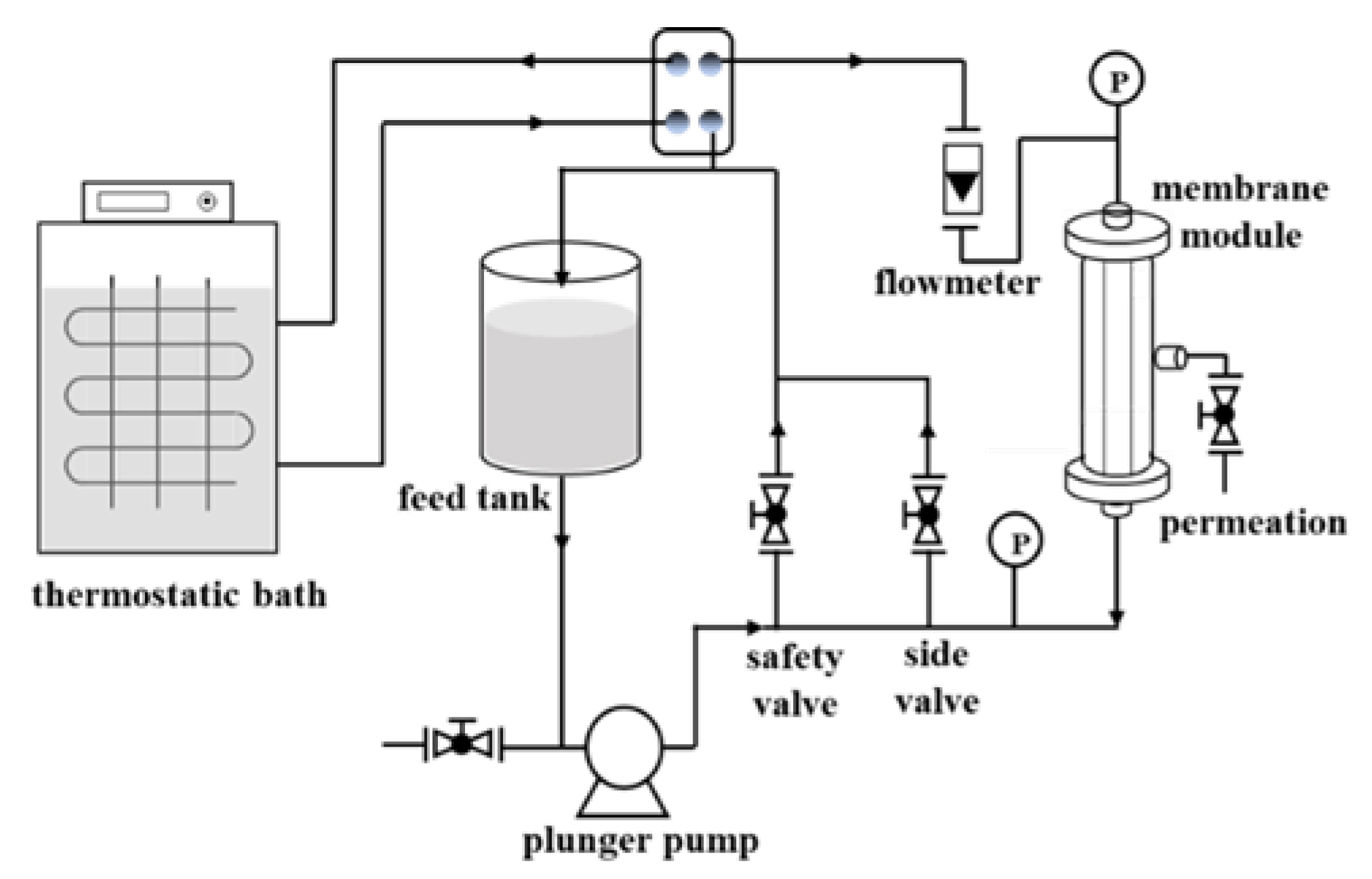
2.5. Evaluation of Membrane Performance
3. Results and Discussion
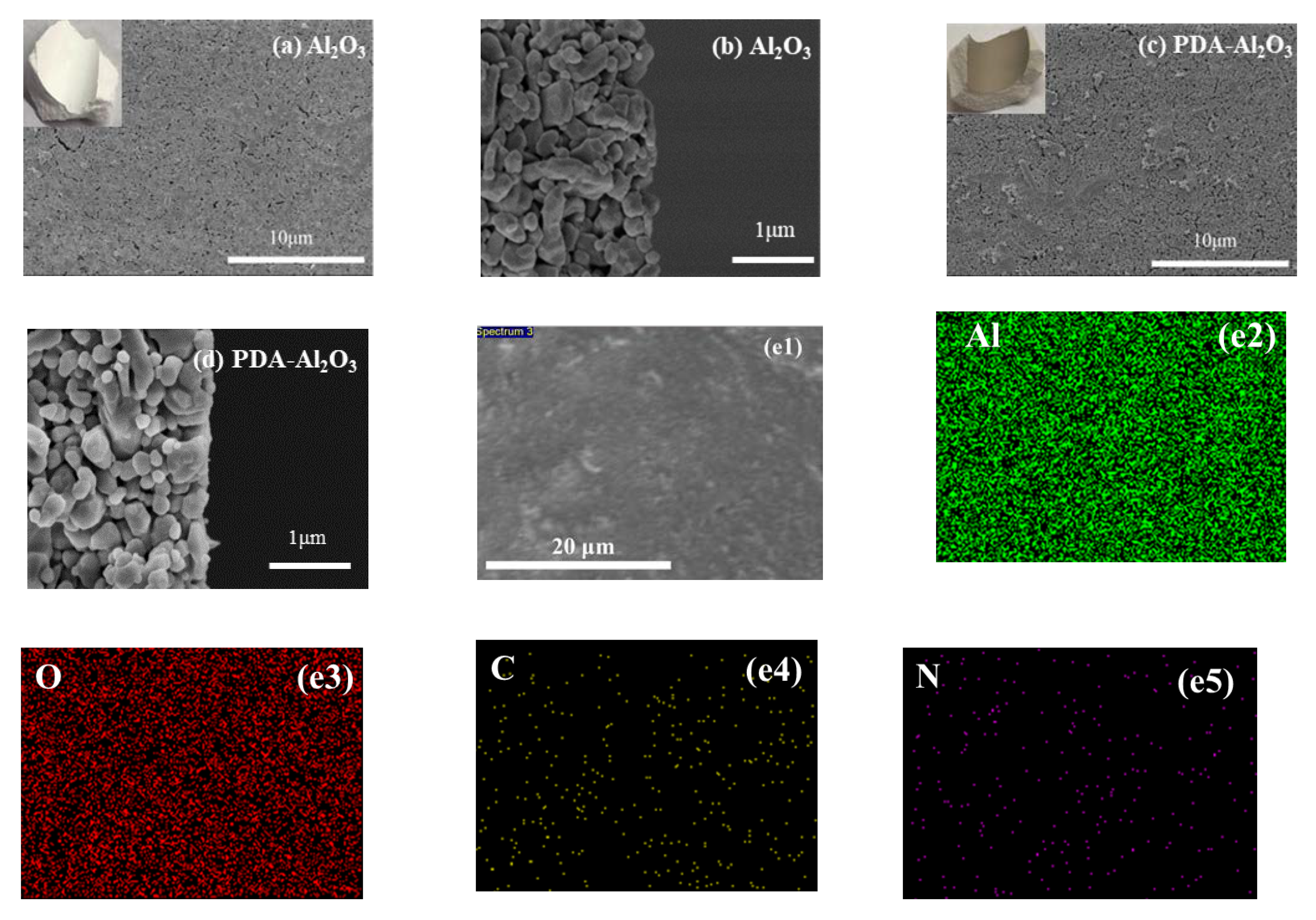
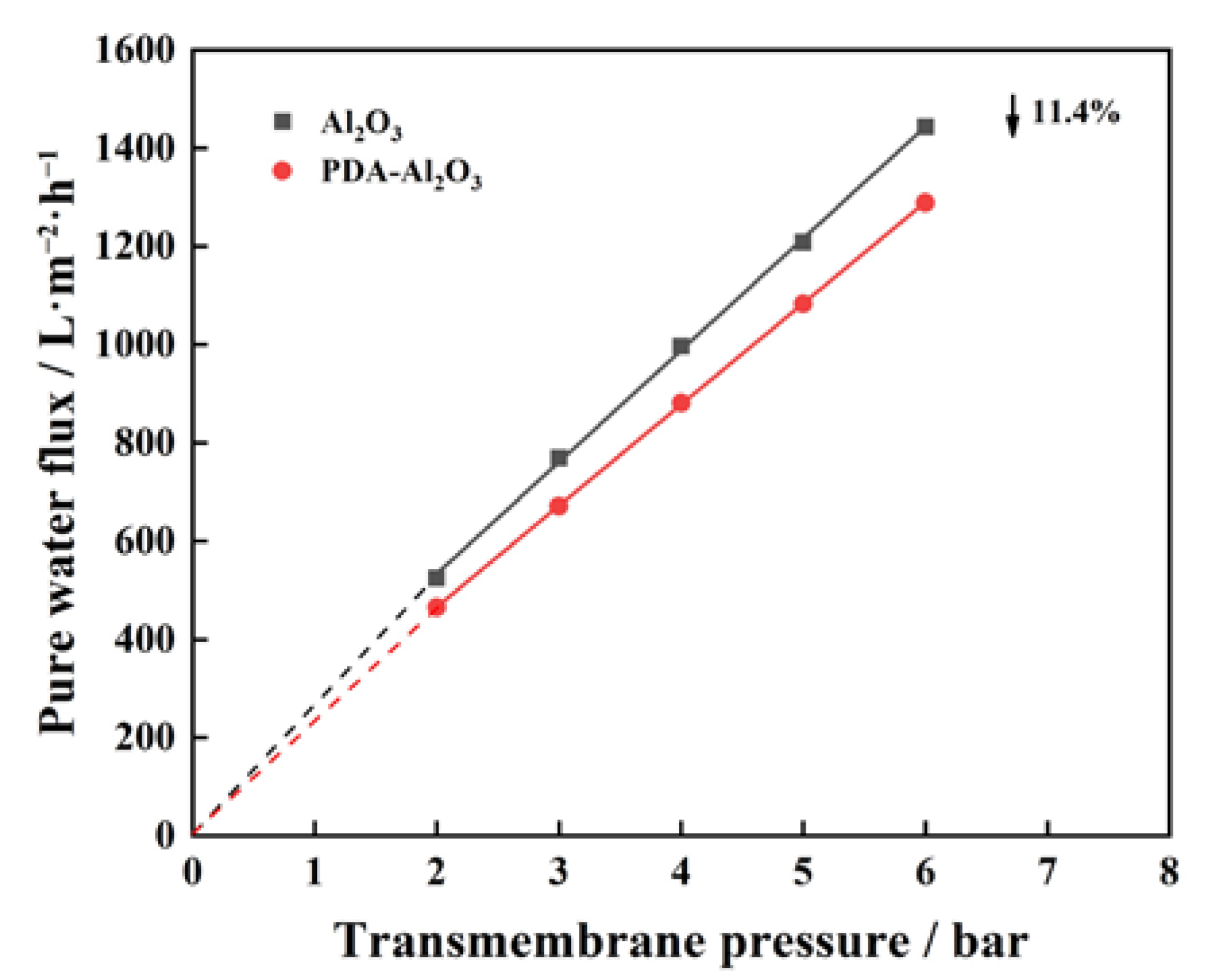
3.1. Characterization and Permeability of PDA-Al2O3 Membrane
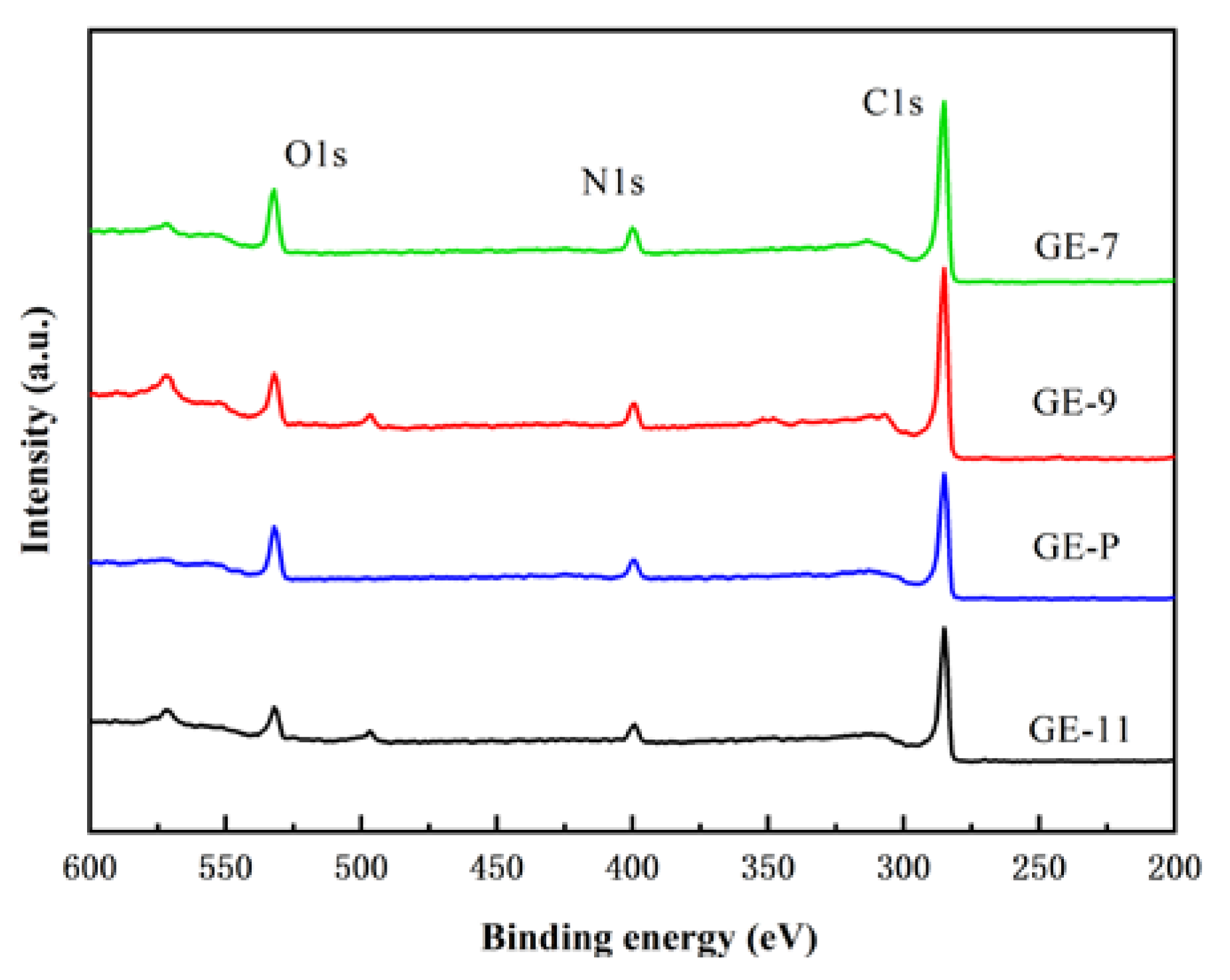
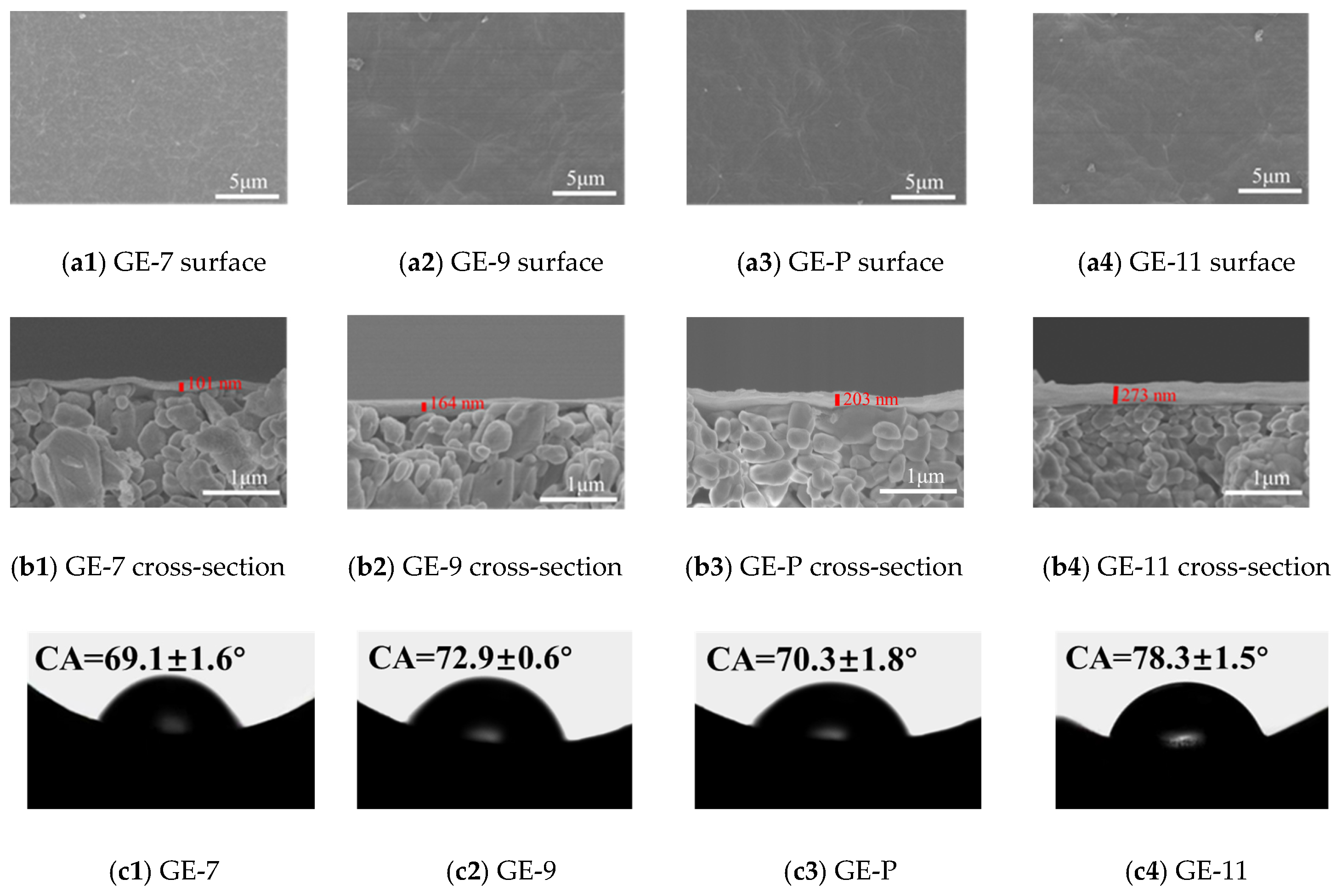
3.2. Characterizations of GO-EDA/Al2O3 Membranes
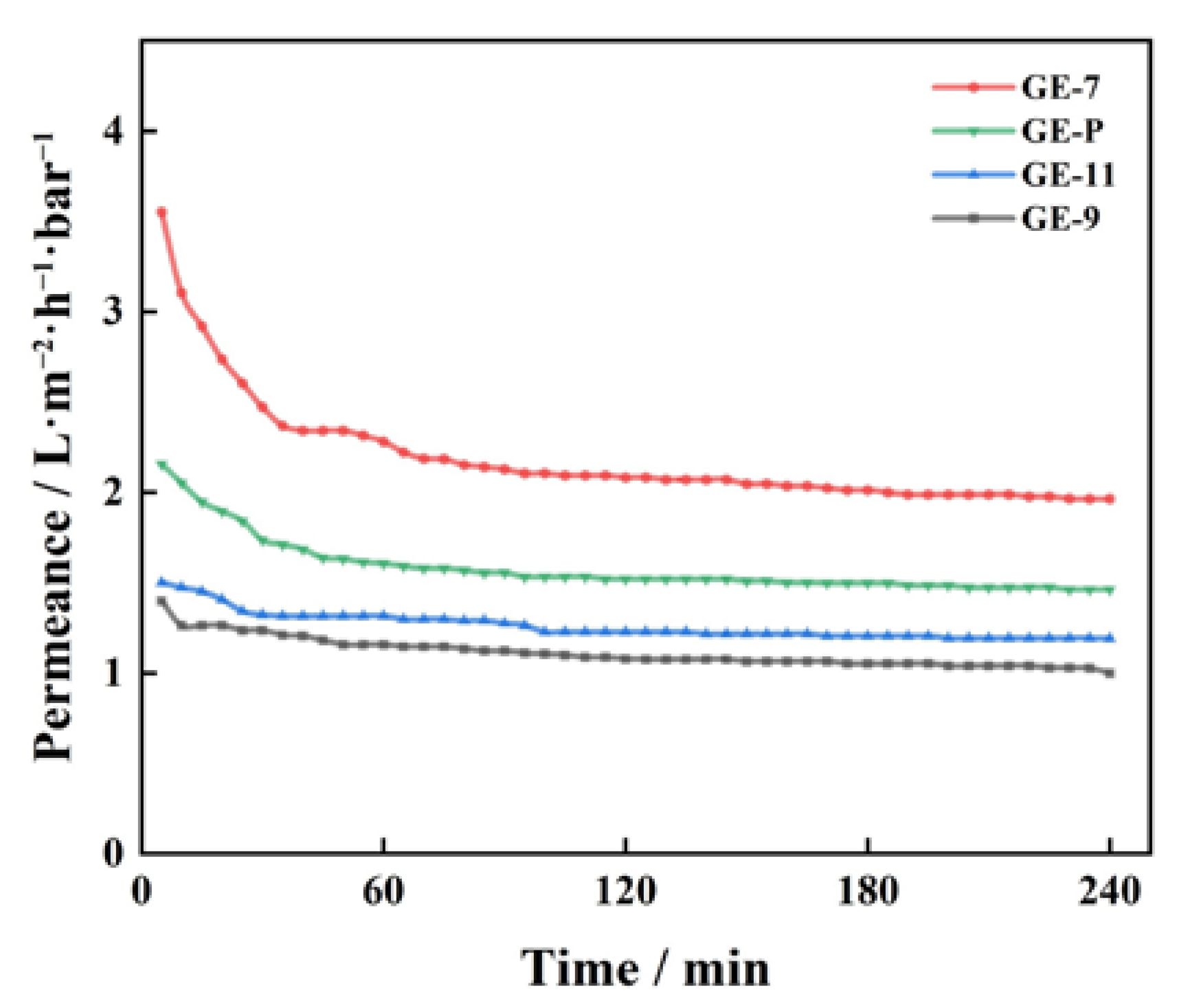
3.3. Pure Water Permeability of GO-EDA/Al2O3 Membranes
3.4. Desalination Performance of GO-EDA/Al2O3 Membranes
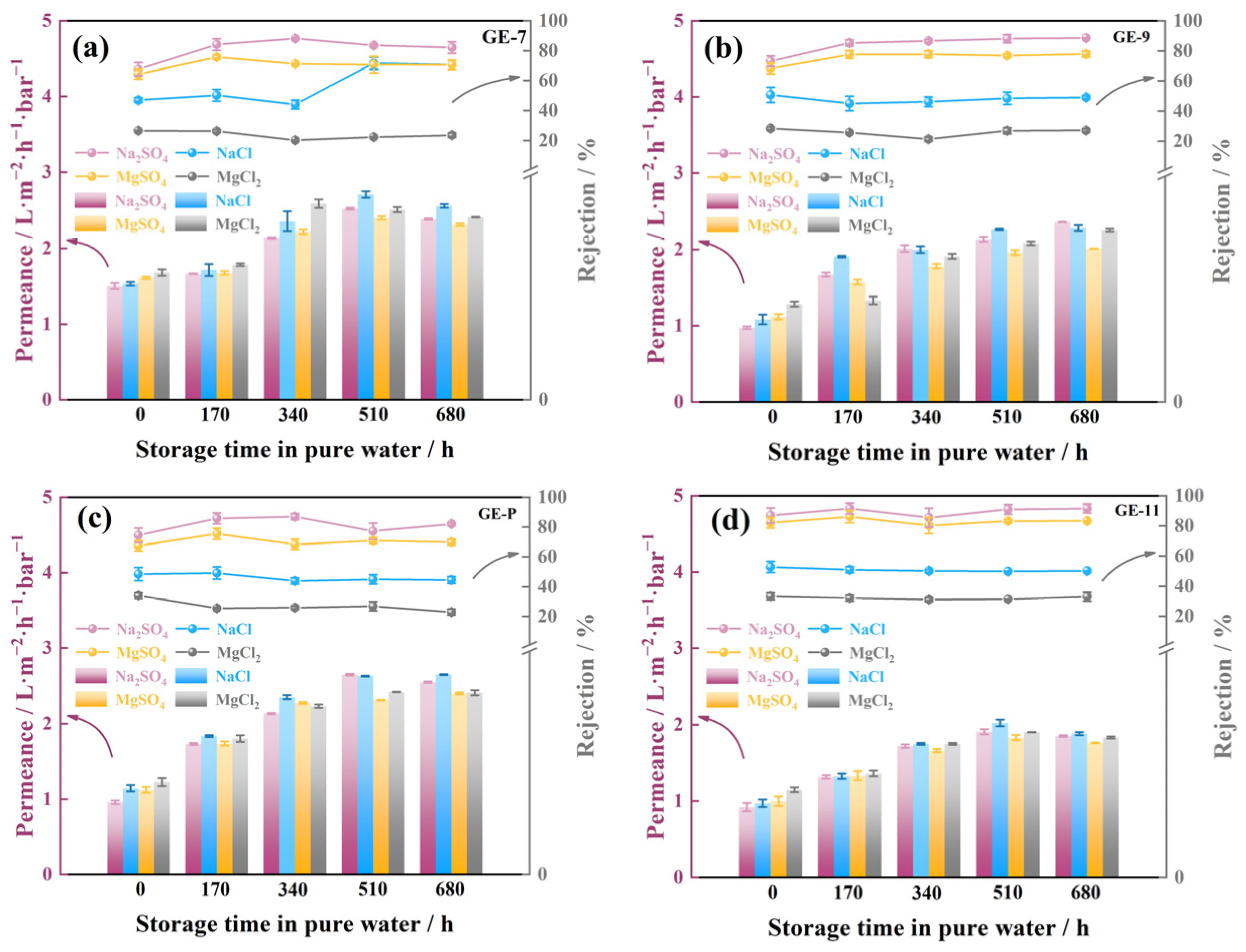
3.5. Stability of GO-EDA/Al2O3 Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Karbassiyazdi, E.; Zhou, J.; Bartocci, P. High-Performance mild annealed CNT/GO-PVA composite membrane for brackish water treatment. Sep. Purif. Technol. 2022, 285, 120361. [Google Scholar] [CrossRef]
- Benfer, S.; Arki, P.; Tomandl, G. Ceramic membranes for filtration applications-Preparation and characterization. Adv. Eng. Mater. 2004, 6, 495–500. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, C.; Huang, A.S. Cross-linking modification with diamine monomers to enhance desalination performance of graphene oxide membranes. Carbon 2018, 136, 28–37. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.S.; Shen, J.; Peng, B.Q.; Zhang, B.W.; Wang, Y.Z.; Bian, F.G.; Wang, J.J.; Li, D.Y.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Memisoglu, G.; Murugesan, R.C.; Zubia, J.; Rozhin, A.G. Graphene Nanocomposite Membranes: Fabrication and Water Treatment Applications. Membranes 2023, 13, 145. [Google Scholar] [CrossRef]
- Zhang, M.C.; Guan, K.C.; Ji, Y.F.; Liu, G.P.; Jin, W.Q.; Xu, N.P. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.P.; Huang, K.; Jin, W.Q.; Lee, K.R.; Xu, N.P. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem. Int. Edit. 2015, 54, 578–582. [Google Scholar] [CrossRef]
- Guan, K.C.; Shen, J.; Liu, G.P.; Zhao, J.; Zhou, H.L.; Jin, W.Q. Spray-evaporation assembled graphene oxide membranes for selective hydrogen transport. Sep. Purif. Technol. 2017, 174, 126–135. [Google Scholar] [CrossRef]
- Wang, N.X.; Sun, H.; Yang, H.Y.; Li, X.T.; Ji, S.L.; An, Q.F. Hollow Polyhedron-Modified Graphene Oxide Membranes for Organic Solvent Nanofiltration with Enhanced Permeance. ACS Appl. Nano Mater. 2020, 3, 5874–5880. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, N.X.; Wang, L.; Liu, H.X.; An, Q.F.; Ji, S.L. Vacuum-assisted assembly of ZIF-8@GO composite membranes on ceramic tube with enhanced organic solvent nanofiltration performance. J. Membr. Sci. 2018, 545, 158–166. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Zhang, M.C.; Mao, Y.Y.; Liu, G.Z.; Liu, G.P.; Fan, Y.Q.; Jin, W.Q. Molecular Bridges Stabilize Graphene Oxide Membranes in Water. Angew. Chem. Int. Ed. 2020, 59, 1689–1695. [Google Scholar] [CrossRef]
- Yuan, L.; Wen, T.P.; Jiang, L.Y.; Liu, Z.L.; Tian, C.; Yu, J.K. Modified superhydrophilic/underwater superoleophobic mullite fiber-based porous ceramic for oil-water separation. Mater. Res. Bull. 2021, 143, 111454. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Wang, N.X.; Ji, S.L.; Zhang, G.J.; Li, J.; Wang, L. Self-assembly of graphene oxide and polyelectrolyte complex nanohybrid membranes for nanofiltration and pervaporation. Chem. Eng. J. 2012, 213, 318–329. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.F.; Zhu, J.H. Cross-linked GO membranes assembled with GO nanosheets of differently sized lateral dimensions for organic dye and chromium separation. J. Membr. Sci. 2020, 598, 117789. [Google Scholar] [CrossRef]
- Lv, X.B.; Xie, R.; Ji, J.Y.; Liu, Z.; Wen, X.Y.; Liu, L.Y.; Hu, J.Q.; Ju, X.J.; Wang, W.; Chu, L.Y. A Novel Strategy to Fabricate Cation-Cross-linked Graphene Oxide Membrane with High Aqueous Stability and High Separation Performance. ACS Appl. Mater. Interfaces 2020, 12, 56269–56280. [Google Scholar] [CrossRef]
- Pan, F.S.; Li, Y.; Song, Y.M.; Wang, M.D.; Zhang, Y.; Yang, H.; Wang, H.J.; Jiang, Z.Y. Graphene oxide membranes with fixed interlayer distance via dual crosslinkers for efficient liquid molecular separations. J. Membr. Sci. 2020, 595, 117486. [Google Scholar] [CrossRef]
- Wang, C.F.; Chen, Y.B.; Yang, K.; Hu, X.Y.; Zhang, Y.F. Fabrication of tight GO/PVDF hollow fiber membranes with improved permeability for efficient fractionation of dyes and salts in textile wastewater. Polym. Bull. 2022, 79, 443–462. [Google Scholar] [CrossRef]
- Park, J.; Enomoto, K.; Yamashita, T.; Takagi, Y.; Todaka, K.; Maekawa, Y. Polymerization mechanism for radiation-induced grafting of styrene into alicyclic polyimide films for preparation of polymer electrolyte membranes. J. Membr. Sci. 2013, 438, 1–7. [Google Scholar] [CrossRef]
- Epsztein, R.; Shaulsky, E.; Dizge, N.; Warsinger, D.M.; Elimelech, M. Role of Ionic Charge Density in Donnan Exclusion of Monovalent Anions by Nanofiltration. Environ. Sci. Technol. 2018, 52, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, N.; Caro, J.; Huang, A. Bio-Inspired Polydopamine: A Versatile and Powerful Platform for Covalent Synthesis of Molecular Sieve Membranes. J. Am. Chem. Soc. 2013, 135, 17679–17682. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Q.; Shi, S.Y.; Cao, H.B.; Li, Y.J.; Duan, F.; Li, Y.P. Surface composite modification of anion exchange membrane by electrodeposition and self-polymerization for improved antifouling performance. Colloids Surf. A 2022, 648, 129402. [Google Scholar] [CrossRef]
- Abbasi, F.; Karimi-Sabet, J.; Ghotbi, C.; Abbasi, Z.; Mousavi, S.A.; Amini, N. The effect of pH on lateral size and surface chemistry of graphene oxide. Sci. Iran. 2017, 24, 3554–3559. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Chung, T.S. Nanometric Graphene Oxide Framework Membranes with Enhanced Heavy Metal Removal via Nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. [Google Scholar] [CrossRef]
- Wang, Q.M.; Zhao, G.J.; Li, C.X.; Meng, H. Orderly stacked ultrathin graphene oxide membranes on a macroporous tubular ceramic substrate. J. Membr. Sci. 2019, 586, 177–184. [Google Scholar] [CrossRef]
- Clark, S.L.; Montague, M.F.; Hammond, P.T. Ionic effects of sodium chloride on the templated deposition of polyelectrolytes using layer-by-layer ionic assembly. Macromolecules 1997, 30, 7237–7244. [Google Scholar] [CrossRef]
- Tai, G.A.; Zeng, T.; Li, H.X.; Liu, J.S.; Kong, J.Z.; Lv, F.Y. Temperature and pH effect on reduction of graphene oxides in aqueous solution. Mater. Res. Express. 2014, 1, 035605. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Li, K. Water transport through graphene oxide membranes: The roles of driving forces. Chem. Commun. 2018, 54, 2554–2557. [Google Scholar] [CrossRef]
- Akther, N.; Yuan, Z.W.; Chen, Y.; Lim, S.; Phuntsho, S.; Ghaffour, N.; Matsuyama, H.; Shon, H. Influence of graphene oxide lateral size on the properties and performances of forward osmosis membrane. Desalination 2020, 484, 114421. [Google Scholar] [CrossRef]
- Wang, S.F.; Yang, L.X.; He, G.W.; Shi, B.B.; Li, Y.F.; Wu, H.; Zhang, R.N.; Nunes, S.; Jiang, Z.Y. Two-dimensional nanochannel membranes for molecular and ionic separations. Chem. Soc. Rev. 2020, 49, 1071–1089. [Google Scholar] [CrossRef]
- Yeh, C.N.; Raidongia, K.; Shao, J.J.; Yang, Q.H.; Huang, J.X. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166–170. [Google Scholar] [CrossRef]
- Kim, I.C.; Jegal, J.; Lee, K.H. Effect of aqueous and organic solutions on the performance of polyamide thin-film-composite nanofiltration membranes. J. Polym. Sci. 2002, 40, 2151–2163. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Tizaoui, C.; Hilal, N. Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desalination 2011, 266, 1–16. [Google Scholar] [CrossRef]
- Popper, K.; Merson, R.L.; Camirand, W.M. Desalination by osmosis–reverse osmosis couple. Science 1968, 159, 1364–1365. [Google Scholar] [CrossRef]
- Ju, H.; Duan, J.Z.; Lu, H.T.; Xu, W.H. Cross-Linking With Diamine Monomers to Prepare Graphene Oxide Composite Membranes With Varying D-Spacing for Enhanced Desalination Properties. Front. Chem. 2021, 9, 1045. [Google Scholar] [CrossRef]
- Yuan, B.Q.; Wang, M.X.; Wang, B.; Yang, F.L.; Quan, X.; Tang, C.; Dong, Y.C. Cross-linked Graphene Oxide Framework Membranes with Robust Nano-Channels for Enhanced Sieving Ability. Environ. Sci. Technol. 2020, 54, 15442–15453. [Google Scholar] [CrossRef]
- Hu, X.B.; Yu, Y.; Lin, N.; Ren, S.; Zhang, X.Z.; Wang, Y.Q.; Zhou, J. Graphene oxide/Al2O3 membrane with efficient salt rejection for water purification. Water Sci. Technol. Water Supply 2018, 18, 2162–2169. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.X.; Gu, P.; Ng, A.J.; Wang, M.N.; Wei, Y.Y.; Urban, J.J.; Mi, B.X. Graphene-polyelectrolyte multilayer membranes with tunable structure and internal charge. Carbon 2020, 160, 219–227. [Google Scholar] [CrossRef]
- Meng, N.; Zhao, W.; Shamsaei, E.; Wang, G.; Zeng, X.K.; Lin, X.C.; Xu, T.W.; Wang, H.T.; Zhang, X.W. A low-pressure GO nanofiltration membrane crosslinked via ethylenediamine. J. Membr. Sci. 2018, 548, 363–371. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Jahim, J.M. New polymeric membrane nanofiltration for succinate recovery: A comparative study. J. Polym. Res. 2017, 24, 197. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Basha, R.K.; Leo, C.P. Nanofiltration of glucose solution containing salts: Effects of membrane characteristics, organic component and salts on retention. J. Food Eng. 2010, 97, 510–518. [Google Scholar] [CrossRef]
- Zhang, M.C.; Guan, K.C.; Shen, J.; Liu, G.P.; Fan, Y.Q.; Jin, W.Q. Nanoparticles@rGO Membrane Enabling Highly Enhanced Water Permeability and Structural Stability with Preserved Selectivity. AIChE J. 2017, 63, 5054–5063. [Google Scholar] [CrossRef]
- Shen, G.S.; Zhao, J.; Guan, K.C.; Shen, J.; Jin, W.Q. Highly Efficient Recovery of Propane by Mixed-Matrix Membrane via Embedding Functionalized Graphene Oxide Nanosheets into Polydimethylsiloxane. AIChE J. 2017, 63, 3501–3510. [Google Scholar] [CrossRef]
- Zhuang, X.L.; Magnone, E.; Shin, M.C.; Lee, J.I.; Hwang, J.Y.; Choi, Y.C.; Park, J.H. Novel TiO2/GO-Al2O3 Hollow Fiber Nanofiltration Membrane for Desalination and Lignin Recovery. Membranes 2022, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Armstrong, D.L.; Finnerty, C.; Zheng, S.X.; Hu, M.; Torrents, A.; Mi, B.X. Understanding the pH-responsive behavior of graphene oxide membrane in removing ions and organic micropollulants. J. Membr. Sci. 2017, 541, 235–243. [Google Scholar] [CrossRef]



| Membrane | Substrate | Feed Condition | Testing Condition | Water Permeance (L·m−2·h−1·bar−1) | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|
| GO-UR | MCE | NaCl | 50 mM, 1 bar | / | 25.74 | [36] |
| RGO | PVDF | Na2SO4, NaCl, MgSO4, MgCl2 | 20 mM, 5 bar | 3.3 | ~60, 30, 20, 40 | [11] |
| Pristine GO | α-Al2O3 | Na2SO4, MgSO4MgCl2, NaCl | 1 mM, 5 bar | 3.68 | 72.6, 58.4, 23.7, 45.8 | [37] |
| GO | Al2O3 | NaCl, MgSO4 | 10 mM, 4 bar | 1.25 | 28.66, 43.52 | [38] |
| GO-PEI | PAN | MgCl2 | 1000 ppm, 5 bar | 4.2 | 86 | [24] |
| GO-PEI | PAN | MgCl2, Na2SO4, NaCl | 10 mM, 3.4 bar | 4.1 | 72, 30, 20 | [39] |
| EDA/GO | BPPO | Na2SO4, MgSO4, NaCl | 1000 ppm, 1 bar | 4.1 | 56.2, 48, 36.3 | [40] |
| Commercial NF1 | PS | Na2SO4, NaCl | 20 mM, 20 bar | 3.45 | 98, 51 | [41] |
| Osmonics CK | / | NaCl | 1.5 mM~100 mM | 2.42 | 45.5~77.7 | [42] |
| Osmonics DK | / | NaCl | 1.5 mM~100 mM | 3.05 | 22.0~75.6 | [42] |
| Commercial NF2 | PAM | Na2SO4, NaCl | 20 mM, 20 bar | 6.5 | 99, 44 | [41] |
| rGO | Al2O3 | / | /, 15 bar | 1.7 | / | [43] |
| GO | PSf | Na2SO4 | 2000 ppm, 15 bar | 11 | 65 | [44] |
| TiO2-GO | Al2O3 | Na2SO4 | 1.4 mM, 5 bar | 5.6 | 9.8 | [45] |
| GO/PAH | PAN | Na2SO4 | 6.7 mM, 6.9 bar | 2 | 68 | [46] |
| GO-EDA | Al2O3 | Na2SO4 | 1 mM, 20 bar | 3.7 | 96.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Qi, H. A Facile Way to Fabricate GO-EDA/Al2O3 Tubular Nanofiltration Membranes with Enhanced Desalination Stability via Fine-Tuning the pH of the Membrane-Forming Suspensions. Membranes 2023, 13, 536. https://doi.org/10.3390/membranes13050536
Ding C, Qi H. A Facile Way to Fabricate GO-EDA/Al2O3 Tubular Nanofiltration Membranes with Enhanced Desalination Stability via Fine-Tuning the pH of the Membrane-Forming Suspensions. Membranes. 2023; 13(5):536. https://doi.org/10.3390/membranes13050536
Chicago/Turabian StyleDing, Chunxiao, and Hong Qi. 2023. "A Facile Way to Fabricate GO-EDA/Al2O3 Tubular Nanofiltration Membranes with Enhanced Desalination Stability via Fine-Tuning the pH of the Membrane-Forming Suspensions" Membranes 13, no. 5: 536. https://doi.org/10.3390/membranes13050536
APA StyleDing, C., & Qi, H. (2023). A Facile Way to Fabricate GO-EDA/Al2O3 Tubular Nanofiltration Membranes with Enhanced Desalination Stability via Fine-Tuning the pH of the Membrane-Forming Suspensions. Membranes, 13(5), 536. https://doi.org/10.3390/membranes13050536






