Modification Approaches of Polyphenylene Oxide Membranes to Enhance Nanofiltration Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
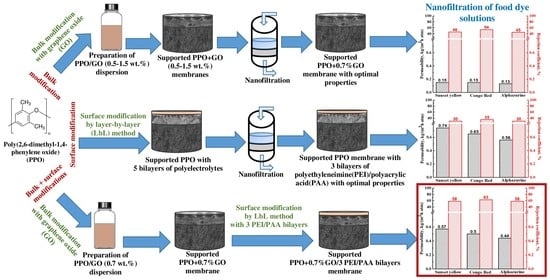
2.2. Membrane Preparation
2.2.1. Supported Membranes
2.2.2. Surface Modification with PEL by Layer-by-Layer Technique
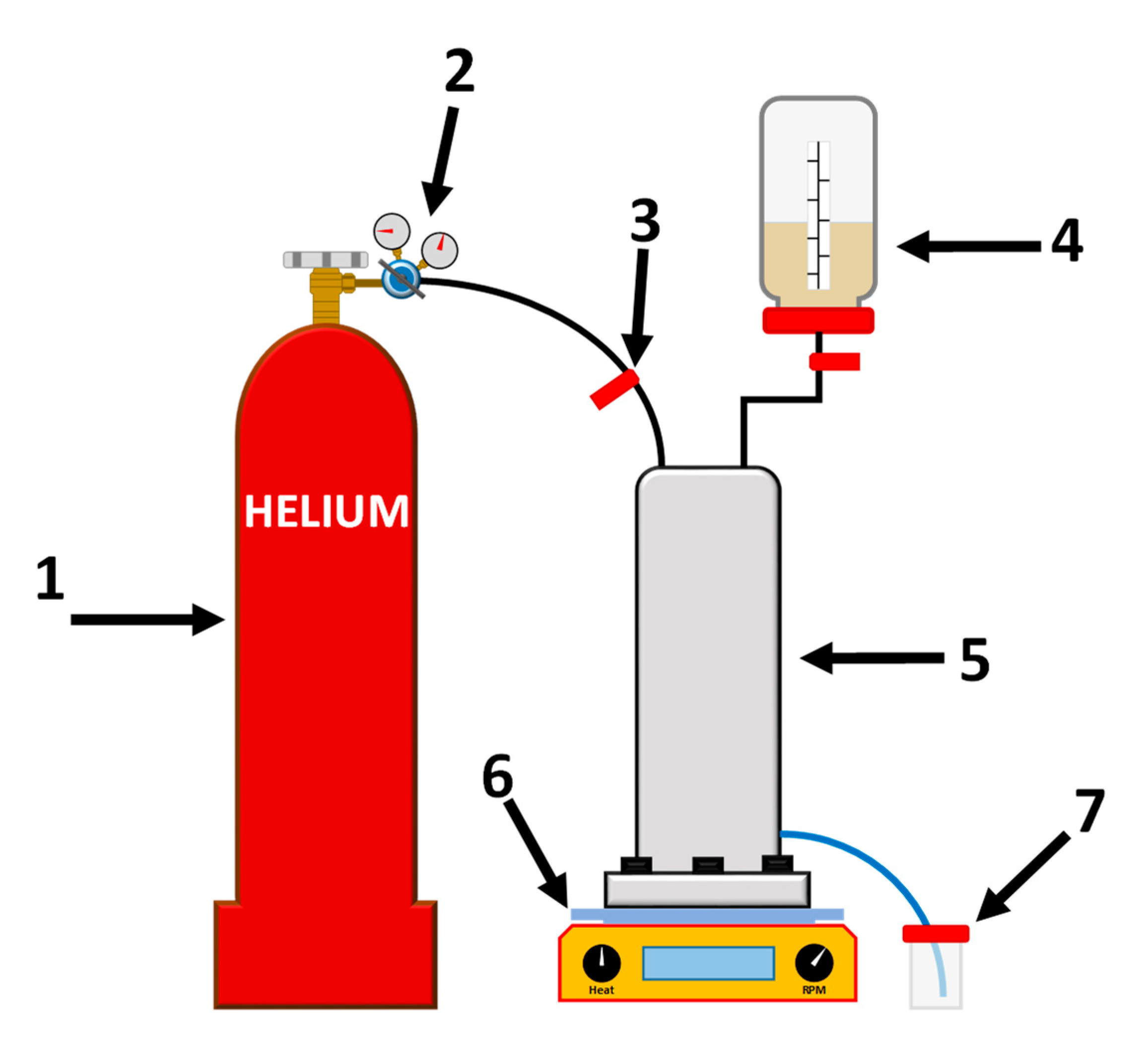
2.3. Nanofiltration (NF)
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Scanning Electron Microscopy (SEM)
2.6. Atomic Force Microscopy (AFM)
2.7. Contact Angle Measurements
3. Results
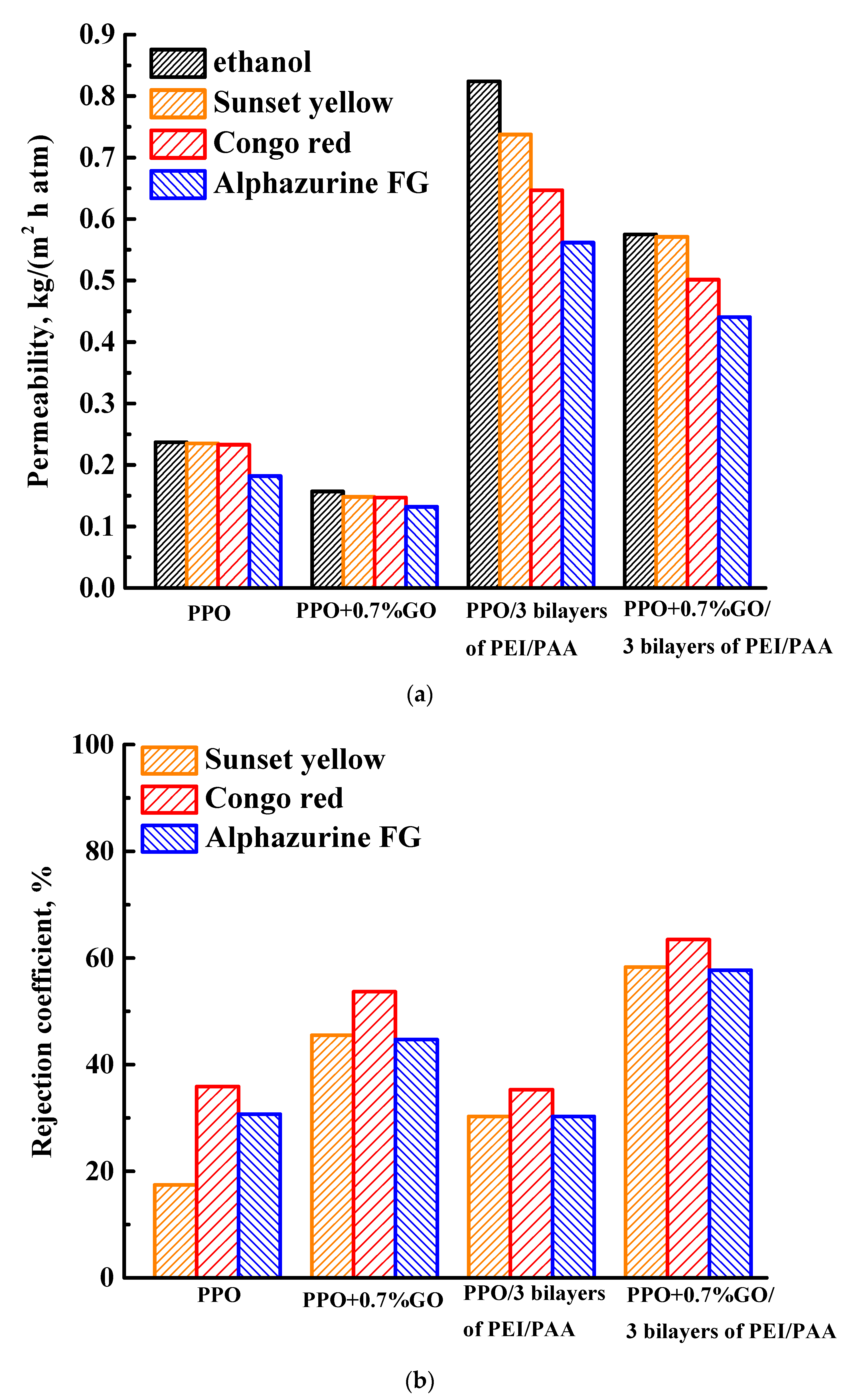
3.1. Nanofiltration Performance
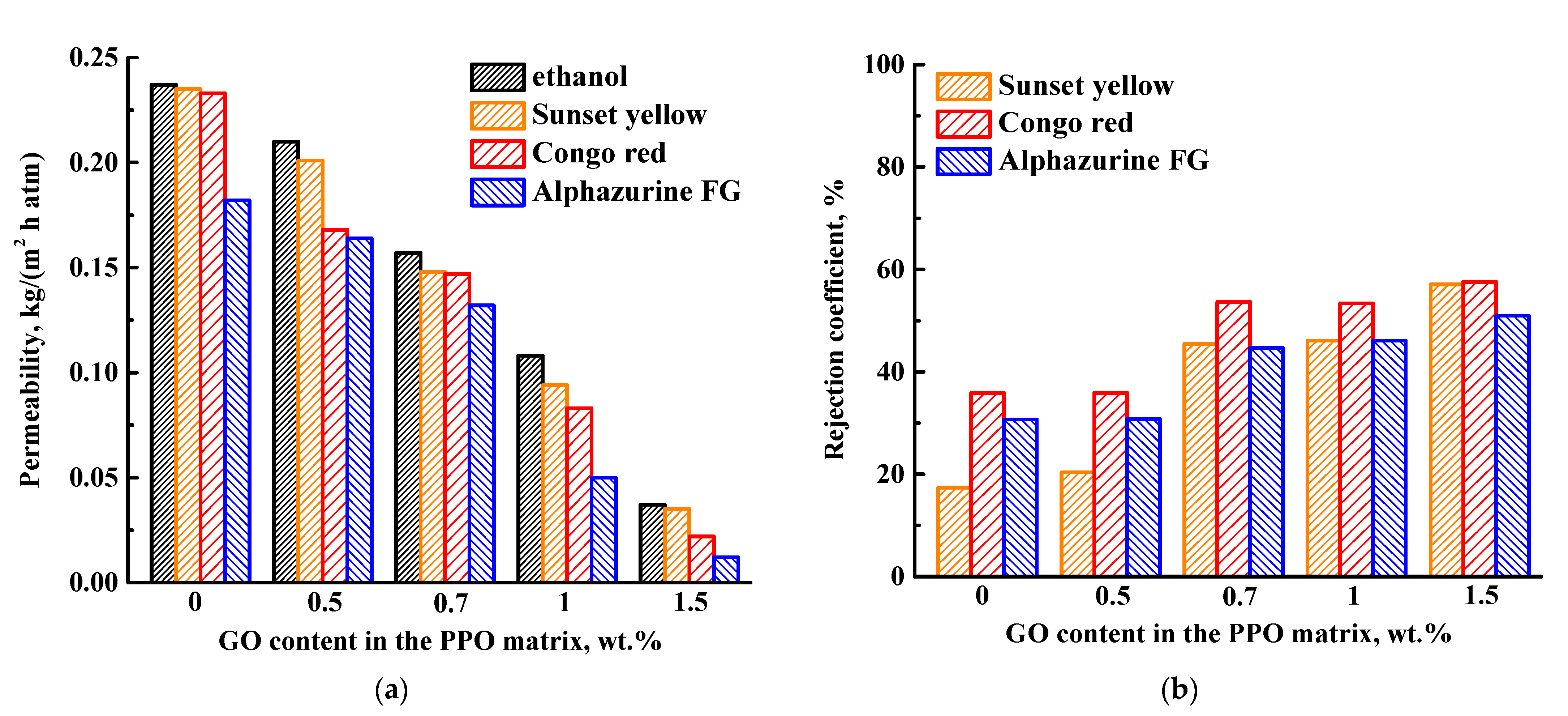
3.1.1. Study of GO Effect
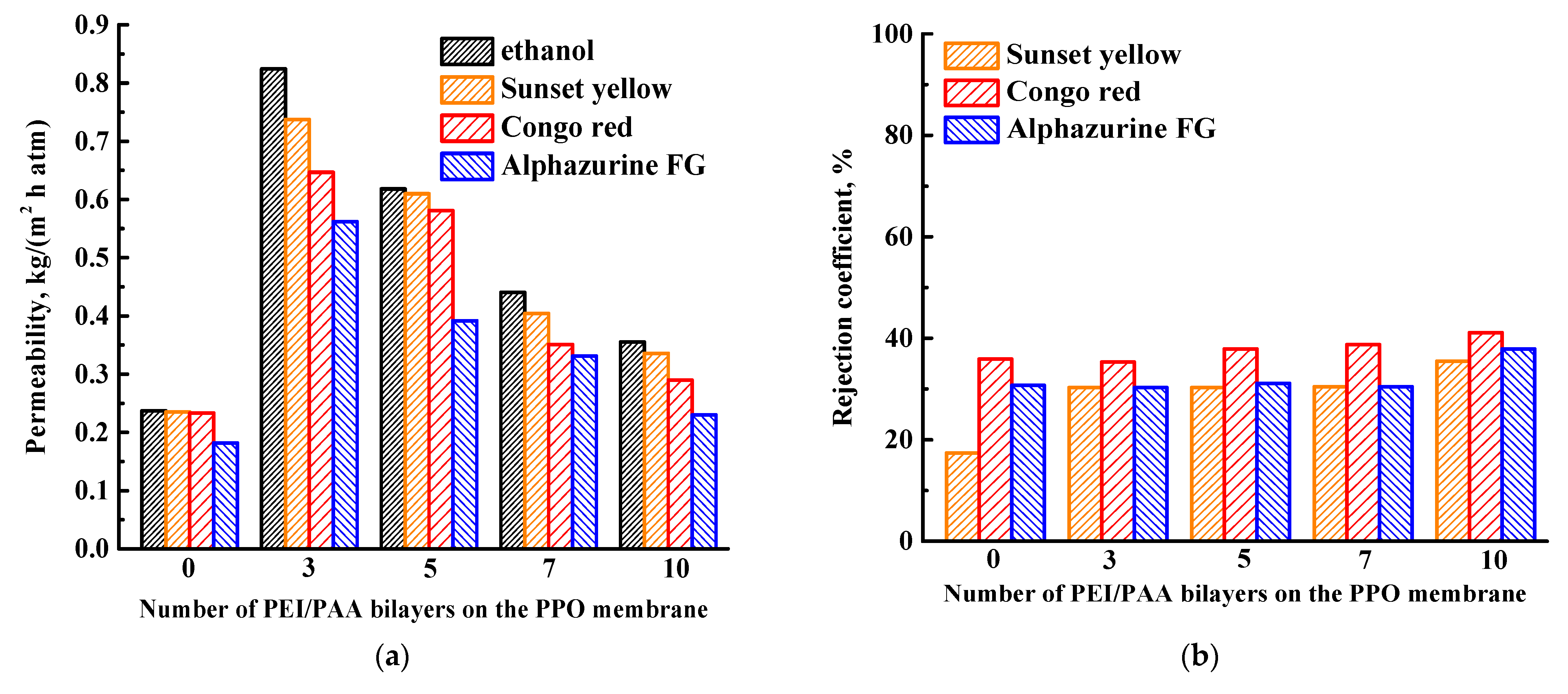
3.1.2. Study of the Effect of PEL Composition and Bilayer Number
3.1.3. Study of Membranes Modified with GO and PEL
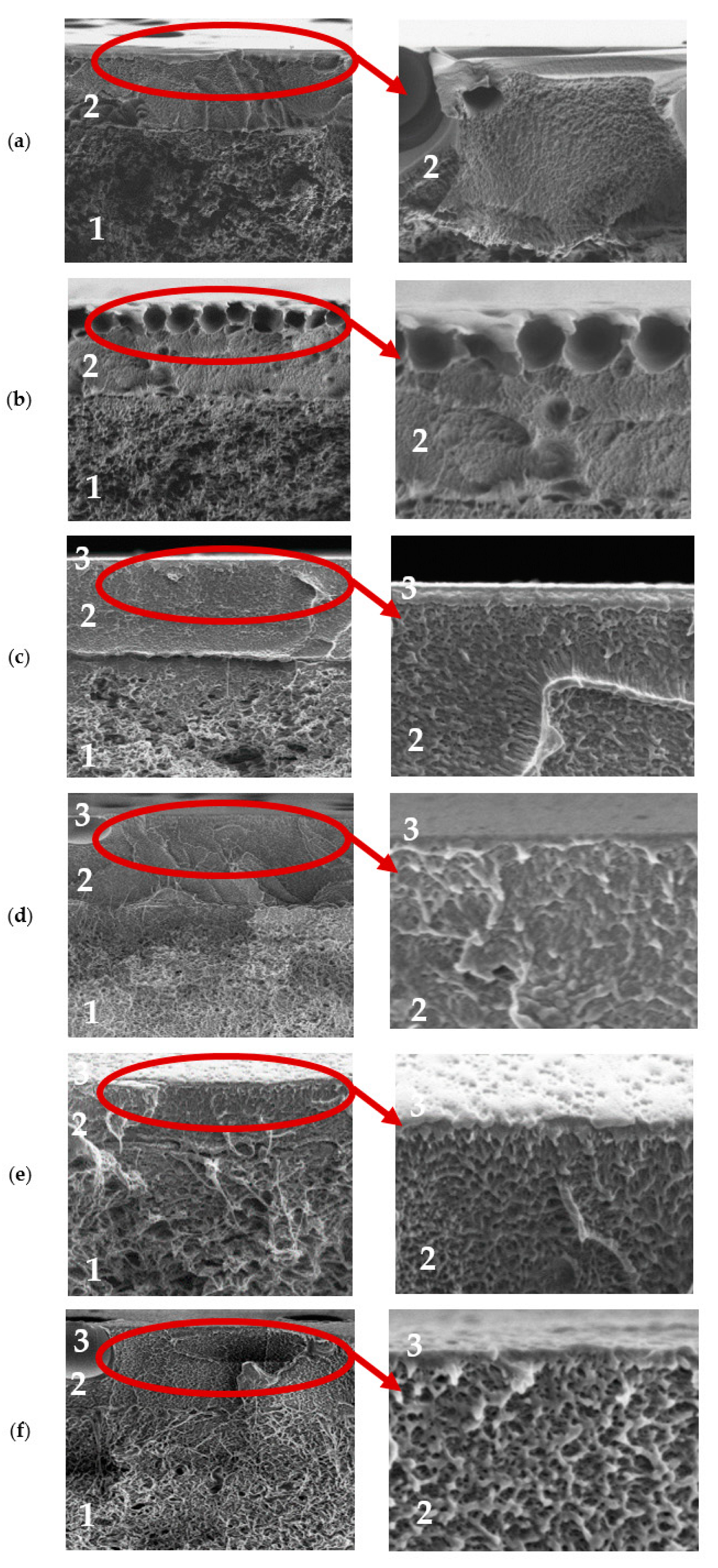
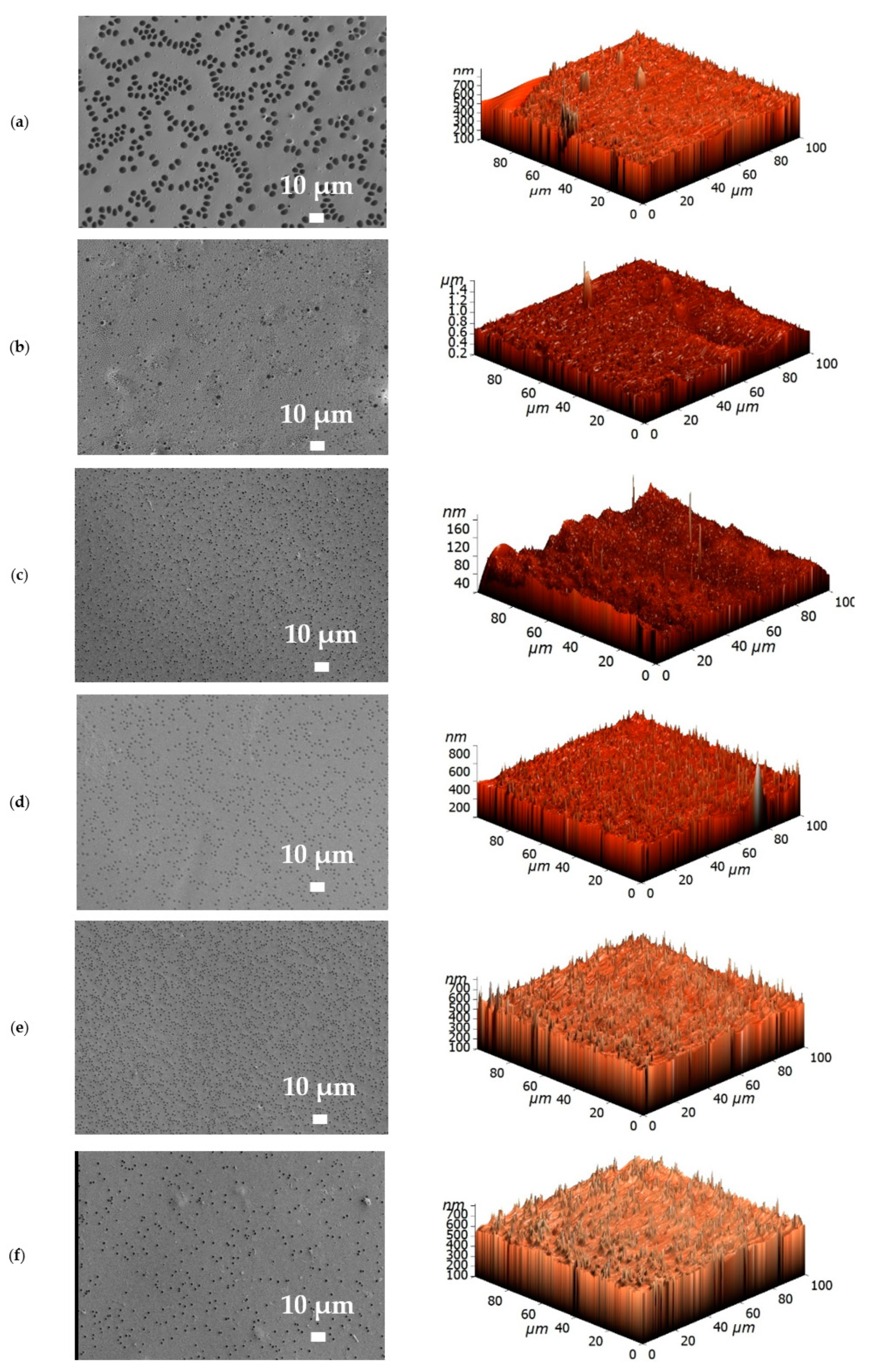
3.2. Membrane Characterization
3.3. Membrane Performance Comparison in Nanofiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahabuddin, S.; Sarih, N.; Afzal Kamboh, M.; Rashidi Nodeh, H.; Mohamad, S. Synthesis of Polyaniline-Coated Graphene Oxide@SrTiO3 Nanocube Nanocomposites for Enhanced Removal of Carcinogenic Dyes from Aqueous Solution. Polymers 2016, 8, 305. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, Kinetics and Mechanism of Removal of Methylene Blue from Aqueous Solution by Adsorption onto Pine Cone Biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Bensalah, N.; Alfaro, M.A.Q.; Martínez-Huitle, C.A. Electrochemical treatment of synthetic wastewaters containing Alphazurine A dye. Chem. Eng. J. 2009, 149, 348–352. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and Characterisation of Novel-Activated Carbon from Waste Biomass Pine Cone and Its Application in the Removal of Congo Red Dye from Aqueous Solution by Adsorption. Water Air Soil Pollut. 2014, 225, 1818. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Carvalho, J.; Zanoni, M.V.B.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact. In Eco-Friendly Textile Dyeing and Finishing; InTech: Vienna, Austria, 2013. [Google Scholar]
- Mondal, S.; Purkait, M.K.; De, S. Nanofiltration of Dyes. In Advances in Dye Removal Technologies; Springer: Singapore, 2018; pp. 153–197. [Google Scholar]
- Peeva, L.G.; Sairam, M.; Livingston, A.G. Nanofiltration Operations in Nonaqueous Systems. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2010; pp. 91–113. [Google Scholar]
- Handbook of Membrane Separations; Pabby, A.K., Rizvi, S.S.H., Requena, A.M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429128066. [Google Scholar]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Postel, S.; Spalding, G.; Chirnside, M.; Wessling, M. On negative retentions in organic solvent nanofiltration. J. Membr. Sci. 2013, 447, 57–65. [Google Scholar] [CrossRef]
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkov, I.I.; Minakov, A.V. Theoretical study of electrolyte transport in nanofiltration membranes with constant surface potential/charge density. J. Membr. Sci. 2016, 520, 515–528. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Rutten, S.B.; Ryzhkov, I.I.; Porada, S.; Elimelech, M. Theory for salt transport in charged reverse osmosis membranes: Novel analytical equations for desalination performance and experimental validation. Desalination 2023, 557, 116580. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Godino, P.; Khayet, M.; Seoane, B.; Mengual, J.I. Pervaporation of Alcohols and Methyl tert -Butyl Ether through a Dense Poly(2,6-dimethyl-1,4-phenylene oxide) Membrane. Ind. Eng. Chem. Res. 2004, 43, 2548–2555. [Google Scholar] [CrossRef]
- Tyan, N.S.; Polotskaya, G.A.; Meleshko, T.K.; Yakimansky, A.V.; Pientka, Z. Influence of the Molecular Polyimide Brush on the Gas Separation Properties of Polyphenylene Oxide. Russ. J. Appl. Chem. 2019, 92, 360–366. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Polotskaya, G.; Vinogradova, L.; Zoolshoev, Z.; Simonova, M.; Hairullin, A.; Toikka, A.; Pientka, Z. Hybrid macromolecular stars incorporated poly(phenylene oxide) membranes: Organization, physical, and gas separation properties. Polymer 2019, 172, 355–364. [Google Scholar] [CrossRef]
- Penkova, A.; Polotskaya, G.; Toikka, A. Pervaporation composite membranes for ethyl acetate production. Chem. Eng. Process. Process Intensif. 2015, 87, 81–87. [Google Scholar] [CrossRef]
- Polotskaya, G.; Pulyalina, A.; Lebedev, V.; Török, G.; Rudakova, D.; Vinogradova, L. Novel view at hybrid membranes containing star macromolecules using neutron scattering and pervaporation dehydration of acetic acid. Mater. Des. 2020, 186, 108352. [Google Scholar] [CrossRef]
- Moulik, S.; Kumar, K.P.; Bohra, S.; Sridhar, S. Pervaporation performance of PPO membranes in dehydration of highly hazardous mmh and udmh liquid propellants. J. Hazard. Mater. 2015, 288, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Mazur, A.; Semenov, K.; Solovyev, N.; Penkova, A. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers 2022, 14, 691. [Google Scholar] [CrossRef]
- Tongwen, X. A novel positively charged composite membranes for nanofiltration prepared from poly(2,6-dimethyl-1,4-phenylene oxide) by in situ amines crosslinking. J. Membr. Sci. 2003, 215, 25–32. [Google Scholar] [CrossRef]
- Meng, N.; Zhao, W.; Shamsaei, E.; Wang, G.; Zeng, X.; Lin, X.; Xu, T.; Wang, H.; Zhang, X. A low-pressure GO nanofiltration membrane crosslinked via ethylenediamine. J. Membr. Sci. 2018, 548, 363–371. [Google Scholar] [CrossRef]
- Nogami, N.; Chowdhury, G.; Matsuura, T. Preparation and performance testing of sulfonated poly(phenylene oxide) based composite membranes for nanofiltration. J. Appl. Polym. Sci. 2004, 91, 2624–2628. [Google Scholar] [CrossRef]
- Noël, I.M.; Lebrun, R.E.; Bouchard, C.R. Nanofiltration of NaCl solutions using a SPPO membrane (BQ01) Part 1. Membrane characterization and separation mechanisms. Desalination 2003, 155, 229–242. [Google Scholar] [CrossRef]
- Kim, K.-J.; Chowdhury, G.; Matsuura, T. Low pressure reverse osmosis performances of sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) thin film composite membranes: Effect of coating conditions and molecular weight of polymer. J. Membr. Sci. 2000, 179, 43–52. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, S.; Peng, G.; Sotto, A.; Ruan, H.; Shen, J.; Gao, C. Novel crosslinked brominated polyphenylene oxide composite nanofiltration membranes with organic solvent permeability and swelling property. J. Membr. Sci. 2021, 620, 118784. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Zhang, Z.; Lin, X.; Li, Q.; Zhu, Y. A high stability GO nanofiltration membrane preparation by co-deposition and crosslinking polydopamine for rejecting dyes. Water Sci. Technol. 2022, 85, 1783–1799. [Google Scholar] [CrossRef]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Shi, G.M.; Zuo, J.; Tang, S.H.; Wei, S.; Chung, T.S. Layer-by-layer (LbL) polyelectrolyte membrane with NexarTM polymer as a polyanion for pervaporation dehydration of ethanol. Sep. Purif. Technol. 2015, 140, 13–22. [Google Scholar] [CrossRef]
- Morshed, M.; Zimmer, A.; Broch, L.; Alem, H.; Roizard, D. PDMS membranes modified by polyelectrolyte multilayer deposition to improve OSN separation of diluted solutes in toluene. Sep. Purif. Technol. 2020, 237, 116331. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced pervaporation properties of PVA-based membranes modified with polyelectrolytes. application to IPA dehydration. Polymers 2020, 12, 14. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Chen, J.C.; Elimelech, M. Coupled model of concentration polarization and pore transport in crossflow nanofiltration. AIChE J. 2001, 47, 2733–2745. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Hidalgo, A.M.; León, G.; Gómez, M.; Murcia, M.D.; Gómez, E.; Macario, J.A. Removal of different dye solutions: A comparison study using a polyamide nf membrane. Membranes 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Influence of Graphene Oxide on the Ethanol Permeability and Ionic Conductivity of QPVA-Based Membrane in Passive Alkaline Direct Ethanol Fuel Cells. Nanoscale Res. Lett. 2019, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.E.; El-Sayed, A.A.; Khalil, A.M. Polysulfone nanofiltration membranes enriched with functionalized graphene oxide for dye removal from wastewater. J. Polym. Eng. 2020, 40, 833–841. [Google Scholar] [CrossRef]
- Church, R.B.; Hu, K.; Magnacca, G.; Cerruti, M. Cations Block Hydrogen-Bonding-Driven Ethanol Permeation through Disordered Drop-Cast Graphene Oxide Membranes. ACS Appl. Nano Mater. 2019, 2, 5389–5398. [Google Scholar] [CrossRef]
- Gholami, N.; Mahdavi, H. Nanofiltration composite membranes of polyethersulfone and graphene oxide and sulfonated graphene oxide. Adv. Polym. Technol. 2018, 37, 3529–3541. [Google Scholar] [CrossRef]
- Vaysizadeh, A.; Zinatizadeh, A.A.; Zinadini, S. Fouling mitigation and enhanced dye rejection in UF and NF membranes via layer-by-layer (LBL) assembly and altering PVP percentage as pore former. Environ. Technol. Innov. 2021, 23, 101698. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Mohammad, A.W.; Kadhum, A.A.H. Nanofiltration of hazardous Congo red dye: Performance and flux decline analysis. J. Water Process Eng. 2014, 4, 99–106. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.Q.; Yang, X.; Deng, H. Preparation of layer-by-layer nanofiltration membranes by dynamic deposition and crosslinking. Membranes 2019, 9, 20. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Yap Ang, M.B.M.; Patra, T.; Castilla-Casadiego, D.A.; Fan, R.; Almodovar, J.; Hung, W.-S.; Wickramasinghe, S.R. High-Performance Polyacrylic Acid-Grafted PVDF Nanofiltration Membrane with Good Antifouling Property for the Textile Industry. Polymers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Q.; Qu, Y.; Chen, M.; Soyekwo, F.; Lin, C.; Zhu, A.; Liu, Q. LBL assembled polyelectrolyte nanofiltration membranes with tunable surface charges and high permeation by employing a nanosheet sacrificial layer. J. Mater. Chem. A 2017, 5, 14819–14827. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Lahderanta, E.; Solovyev, N.; Penkova, A. Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes. Membranes 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Polotskaya, G.A.; Lebedev, V.T.; Pulyalina, A.Y.; Vinogradova, L.V. Structure and transport properties of pervaporation membranes based on polyphenylene oxide and heteroarm star polymers. Pet. Chem. 2016, 56, 920–930. [Google Scholar] [CrossRef]
- Rostovtseva, V.; Pulyalina, A.; Dubovenko, R.; Faykov, I.; Subbotina, K.; Saprykina, N.; Novikov, A.; Vinogradova, L.; Polotskaya, G. Enhancing Pervaporation Membrane Selectivity by Incorporating Star Macromolecules Modified with Ionic Liquid for Intensification of Lactic Acid Dehydration. Polymers 2021, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Hänni-Ciunel, K.; Findenegg, G.H.; von Klitzing, R. Water Contact Angle On Polyelectrolyte-Coated Surfaces: Effects of Film Swelling and Droplet Evaporation. Soft Mater. 2007, 5, 61–73. [Google Scholar] [CrossRef]
- Vanherck, K.; Hermans, S.; Verbiest, T.; Vankelecom, I. Using the photothermal effect to improve membrane separations via localized heating. J. Mater. Chem. 2011, 21, 6079. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Anokhina, T.S.; Volkov, A.V. Application of cellophane films as nanofiltration membranes. Pet. Chem. 2015, 55, 746–752. [Google Scholar] [CrossRef]
- Vanherck, K.; Verbiest, T.; Vankelecom, I. Comparison of Two Synthesis Routes to Obtain Gold Nanoparticles in Polyimide. J. Phys. Chem. C 2012, 116, 115–125. [Google Scholar] [CrossRef]
- Vanherck, K.; Vankelecom, I.; Verbiest, T. Improving fluxes of polyimide membranes containing gold nanoparticles by photothermal heating. J. Membr. Sci. 2011, 373, 5–13. [Google Scholar] [CrossRef]
- Li, H.; Ouyang, G.; Li, X.; Li, L.; Zhong, Z.; Cai, M.; Li, W.; Huang, W. Ultrathin Organic Solvent Nanofiltration with Polydopamine-Hkust-1 Interlayer for Organic Solvent Separation. SSRN Electron. J. 2022, 26. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Y.; Li, Y.; Jiang, Z. Fabrication of composite nanofiltration membranes with enhanced structural stability for concentrating oligomeric proanthocyanidins in ethanol aqueous solution. Korean J. Chem. Eng. 2015, 32, 1902–1909. [Google Scholar] [CrossRef]
- Yang, S.; Zhen, H.; Su, B. Polyimide thin film composite (TFC) membranes via interfacial polymerization on hydrolyzed polyacrylonitrile support for solvent resistant nanofiltration. RSC Adv. 2017, 7, 42800–42810. [Google Scholar] [CrossRef]
- Paseta, L.; Luque-Alled, J.M.; Malankowska, M.; Navarro, M.; Gorgojo, P.; Coronas, J.; Téllez, C. Functionalized graphene-based polyamide thin film nanocomposite membranes for organic solvent nanofiltration. Sep. Purif. Technol. 2020, 247, 116995. [Google Scholar] [CrossRef]



| PAA | PDADMAC | PAH | PEI | |
|---|---|---|---|---|
| Monomer unit | (C3H4O2)n | (C8H16ClN)n | (CH2CH(CH2NH2 · HCl))n | (C22N11H55)n |
| Mw unit, g/mol | 72 | 161.5 | 93.5 | 473 |
| PEL structure | 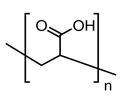 | 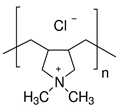 | 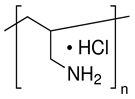 | 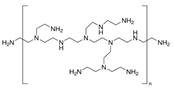 |
| Mn a | 100,000 | 200,000–350,000 | 150,000 | 60,000 |
| Charge density by weight (×103) b [31] | - | PDADMAC/PAA 4.3 | PAH/PAA 6.0 | PEI/PAA 1.8 |
| Dye | Molecular Formula | Structure | Molar Mass, g/mol |
|---|---|---|---|
| Sunset yellow (SY, E110) | C16H10N2Na2O7S2 | 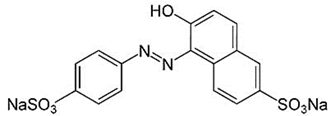 | 429 |
| Congo red (CR, E129) | C32H22N6Na2O6S2 | 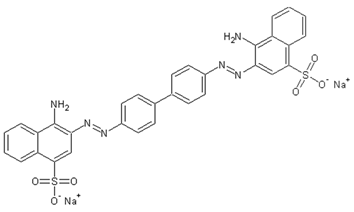 | 697 |
| Alphazurine (AZ, E133) | C37H34Na2N2O9S3 | 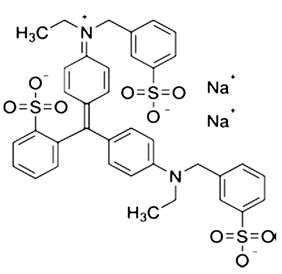 | 793 |
| Membrane | Ra, nm | Rq, nm | Contact Angle of Water, ° |
|---|---|---|---|
| PPO | 24.4 | 49.1 | 88 ± 2 |
| PPO + 0.7% GO | 44.2 | 73.0 | 86 ± 2 |
| PPO/5 bilayers of PDADMAC/PAA | 25.1 | 50.1 | 75 ± 2 |
| PPO/5 bilayers of PAH/PAA | 26.8 | 50.9 | 74 ± 2 |
| PPO/5 bilayers of PEI/PAA | 28.8 | 53.4 | 71 ± 2 |
| PPO + 0.7% GO/3 bilayers of PEI/PAA | 26.4 | 53.1 | 71 ± 2 |
| Membrane | Ethanol Permeability, kg/(m2h atm) | Dye Solution, Concentration | Solution Permeability, kg/(m2h atm) | Rejection Coefficient, % | Ref. |
|---|---|---|---|---|---|
| PPO + 0.7% GO/3 bilayers of PEI/PAA | 0.58 | Sunset yellow 10 mg/L | 0.57 | 58 | This study |
| Congo red 10 mg/L | 0.50 | 63 | |||
| Alphazurine FG 10 mg/L | 0.44 | 58 | |||
| PIM-1/MIL-125 | - | Alphazurine FG 10 mg/L | 0.19 | 99 | [33] |
| - | Sunset yellow 10 mg/L | 0.19 | 99 | ||
| PIM-1/MIL-140A | - | Alphazurine FG 10 mg/L | 0.25 | 89 | |
| - | Sunset yellow 10 mg/L | 0.23 | 91 | ||
| Cellulose acetate/gold nanoparticles | 0.06 | Bromothymol Blue | - | 82 | [50] |
| Cellophane | 0.05 | Remazol Brilliant Blue R | - | 79 | [51] |
| Orange II | - | 55 | |||
| Polyimide (PI)/gold nanoparticles | - | Methyl Orange | 0.16 | 82 | [52] |
| Bromothymol Blue | 2.3 | 58 | [53] | ||
| Thin- film nanocomposite membrane (TFCM) polyamide (PA)/polydopamine-HKUST-10.6/polyetherimide | 3.6 | Congo red 100 mg/L | 2.5 | 93 | [54] |
| Rose Bengal 100 mg/L | - | 91 | |||
| Methyl Orange 100 mg/L | - | 80 | |||
| TFCM PA-polyether- sulfone/polyvinyl formal | 1.6 | Orange GII 100 mg/L | - | 68 | [55] |
| TFCM PI/hydrolyzed polyacrylonitrile (PAN) | - | Coomassie brilliant blue 100 mg/L | 0.55 | 99 | [56] |
| TFCM PA/octadecylamine (ODA)-functionalized reduced graphene oxide (rGO)/PI | - | Sunset Yellow 20 mg/L | 3.6 | 99 | [57] |
| Rose Bengal 20 mg/L | 3.9 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Sushkova, X.; Chepeleva, A.; Liamin, V.; Mikhailovskaya, O.; Kuzminova, A.; Semenov, K.; Ermakov, S.; Penkova, A. Modification Approaches of Polyphenylene Oxide Membranes to Enhance Nanofiltration Performance. Membranes 2023, 13, 534. https://doi.org/10.3390/membranes13050534
Dmitrenko M, Sushkova X, Chepeleva A, Liamin V, Mikhailovskaya O, Kuzminova A, Semenov K, Ermakov S, Penkova A. Modification Approaches of Polyphenylene Oxide Membranes to Enhance Nanofiltration Performance. Membranes. 2023; 13(5):534. https://doi.org/10.3390/membranes13050534
Chicago/Turabian StyleDmitrenko, Mariia, Xeniya Sushkova, Anastasia Chepeleva, Vladislav Liamin, Olga Mikhailovskaya, Anna Kuzminova, Konstantin Semenov, Sergey Ermakov, and Anastasia Penkova. 2023. "Modification Approaches of Polyphenylene Oxide Membranes to Enhance Nanofiltration Performance" Membranes 13, no. 5: 534. https://doi.org/10.3390/membranes13050534
APA StyleDmitrenko, M., Sushkova, X., Chepeleva, A., Liamin, V., Mikhailovskaya, O., Kuzminova, A., Semenov, K., Ermakov, S., & Penkova, A. (2023). Modification Approaches of Polyphenylene Oxide Membranes to Enhance Nanofiltration Performance. Membranes, 13(5), 534. https://doi.org/10.3390/membranes13050534