Role of Plasma Membrane at Dielectric Relaxations and Intermembrane Interaction in Human Erythrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Erythrocytes
2.3. Isolation of Erythrocyte Ghost Membranes
2.4. Preparation of Triton-X-100 Shells of Erythrocyte Ghost Membranes
2.5. Dielectric Spectroscopy of the Suspensions of Erythrocytes, Erythrocyte Ghost Membranes, and Triton Shells
2.6. Dielectric Loss Curve of Triton Shells and the Suspensions of Erythrocytes and Erythrocyte Ghost Membranes
2.7. Contribution of the Spectrin Network to the Dielectric Properties of Erythrocytes, Erythrocyte Ghost Membrane, and Triton Shells
2.8. Reduction of Electrode Polarization and Measurement Errors
3. Results
3.1. Model Presentation of the Dielectric Relaxations on the Spectrin Network of Erythrocytes and Erythrocyte Ghost Membranes
3.2. Complex Admittance and Capacitance Contribution of Spectrin Network as Affected by the Modification of Lipid Membrane
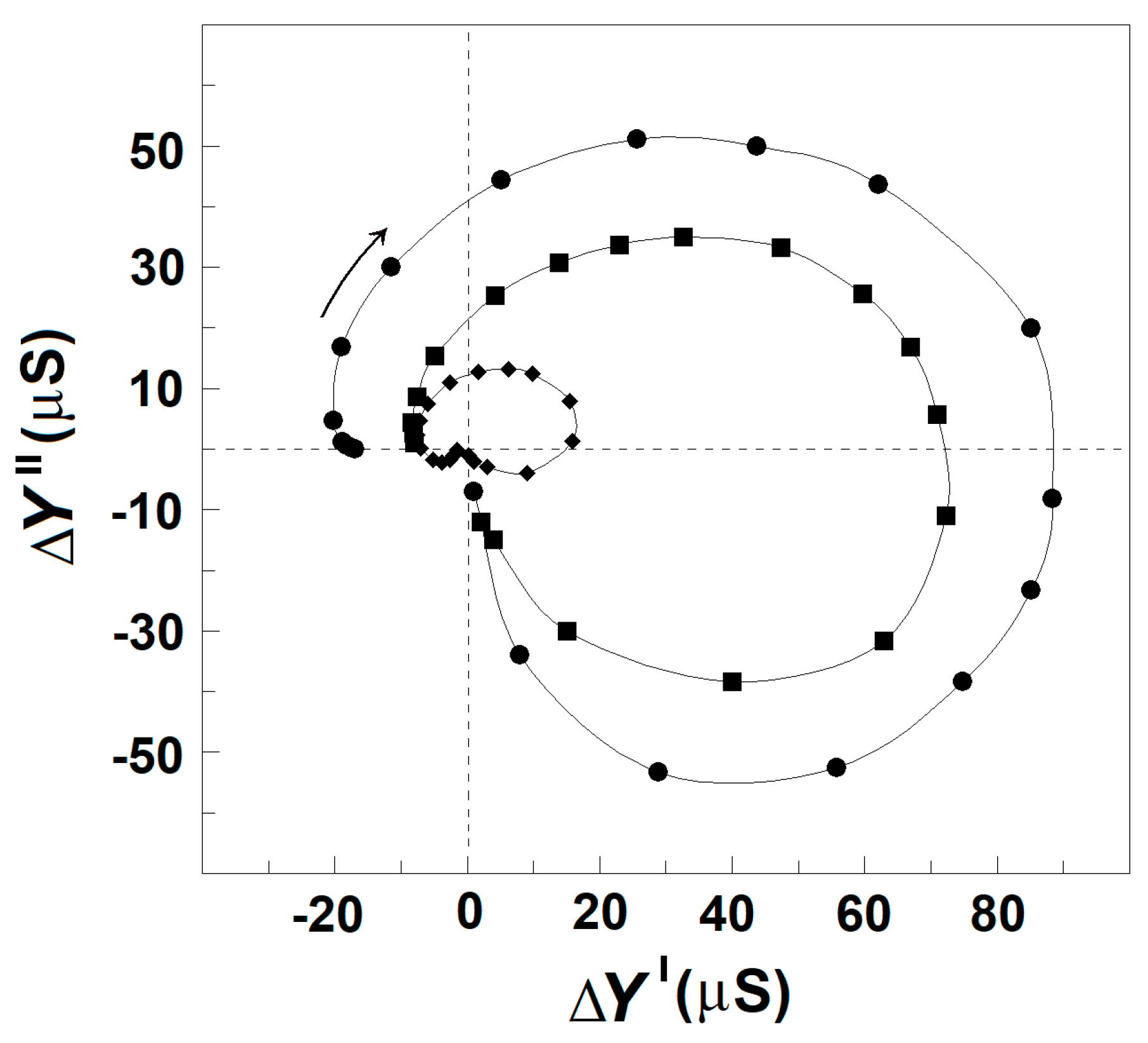
3.2.1. Effect of Acidification of Erythrocytes on the Spectrin Relaxations
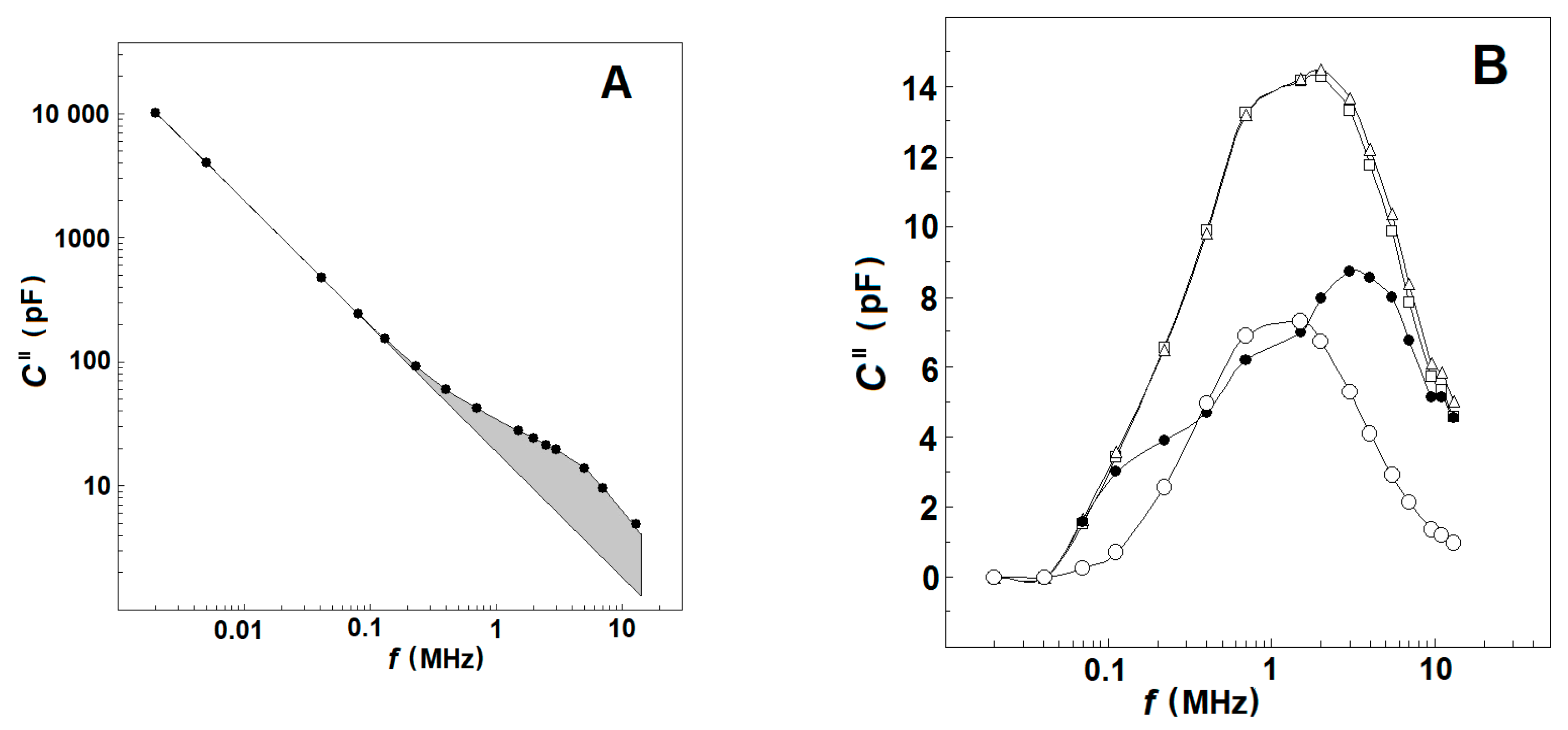
3.2.2. Effect of Mild Delipidation of Erythrocyte Ghost Membranes on the Spectrin Relaxations
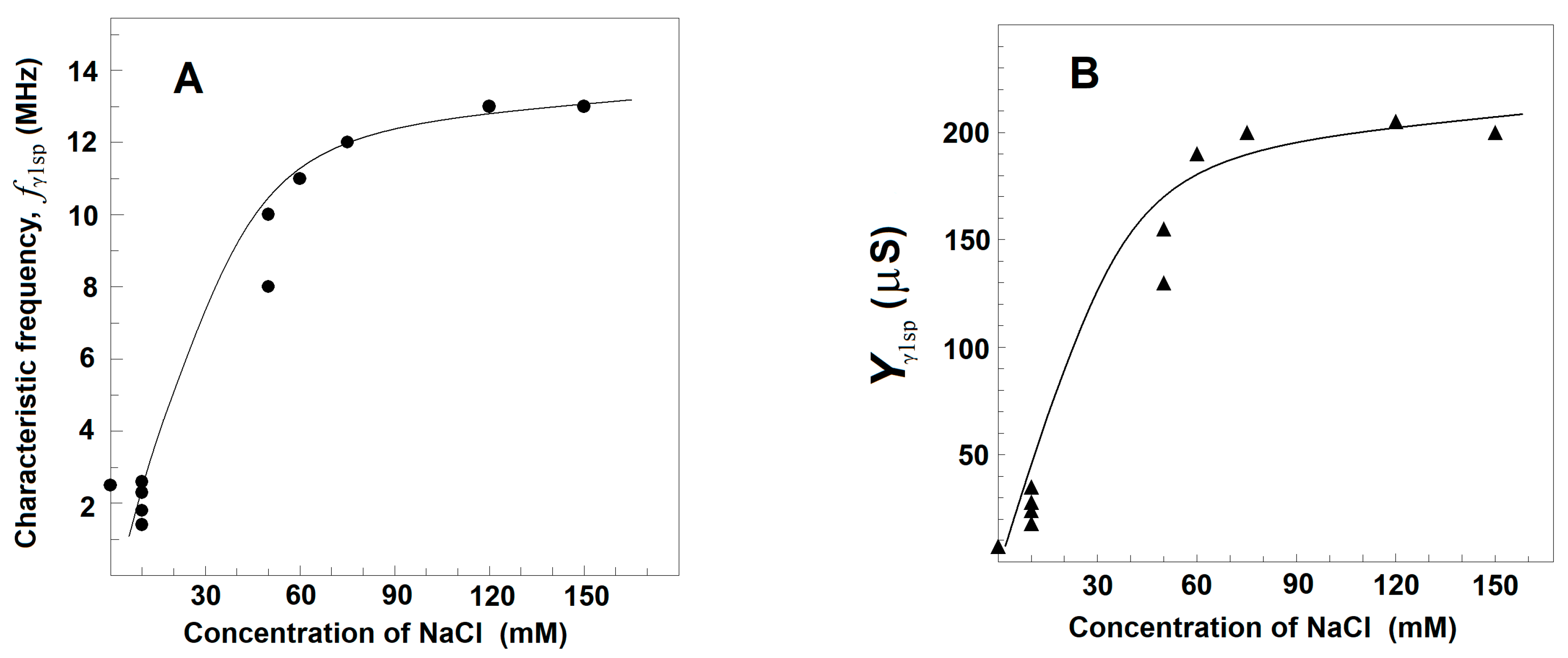
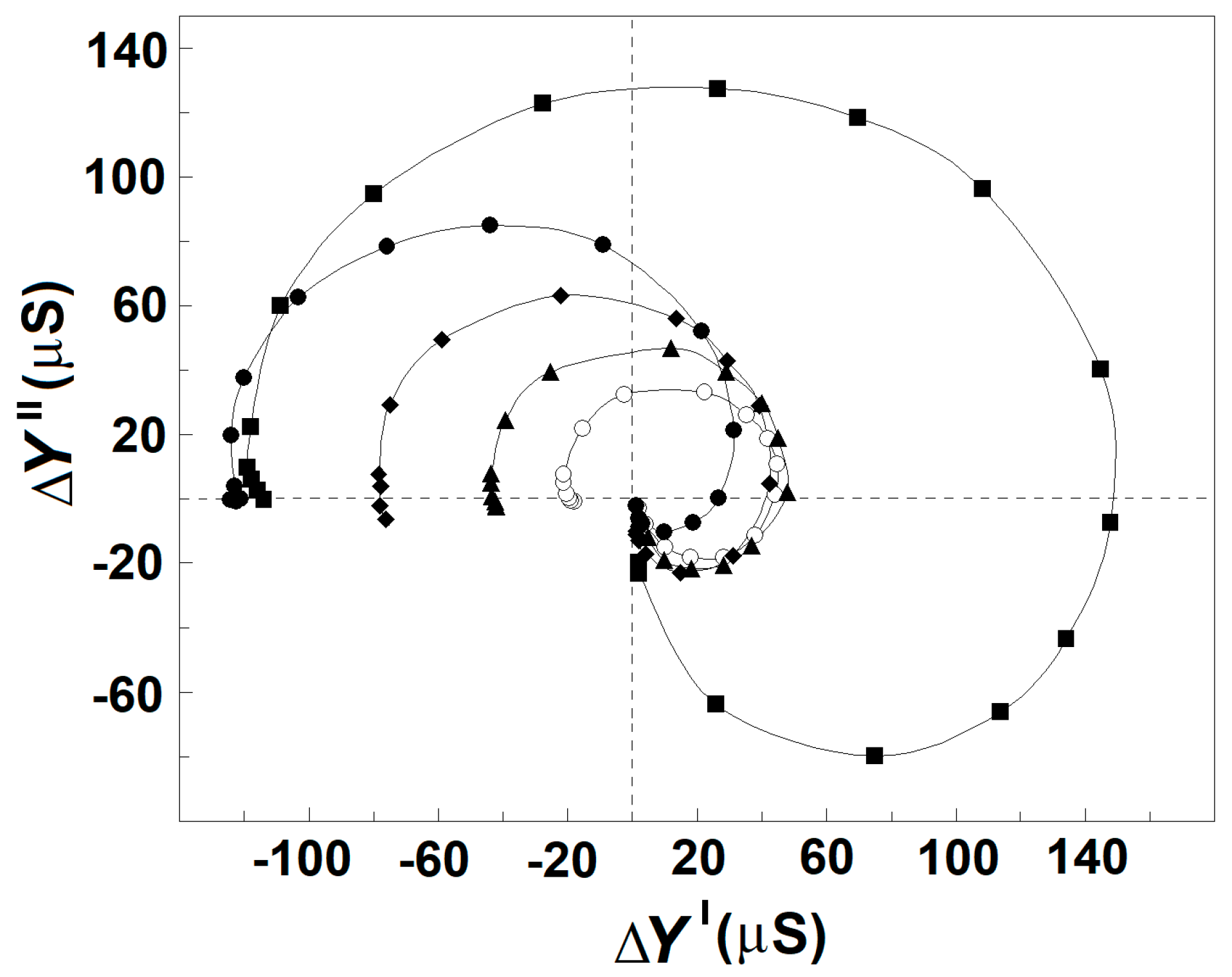
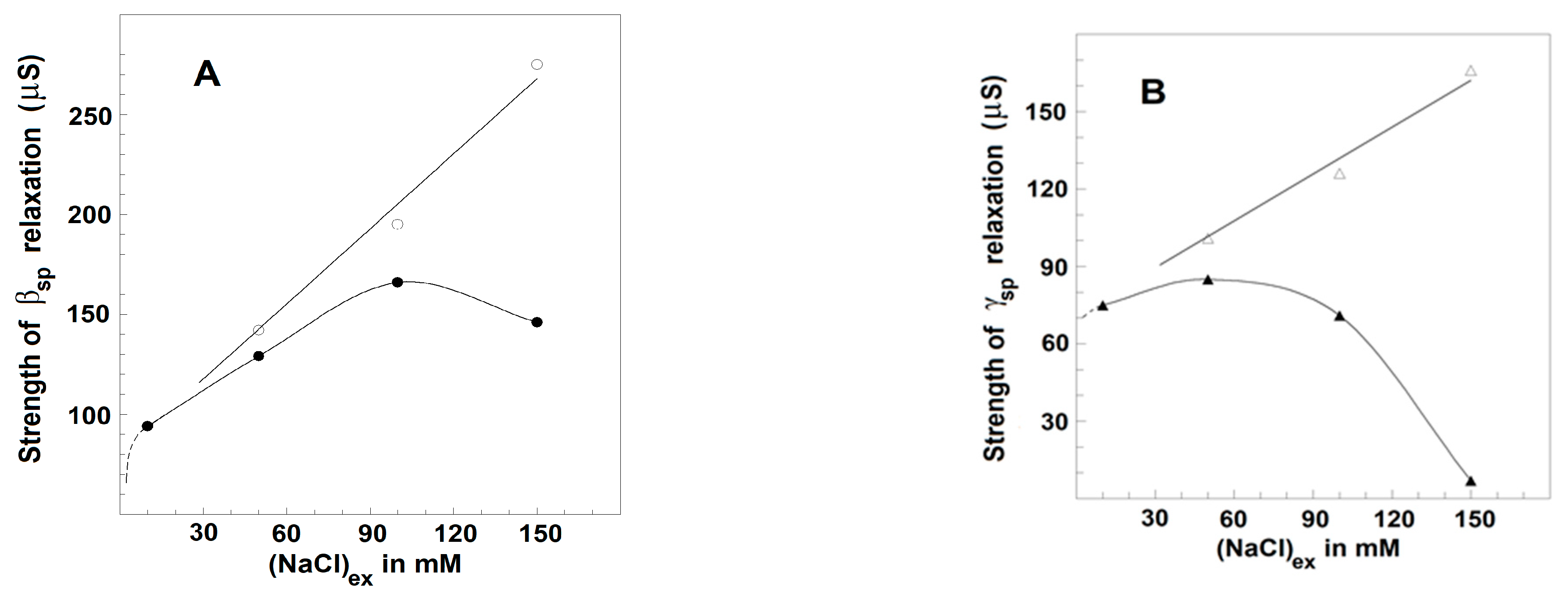
3.2.3. Effects of NaCl Concentration on the Strength, Yγ1sp, and Characteristic Frequency, fγ1sp, of γ1sp-Relaxation in Moderately Delipidated Triton Shells
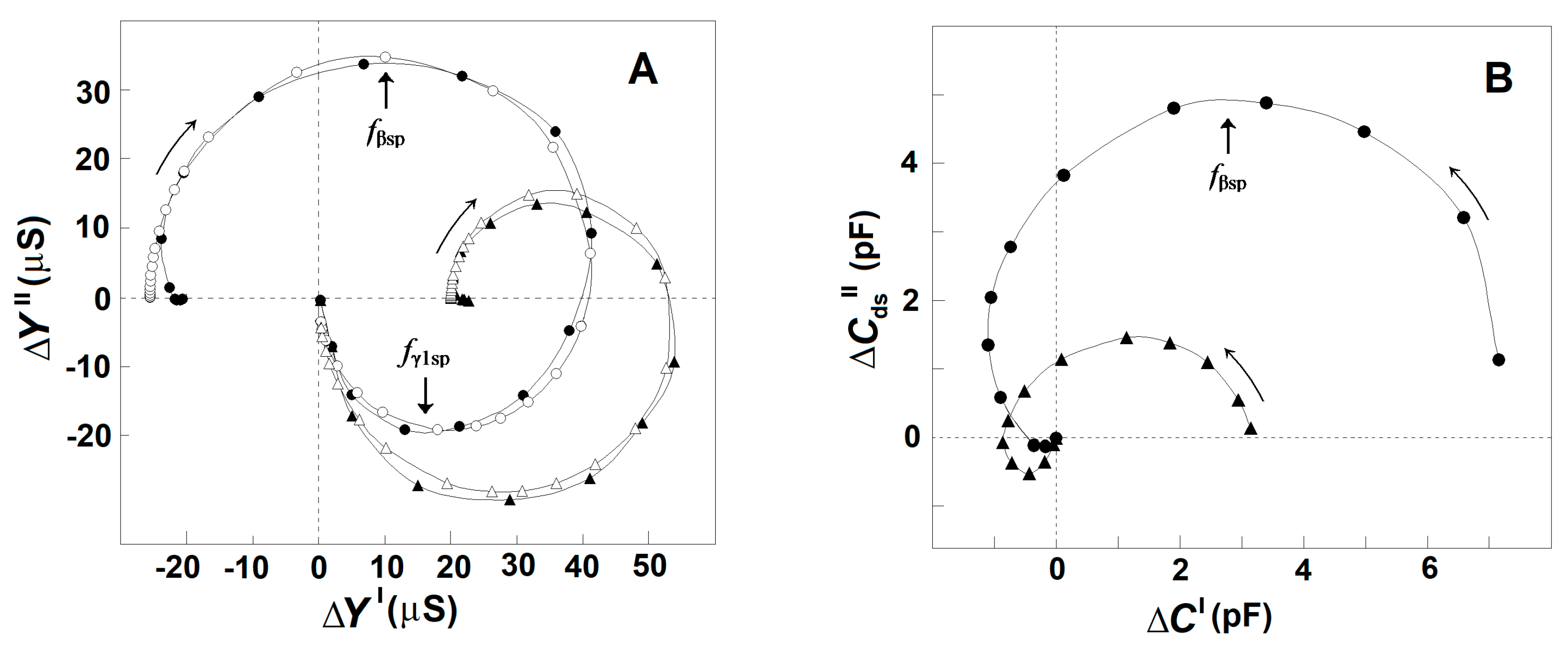
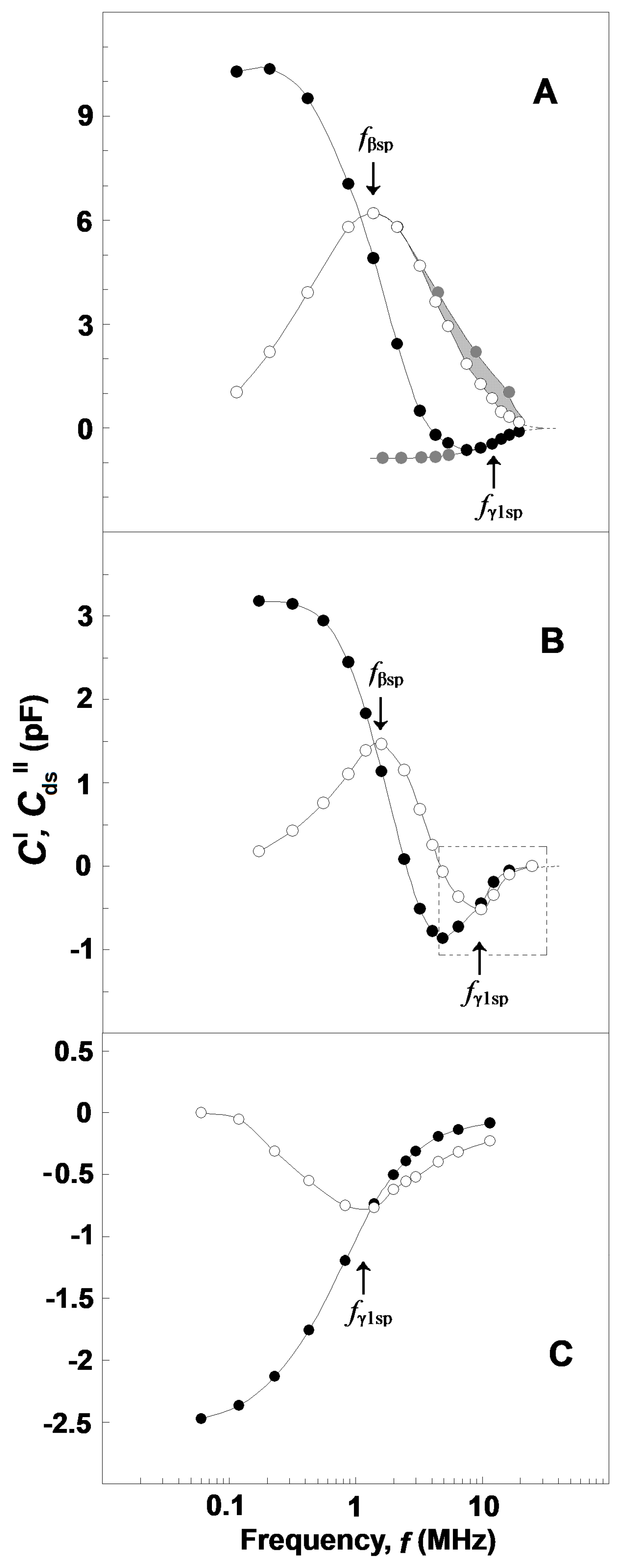
3.2.4. Complex Capacitance Contribution of the Spectrin Network, ΔC′ vs. f and ΔCds″ vs. f in Erythrocytes, Erythrocyte Ghost Membranes, and Triton Shells
3.3. Complex Admittance Contribution of Spectrin Network as Affected by the Inter-Membrane Interaction
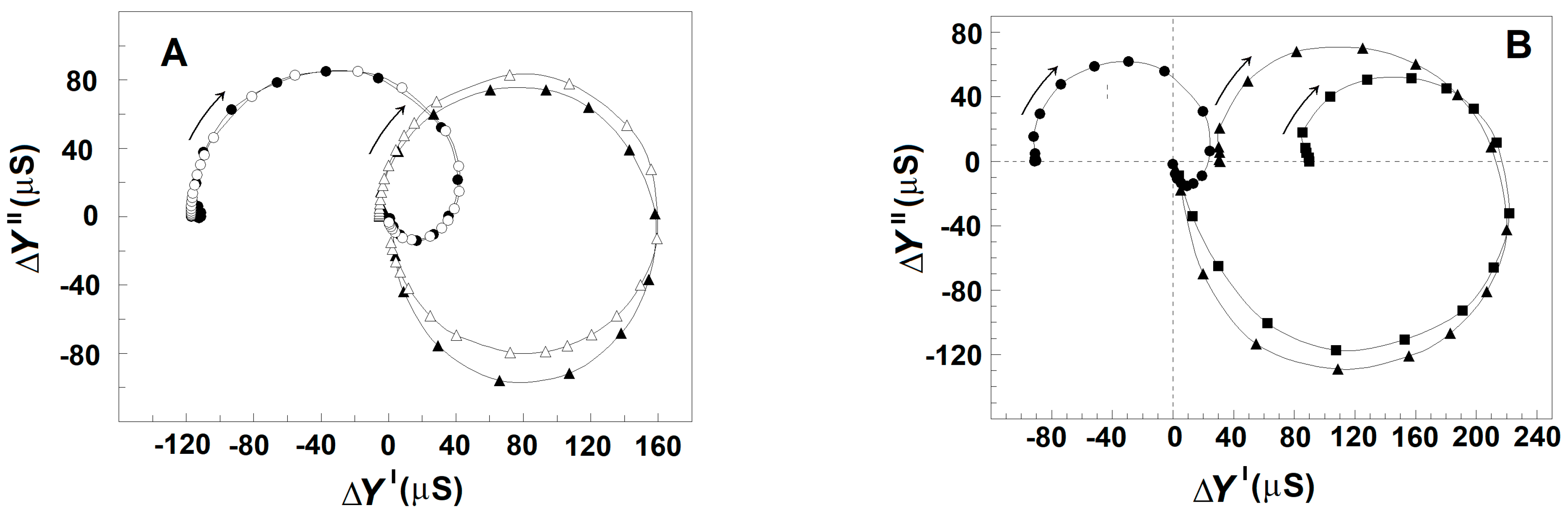
3.3.1. Effect of Extracellular and Intracellular NaCl on the Strengths of βsp- and γ1sp-Relaxations in Erythrocytes and Erythrocyte Ghost Membranes
3.3.2. Effect of Extracellular Albumin on the Strengths of βsp- and γ1sp-Relaxations in Erythrocytes
3.3.3. Effect of Extracellular Synthetic Polymers on the Strengths of βsp- and γ1sp-Relaxations in Erythrocytes
3.3.4. Effect of Cellular Packing on the Strength of Relaxations in Erythrocytes
4. Discussion
4.1. Role of Lipid Membrane in the Dielectric Relaxations on the Spectrin Network
4.2. Biophysical Characteristics of βsp-Relaxation
4.3. Biophysical Characteristics of γ1sp-Relaxation
4.4. Dielectric Relaxations in Erythrocytes as Sensitive Markers of Erythrocyte Membrane Deformability
4.5. Dielectric Relaxations in Erythrocytes as Sensitive Markers of Inter-Membrane Interaction
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
References
- Ivanov, I.T.; Paarvanova, B.K. Thermal dielectroscopy study on the vertical and horizontal interactions in erythrocyte submembrane skeleton. Electrochim. Acta 2019, 317, 289–300. [Google Scholar] [CrossRef]
- Ivanov, I.T.; Paarvanova, B.K. Segmental flexibility of spectrin reflects erythrocyte membrane deformability. Gen. Physiol. Biophys. 2022, 41, 87–100. [Google Scholar] [CrossRef]
- Blanc, L.; Salomao, M.; Guo, X.; An, X.; Gratzer, W.; Mohandas, N. Control of erythrocyte membrane-skeletal cohesion by the spectrin-membrane linkage. Biochemistry 2010, 49, 4516–4523. [Google Scholar] [CrossRef]
- de Oliveira, S.; Saldanha, C. An overview about erythrocyte membrane. Clin. Hemorheol. Microcirc. 2010, 44, 63–74. [Google Scholar] [CrossRef]
- Mohandas, N.; Chasis, J.A.; Shohet, S. The influence of membrane skeleton on red cell deformability, membrane material properties and shape. Semin. Hematol. 1983, 20, 225–242. [Google Scholar]
- Sheetz, M.P. Membrane skeletal dynamics: Role in modulation of red cell deformability, mobility of transmembrane proteins, and shape. Semin. Hematol. 1983, 20, 175–188. [Google Scholar]
- Mohandas, N.; Gallagher, P.G. Red cell membrane: Past, present, and future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.G.; Wortis, M.; Mukhopadhyay, R. Red Blood Cell Shapes and Shape Transformations: Newtonian Mechanics of a Composite Membrane: Sections 2.1–2.4. In Soft Matter: Lipid Bilayers and Red Blood Cells; Gompper, G., Schick, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2008; Volume 4, pp. 83–139. [Google Scholar] [CrossRef]
- Hale, J.P.; Winlove, C.P.; Petrov, P.G. Effect of hydroperoxides on red blood cell membrane mechanical properties. Biophys. J. 2011, 101, 1921–1929. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Chen, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Schwan, H.P.; Takashima, S. Electrical conduction and dielectric behavior in biological systems. Encycl. Appl. Phys. 1993, 5, 177–200. [Google Scholar]
- Martinsen, O.G.; Grimnes, S.; Schwan, H.P. Interface Phenomena and Dielectric Properties of Biological tissue. In Encyclopedia of Surface and Colloid Science Anonymous; Somasundaran, P., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 2643–2652. [Google Scholar]
- Bone, S.; Ginzburg, B.Z.; Morgan, H.; Wilson, G.; Zaba, B. Time-domain dielectric spectroscopy applied to cell suspensions. Phys. Med. Biol. 1993, 38, 511–520. [Google Scholar] [CrossRef]
- Asami, K. Radio-Frequency Dielectric Properties of Cell Suspensions. In Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Raicu, V., Feldman, Y., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 340–362. [Google Scholar]
- Abasi, S.; Aggas, J.R.; Garayar-Leyva, G.G.; Walther, B.K.; Guiseppi-Elie, A. Bioelectrical Impedance Spectroscopy for Monitoring Mammalian Cells and Tissues under Different Frequency Domains: A Review. ACS Meas. Sci. Au 2022, 2, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.M.; Kell, D.B. On the dielectrically observable consequences of the diffusional motions of lipids and proteins in membranes. 2. Experiments with microbial cells, protoplasts and membrane vesicles. Eur. Biophys. J. 1985, 13, 11–24. [Google Scholar] [CrossRef]
- Ferris, L.E.; Davy, C.L.; Kell, D.B. Evidence from its temperature dependence that the β-dielectric dispersion of cell suspensions is not due solely to the charging of a static membrane capacitance. Eur. Biophys. J. 1990, 18, 267–276. [Google Scholar] [CrossRef]
- Gimsa, J. Electric and magnetic fields in cells and tissues. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2017; pp. 1–10. [Google Scholar] [CrossRef]
- Brandts, J.F.; Erickson, L.; Lysko, K.; Schwartz, A.T.; Taverna, R.D. Calorimetric studies of the structural transitions of the human erythrocyte membrane. The involvement of spectrin in the A transition. Biochemistry 1977, 16, 3450–3454. [Google Scholar] [CrossRef]
- Ivanov, I.T.; Paarvanova, B.K. Differential dielectroscopic data on the relation of erythrocyte membrane skeleton to erythrocyte deformability and flicker. Eur. Biophys. J. 2021, 50, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Ramanathan, T.; Machado, D.; Sundararajan, R. Electrical impedance spectroscopy study of biological tissues. J. Electrost. 2008, 66, 165–177. [Google Scholar] [CrossRef]
- Ivanov, I.T.; Lyutskanov, V.G. Thermotropic bahaviour of intact human erythrocyte membranes revealed by differential scanning conductometry. Mol. Cryst. Liq. Crist. 1987, 152, 327–332. [Google Scholar]
- Hianik, T.; Dlugopolsky, J.; Passechnik, V.I.; Sargent, D.F.; Ivanov, S.A. Electrostriction and membrane potential of lipid bilayers on a metal support. Colloids Surf. A Physicochem. Eng. Asp. 1996, 106, 109–118. [Google Scholar] [CrossRef]
- Ivanov, I.T.; Paarvanova, B.K. Dielectric relaxations on erythrocyte membrane as revealed by spectrin denaturation. Bioelectrochemistry 2016, 110, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.T.; Paarvanova, B.K.; Tacheva, B.T.; Slavov, T. Species-dependent variations in the dielectric activity of membrane skeleton of erythrocytes. Gen. Physiol. Biophys. 2020, 39, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Yu, J.; Fischman, D.A.; Steck, T.L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J. Supramol. Struct. 1973, 1, 233–248. [Google Scholar] [CrossRef]
- Ciana, A.; Balduini, C.; Minetti, G. Detergent-resistant membranes in human erythrocytes and their connection to the membrane-skeleton. J. Biosci. 2005, 30, 317–328. [Google Scholar] [CrossRef]
- Thom, F.; Gollek, H. Calculation of mechanical properties of human red cells based on electrically induced deformation experiments. J. Electrost. 2006, 64, 53–61. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Kahana, E.; Streichman, E.; Silver, B.L. The role of electrostatic forces in the interaction between the membrane and cytoskeleton of human erythrocytes. Biochim. Biophys. Acta (BBA)—Biomembr. 1991, 1066, 1–5. [Google Scholar] [CrossRef]
- Pethig, R. Dielectric and Electronic Properties of Biological Materials; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar] [CrossRef]
- Feldman, Y.; Ishai, P.B.; Raicu, V. Electrode Polarization. In Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Raicu, V., Feldman, Y., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 140–169. [Google Scholar]
- Feldman, Y.; Ermolina, I.; Hayashi, Y. Time Domain Dielectric Spectroscopy Study of Biological Systems. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 728–753. [Google Scholar] [CrossRef]
- Kell, D.B. The principles and potential of electrical admittance spectroscopy: An introduction. In Biosensors: Fundamenlals and Applications; Turner, A.P.F., Karube, I., Wilson, J.S., Eds.; Oxford University Press: Oxford, UK, 1987; pp. 427–468. [Google Scholar]
- Klösgen, B.; Rümenapp, C.; Gleich, B. Bioimpedance Spectroscopy. In BetaSys: Systems Biology of Regulated Exocytosis in Pancreatic β-Cells; Booβ-Bavnbek, B., Klösgen, B., Larsen, J., Pociot, F., Renström, E., Eds.; Systems Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 241–271. [Google Scholar] [CrossRef]
- Ivanov, I.T.; Paarvanova, B.K. Effect of permeant cryoprotectants on membrane skeleton of erythrocytes. Probl. Cryobiol. Cryomed. 2019, 29, 237–245. [Google Scholar] [CrossRef]
- Sheetz, M.P. DNase-I-dependent dissociation of erythrocyte cytoskeletons. J. Cell Biol. 1979, 81, 266–270. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Casaly, J. 2,3-Diphosphoglycerate and ATP dissociate erythrocyte membrane skeletons. J. Biol. Chem. 1980, 255, 9955–9960. [Google Scholar] [CrossRef]
- Levitsky, D.I.; Pivovarova, A.V.; Mikhailova, V.V.; Nikolaeva, O.P. Thermal unfolding and aggregation of actin. FEBS J. 2008, 275, 4280–4295. [Google Scholar] [CrossRef]
- Patra, M.; Mukhopadhyay, C.; Chakrabarti, A. Probing conformational stability and dynamics of erythroid and nonerythroid spectrin: Effects of urea and guanidine hydrochloride. PLoS ONE 2015, 10, e0116991. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Low, P.S. Regulation of the Glycophorin C-Protein 4.1 Membrane-to-Skeleton Bridge and Evaluation of Its Contribution to Erythrocyte Membrane Stability. J. Biol. Chem. 2001, 276, 22223–22230. [Google Scholar] [CrossRef]
- Swietach, P.; Tiffert, T.; Mauritz, J.M.A.; Seear, R.; Esposito, A.; Kaminski, C.F.; Lew, V.L.; Vaughan-Jones, R.D. Hydrogen ion dynamics in human red blood cells. J. Physiol. 2010, 24, 4995–5014. [Google Scholar] [CrossRef]
- Habibi, S.; Lee, H.Y.; Moncada-Hernandez, H.; Gooding, J.; Minerick, A.R. Impacts of low concentration surfactant on red blood cell dielectrophoretic responses. Biomicrofluidics 2019, 13, 054101. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.T.; Paarvanova, B.K.; Ivanov, V.; Smuda, K.; Bäumler, H.; Georgieva, G. Effects of heat and freeze on isolated erythrocyte submembrane skeletons. Gen. Physiol. Biophys. 2017, 36, 155–165. [Google Scholar] [CrossRef]
- Lange, Y.; Hadesman, R.A.; Steck, T.L. Role of the Reticulum in the Stability and Shape of the Isolated Human Erythrocyte Membrane. J. Cell Biol. 1982, 92, 714–721. [Google Scholar] [CrossRef]
- Vertessy, B.G.; Steck, T.L. Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys. J. 1989, 55, 255–262. [Google Scholar] [CrossRef]
- Fuhrmann, G.F.; Netter, K.J. A Hundred-Year Researching History on the Low Ionic Strength in Red Blood Cells: Literature Review. J. Biomed. Res. Environ. Sci. 2021, 2, 139–168. [Google Scholar] [CrossRef]
- Abidor, I.G.; Barbul, A.I.; Zhelev, D.V.; Doinov, P.; Bandrina, I.N.; Osipova, E.M.; Sukharev, S.I. Electrical properties of cell pellets and cell electrofusion in a centrifuge. Biochim. Biophys. Acta (BBA)—Biomembr. 1993, 1152, 207–218. [Google Scholar] [CrossRef]
- Itagaki, M.; Suzuki, S.; Shitanda, I.; Watanabe, K. Electrochemical Impedance and Complex Capacitance to Interpret Electrochemical Capacitor. Electrochemistry 2007, 75, 649–655. [Google Scholar] [CrossRef]
- Smith, B.D.; La Celle, P.L. Parallel decrease of erythrocyte membrane deformability and spectrin solubility at low pH. Blood 1979, 53, 15–18. [Google Scholar] [CrossRef]
- Sosa, J.M.; Nielsen, N.D.; Vignes, S.M.; Chen, T.G.; Shevkoplyas, S.S. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin. Hemorheol. Microcirc. 2014, 57, 291–305. [Google Scholar] [CrossRef]
- Auth, T.; Safran, S.A.; Gov, N.S. Fluctuations of coupled fluid and solid membranes with application to red blood cells. Phys. Rev. E 2007, 76, 051910. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Wenby, R.B.; Meiselman, H.J.; Fisher, T.C. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys. J. 2004, 87, 4259–4270. [Google Scholar] [CrossRef] [PubMed]
- Sümpelmann, R.; Schürholz, T.; Marx, G.; Zander, R. Protective effects of plasma replacement fluids on erythrocytes exposed to mechanical stress. Anaesthesia 2000, 55, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Kameneva, M.V.; Repko, B.M.; Krasik, E.F.; Perricelli, B.C.; Borovetz, H.S. Polyethylene glycol additives reduce hemolysis in red blood cell suspensions exposed to mechanical stress. ASAIO J. 2003, 49, 537–542. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Barshtein, G.; Mardi, T.; Deutch, V.; Elkayam, O.; Yedgar, S.; Berliner, S. A synergistic effect of albumin and fibrinogen on immunoglobulin-induced red blood cell aggregation. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2663–H2669. [Google Scholar] [CrossRef]
- Carrique, F.; Arroyo, F.J.; Delgado, A.V. Electrokinetics of Concentrated Suspensions of Spherical Colloidal Particles with Surface Conductance, Arbitrary Zeta Potential, and Double-Layer Thickness in Static Electric Fields. J. Colloid Interface Sci. 2002, 252, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.L.; Davey, H.M.; Kell, D.B. On the dielectric properties of cell suspensions at high volume fractions. Bioelectrochem. Bioenerg. 1992, 28, 319–340. [Google Scholar] [CrossRef]
- Gimsa, J.; Wachner, D. A Polarization Model Overcoming the Geometric Restrictions of the Laplace Solution for Spheroidal Cells: Obtaining New Equations for Field-Induced Forces and Transmembrane Potential. Biophys. J. 1999, 77, 1316–1326. [Google Scholar] [CrossRef]
- Pavlin, M.; Miklavčič, D. Effective Conductivity of a Suspension of Permeabilized Cells: A Theoretical Analysis. Biophys. J. 2003, 85, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Chasis, J.A.; Reid, M.E.; Jensen, R.H.; Mohandas, N. Signal transduction by glycophorin A: Role of extracellular and cytoplasmic domains in a modulatable process. J. Cell Biol. 1988, 107, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Himbert, S.; D’Alessandro, A.; Qadri, S.M.; Majcher, M.J.; Hoare, T.; Sheffield, W.P.; Nagao, M.; Nagle, J.F.; Rheinstädter, M.C. The bending rigidity of the red blood cell cytoplasmic membrane. PLoS ONE 2022, 17, e0269619. [Google Scholar] [CrossRef]


| −Yβsp, (μS) | −Cβsp, (pF) | Yγ1sp, (μS) | Cγ1sp, (pF) | −Yβsp/Yγ1sp | −Cβsp/Cγ1sp | |
|---|---|---|---|---|---|---|
| pH 7.4 | 90.50 | 12.01 | 65.00 | 1.59 | 1.39 | 7.52 |
| pH 5.2 | 57.00 | 6.98 | 77.00 | 2.04 | 0.74 | 3.41 |
| Concentration of Triton-X-100 (%Vol.) | −Yβsp, (μS) | −Cβsp, (pF) | Yγ1sp, (μS) | Cγ1sp, (pF) | −Yβsp/Yγ1sp | −Cβsp/Cγ1sp | |
|---|---|---|---|---|---|---|---|
| Erythrocyte ghost membranes | - | 103.00 | 12.62 | 85.00 | 1.50 | 1.21 | 8.40 |
| Triton shells (low delipidated) | 0.07 | 105 | 10.45 | 184.7 | 2.45 | 0.57 | 4.29 |
| Triton shells (moderate delipidated) | 0.10–0.20 | 5.0 | 0.72 | 110 | 2.2 | 22 | 3.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, I.T.; Paarvanova, B.K. Role of Plasma Membrane at Dielectric Relaxations and Intermembrane Interaction in Human Erythrocytes. Membranes 2023, 13, 658. https://doi.org/10.3390/membranes13070658
Ivanov IT, Paarvanova BK. Role of Plasma Membrane at Dielectric Relaxations and Intermembrane Interaction in Human Erythrocytes. Membranes. 2023; 13(7):658. https://doi.org/10.3390/membranes13070658
Chicago/Turabian StyleIvanov, Ivan T., and Boyana K. Paarvanova. 2023. "Role of Plasma Membrane at Dielectric Relaxations and Intermembrane Interaction in Human Erythrocytes" Membranes 13, no. 7: 658. https://doi.org/10.3390/membranes13070658
APA StyleIvanov, I. T., & Paarvanova, B. K. (2023). Role of Plasma Membrane at Dielectric Relaxations and Intermembrane Interaction in Human Erythrocytes. Membranes, 13(7), 658. https://doi.org/10.3390/membranes13070658







