Mitigation of Physical Aging of Polymeric Membrane Materials for Gas Separation: A Review
Abstract
1. Introduction
| Gas Pair | Application |
|---|---|
| CO2/CH4 | Acid gas treatment |
| Biogas separation | |
| N2/O2 | Nitrogen generation from the air |
| H2/N2 | Ammonia purge gas recovery |
| H2/CH4 | Refinery gas purification |
| H2/CO | Syngas ratio adjustment |
| H2O/Air | Dehydration |
| Membrane | Supplier | Material | Gas Separation | Module Type |
|---|---|---|---|---|
| Prism | Air Products/Permea | Polysulfone | H2/CO | Hollow fiber |
| H2/N2 | ||||
| H2/CH4 | ||||
| O2/N2 | ||||
| Cynara | Cameron | Cellulose acetate | CO2/CH4 | Hollow fiber |
| Medal | Air Liquide | Polyimide/polyaramide | CO2/CH4 | Hollow fiber |
| N2/CH4 | ||||
| H2S/CH4 | ||||
| Separex | UOP | Cellulose acetate | CO2/CH4 | Spiral wound |
| H2O/CH4 | ||||
| Grace | UOP | Cellulose acetate | CO2/CH4 | Spiral wound |
| H2O/CH4 | ||||
| Generon | IGS, Inc. | Polycarbonate, incl tetrabromo | O2/N2, | Hollow fiber |
| H2/N2, | ||||
| H2/CH4 | ||||
| IMS | Praxair | Polyimide | O2/N2 | Hollow fiber |
| (N2 generation) | ||||
| UBE | Ube Industries | Polyimide | H2O/Air | Hollow fiber |
| O2/N2 | ||||
| (N2 generation) | ||||
| CO2/CH4 | ||||
| Parker | Parker Hannifin | poly(phenylene oxide) | O2/N2 | Hollow fiber |
| (N2 generation) | ||||
| PVTMS 1 | Cryogenmash | Polyvinyltrimethylsilane | H2/N2 | Plate and frame |
| O2/N2 |
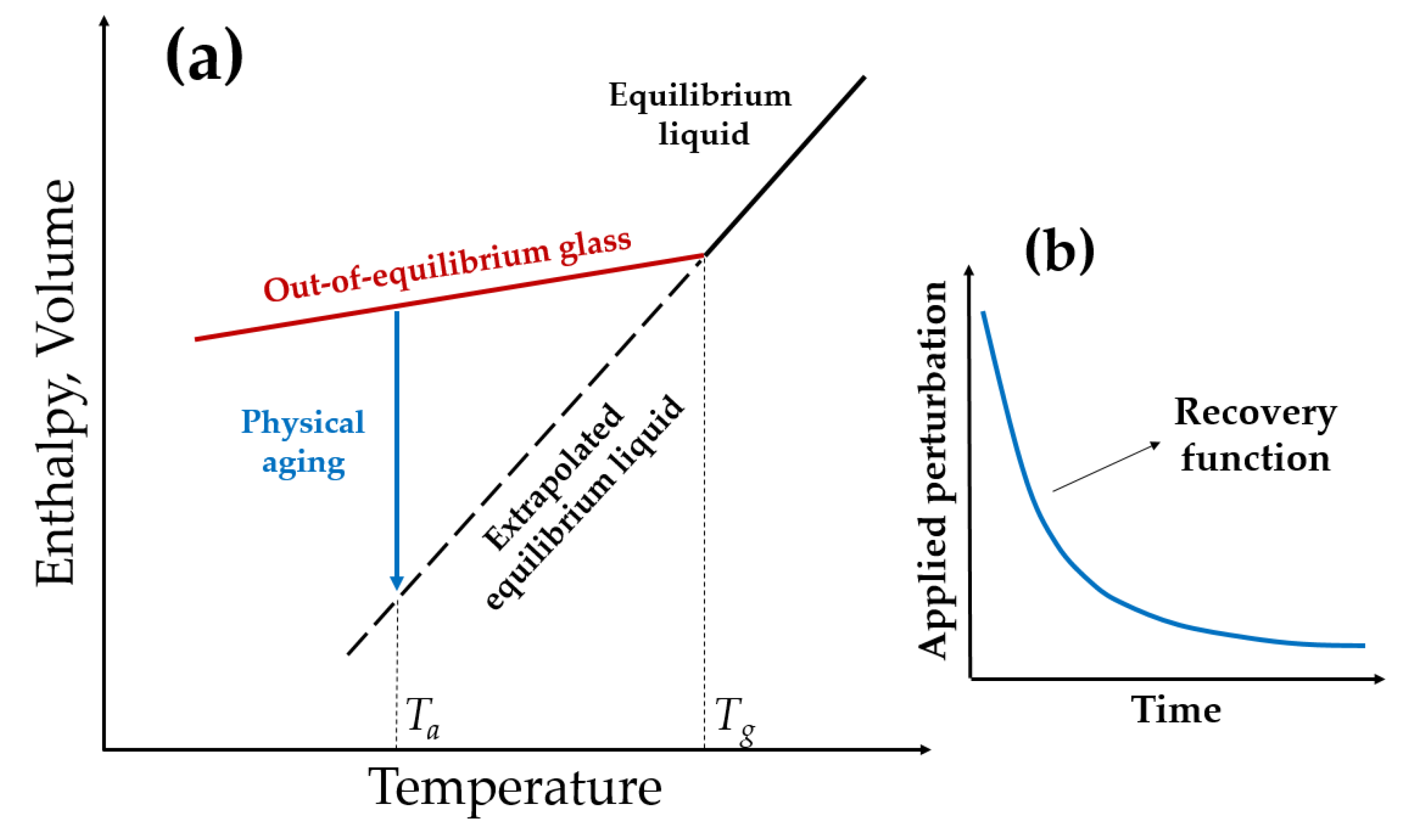
2. Physical Aging
2.1. Physical Aging of Glassy Polymers
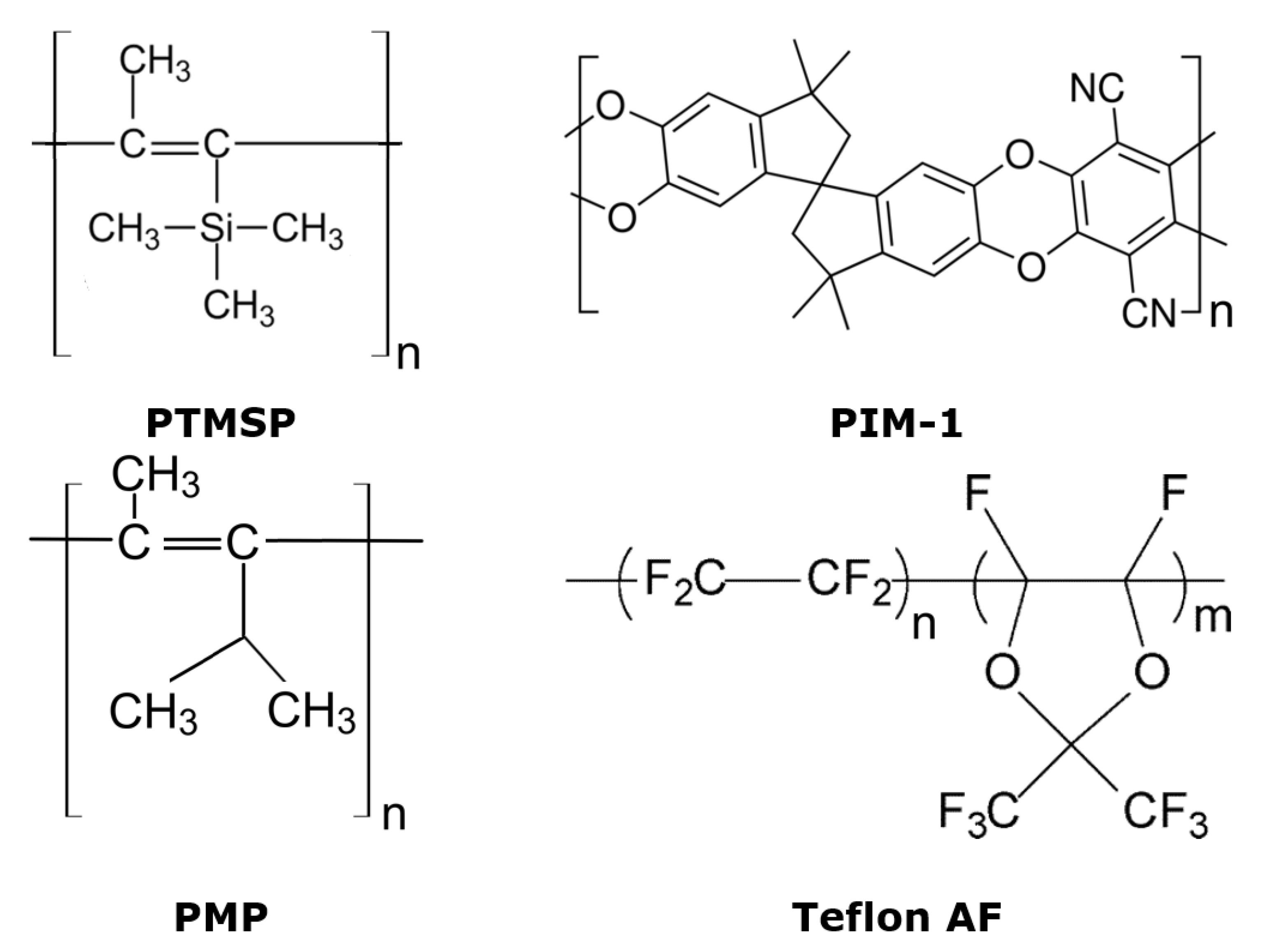
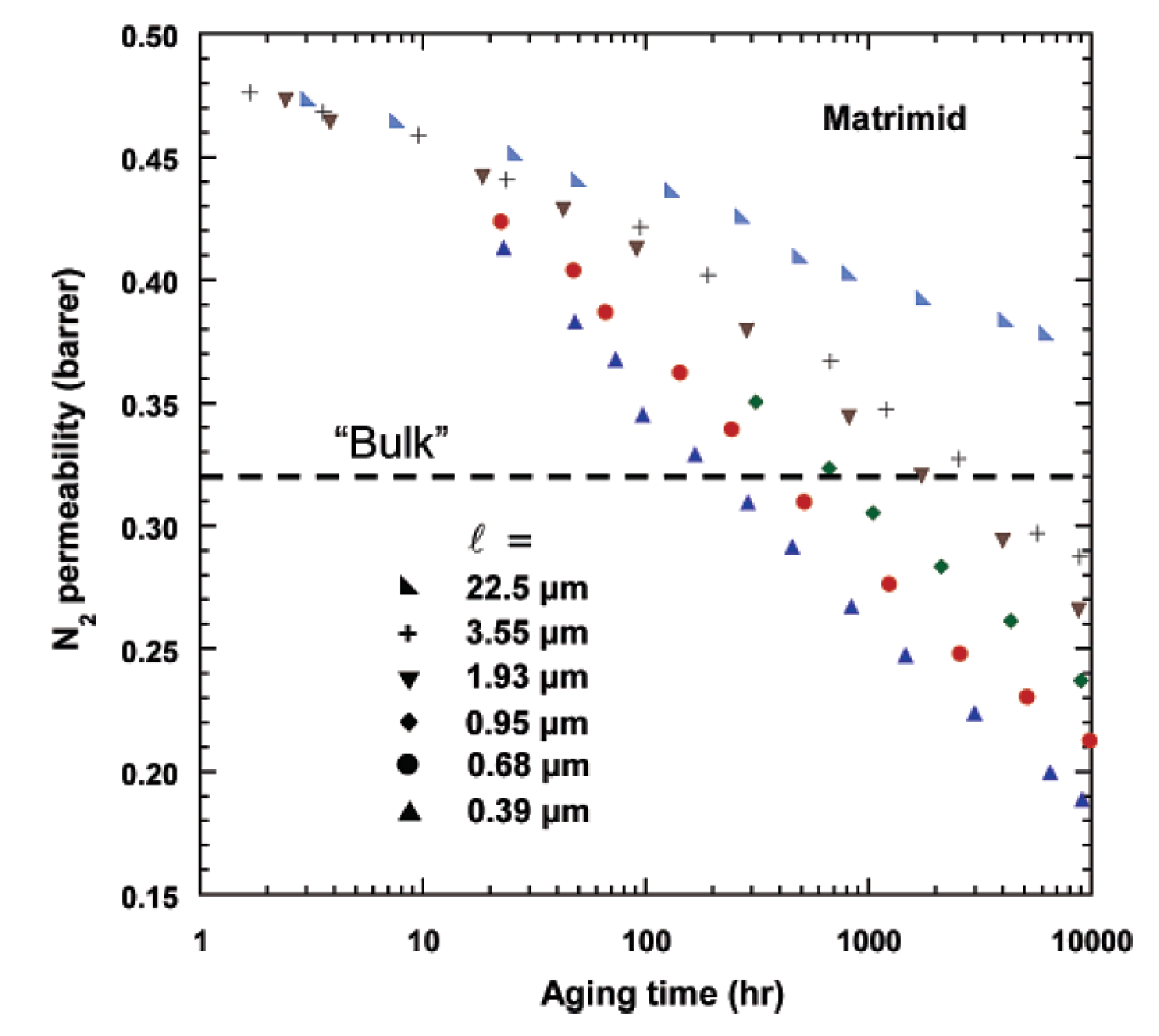
2.2. Physical Aging of Dense Membranes
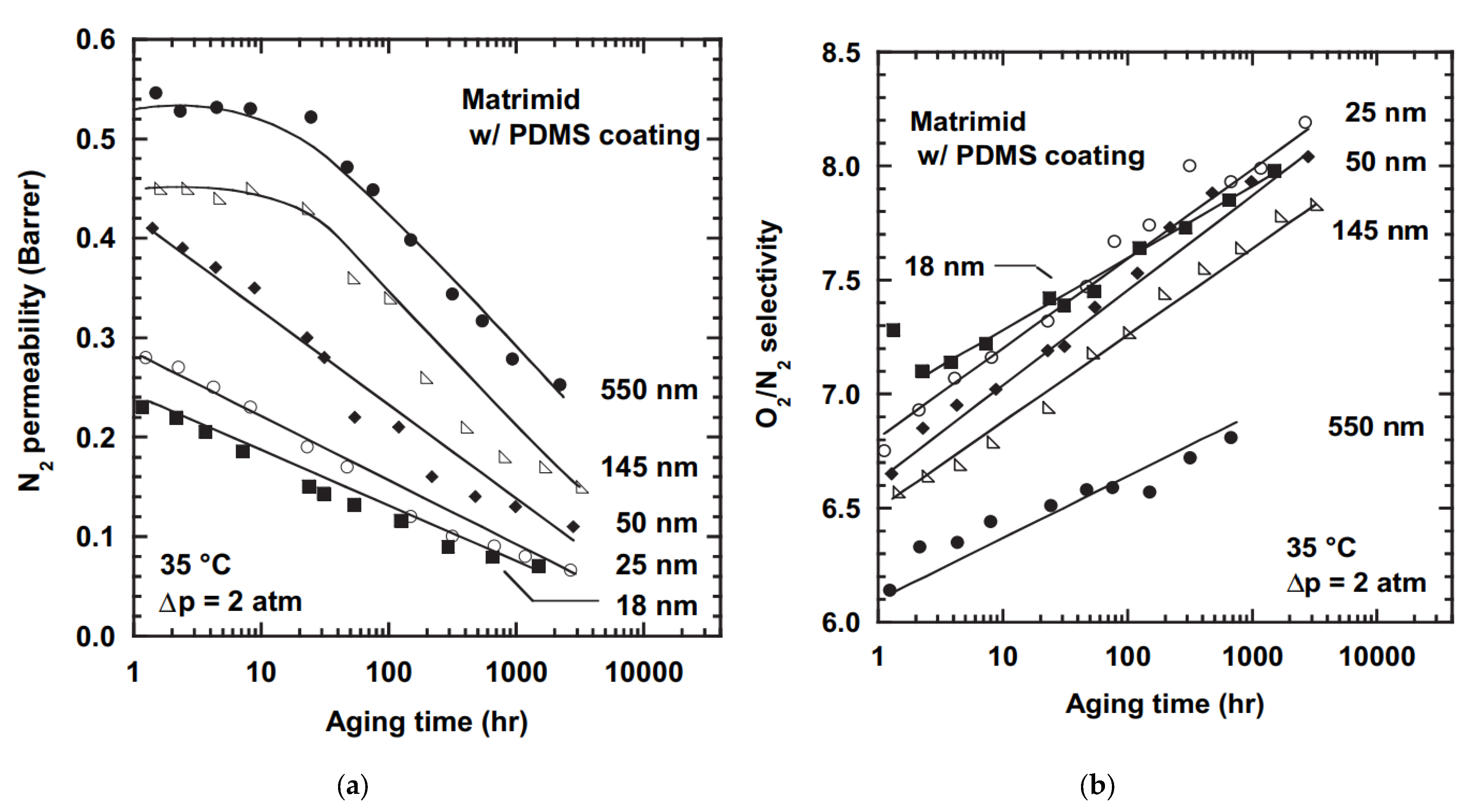
2.3. Physical Aging of Thin-Film Membranes
3. Mitigating Physical Aging
3.1. Dense Membranes
3.2. Thin Film Composite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Yampol’skii, Y.P.; Volkov, V.V. Studies in gas permeability and membrane gas separation in the Soviet Union. J. Membr. Sci. 1991, 64, 191–228. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Valappil, R.S.K.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.M.; Alegria, A.; Colmenero, J. Physical aging in polymers and polymer nanocomposites: Recent results and open questions. Soft Matter 2013, 9, 8619–8630. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T. Poly [1-(trimethylsilyl)-1-propyne]: A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Khotimsky, V.S.; Tchirkova, M.V.; Litvinova, E.G.; Rebrov, A.I.; Bondarenko, G.N. Poly [1-(trimethylgermyl)-1-propyne] and poly [1-(trimethylsilyl)-1-propyne] with various geometries: Their synthesis and properties. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2133–2155. [Google Scholar] [CrossRef]
- Shtennikova, I.N.; Kolbina, G.F.; Yakimansky, A.V.; Plate, N.A.; Khotimsky, V.S.; Litvinova, E.G. Experimental and theoretical investigation of optical properties of poly-(1-trimethylsilyl-1 propyne) molecules in solution. Eur. Polym. J. 1999, 35, 2073–2078. [Google Scholar] [CrossRef]
- Volkov, V.V. Free volume structure and transport properties of glassy polymers—Materials for separating membranes. Polym. J. 1991, 23, 457–466. [Google Scholar] [CrossRef]
- Srinivasan, R.; Auvil, S.R.; Burban, P.M. Elucidating the mechanism(s) of gas transport in poly [1-(trimethylsilyl)-1-propyne](PTMSP) membranes. J. Membr. Sci. 1994, 86, 67–86. [Google Scholar] [CrossRef]
- Hofmann, D.; Entrialgo-Castano, M.; Lerbret, A.; Heuchel, M.; Yampolskii, Y. Molecular modeling investigation of free volume distributions in stiff chain polymers with conventional and ultrahigh free volume: Comparison between molecular modeling and positron lifetime studies. Macromolecules 2003, 36, 8528–8538. [Google Scholar] [CrossRef]
- Consolati, G.; Genco, I.; Pegoraro, M.; Zanderighi, L. Positron annihilation lifetime (PAL) in poly [1-(trimethyl-silyl) propine](PTMSP): Free volume determination and time dependence of permeability. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 357–368. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Castro-Muñoz, R.; Budd, P.M. Boosting gas separation performance and suppressing the physical aging of polymers of intrinsic microporosity (PIM-1) by nanomaterial blending. Nanoscale 2020, 12, 23333–23370. [Google Scholar] [CrossRef]
- Morisato, A.; He, Z.; Pinnau, I. Mixed-Gas Properties and Physical Aging of Poly(4-methyl-2-pentyne). In Polymer Membranes for Gas and Vapor Separation: Chemistry and Materials Science; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1999; Volume 733. [Google Scholar]
- Nagai, K.; Toy, L.G.; Freeman, B.D.; Teraguchi, M.; Kwak, G.; Masuda, T.; Pinnau, I. Gas permeability and n-butane solubility of poly (1-trimethylgermyl-1-propyne). J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2228–2236. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Borisov, I.L.; Volkov, A.V.; Petukhov, D.I.; Gavrilova, N.N.; Shantarovich, V.P.; Asachenko, A.F.; Topchiy, M.A.; Finkelshtein, E.S. Switching on/switching off solubility controlled permeation of hydrocarbons through glassy polynorbornenes by the length of side alkyl groups. J. Membr. Sci. 2022, 641, 119848. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Syromolotov, A.V.; Gringolts, M.L.; Starannikova, L.E.; Yampolskii, Y.P.; Finkelshtein, E.S. Synthesis of high molecular weight poly [3-{tris (trimethylsiloxy) silyl} tricyclononenes-7] and their gas permeation properties. Macromolecules 2011, 44, 6637. [Google Scholar] [CrossRef]
- Merkel, T.C.; Pinnau, I.; Prabhakar, R.; Freeman, B.D. Gas and vapor transport properties of perfluoropolymers. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Pinnau, I., Freeman, B.D., Eds.; John Wiley & Sons: Chichester, UK, 2006; Chapter 9; pp. 251–270. [Google Scholar]
- Pinnau, I.; Toy, L.G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2, 2-bistrifluoromethyl-4, 5-difluoro-1, 3-dioxole/tetrafluoroethylene. J. Membr. Sci. 1996, 109, 125–133. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Yampolskii, Y.P.; Shantarovich, V.P.; Nemser, S.M.; Plate, N.A. High transport parameters and free volume of perfluorodioxole copolymers. J. Membr. Sci. 1997, 126, 123–132. [Google Scholar] [CrossRef]
- Neki, K.; Geil, P.H. Morphology-property studies of amorphous polycarbonate. J. Macromol. Sci. Part B Phys. 1973, 8, 295–341. [Google Scholar] [CrossRef]
- Yeh, G.S.Y. Yielding of glassy polymer on a microstructural level. J. Macromol. Sci. Part B Phys. 1973, 7, 729–746. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by optical properties. Macromolecules 2006, 39, 1554–1559. [Google Scholar] [CrossRef]
- McCaig, M.S.; Paul, D.R. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical aging Part I. Experimental observations. Polymer 2000, 41, 629–637. [Google Scholar] [CrossRef]
- Rowe, B.W.; Freeman, B.D.; Paul, D.R. Physical aging of ultrathin glassy polymer films tracked by gas permeability. Polymer 2009, 50, 5565–5575. [Google Scholar] [CrossRef]
- McCaig, M.S.; Paul, D.R.; Barlow, J.W. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical aging Part II. Mathematical model. Polymer 2000, 41, 639–648. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by gas permeability. Polymer 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Kelman, S.D.; Rowe, B.W.; Bielawski, C.W.; Pas, S.J.; Hill, A.J.; Paul, D.R.; Freeman, B.D. Crosslinking poly [1-(trimethylsilyl)-1-propyne] and its effect on physical stability. J. Membr. Sci. 2008, 320, 123–134. [Google Scholar] [CrossRef]
- Horn, N.R.; Paul, D.R. Carbon dioxide plasticization of thin glassy polymer films. Polymer 2011, 52, 5587–5594. [Google Scholar] [CrossRef]
- Horn, N.R.; Paul, D.R. Carbon dioxide sorption and plasticization of thin glassy polymer films tracked by optical methods. Macromolecules 2012, 45, 2820–2834. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Smith, Z.P.; Lin, H.; Freeman, B.D.; Paul, D.R. Gas permeation in thin films of “high free-volume” glassy perfluoropolymers: Part I. Physical aging. Polymer 2014, 55, 5788–5800. [Google Scholar] [CrossRef]
- Wang, H.; Chung, T.-S.; Paul, D.R. Physical aging and plasticization of thick and thin films of the thermally rearranged ortho-functional polyimide 6FDA−HAB. J. Membr. Sci. 2014, 458, 27–35. [Google Scholar] [CrossRef]
- Rowe, B.W.; Pas, S.J.; Hill, A.J.; Suzuki, R.; Freeman, B.D.; Paul, D. A variable energy positron annihilation lifetime spectroscopy study of physical aging in thin glassy polymer films. Polymer 2009, 50, 6149–6156. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Jin, J.Y.; Freeman, B.D.; Paul, D.R. Physical aging, CO2 sorption and plasticization in thin films of polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2017, 537, 362–371. [Google Scholar] [CrossRef]
- Merrick, M.M.; Sujanani, R.; Freeman, B.D. Glassy polymers: Historical findings, membrane applications, and unresolved questions regarding physical aging. Polymer 2020, 211, 123176. [Google Scholar] [CrossRef]
- Low, Z.X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.Y.; Mudie, S.; Kennedy, D.F.; Shaun, C.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-separation membranes loaded with porous aromatic frameworks that improve with age. Angew. Chem. 2015, 127, 2707–2711. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Ghanem, B.S.; Alghunaimi, F.; Pinnau, I.; Han, Y. Polymers of intrinsic microporosity for energy-intensive membrane-based gas separations. Mater. Today Nano 2018, 3, 69–95. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef]
- León, N.E.; Liu, Z.; Irani, M.; Koros, W.J. How to get the best gas separation membranes from state-of-the-art glassy polymers. Macromolecules 2022, 55, 1457–1473. [Google Scholar] [CrossRef]
- Yampol’skii, Y.P.; Shishatskii, S.M.; Shantorovich, V.P.; Antipov, E.M.; Kuzmin, N.N.; Rykov, S.V.; Plate, N.A. Transport characteristics and other physicochemical properties of aged poly (1-(trimethylsilyl)-1-propyne). J. Appl. Polym. Sci. 1993, 48, 1935–1944. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Glassy Polymers and Other Materials; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Pfromm, P.H.; Koros, W.J. Accelerated physical ageing of thin glassy polymer films: Evidence from gas transport measurements. Polymer 1995, 36, 2379–2387. [Google Scholar] [CrossRef]
- Dorkenoo, K.D.; Pfromm, P.H. Experimental evidence and theoretical analysis of physical aging in thin and thick amorphous glassy polymer films. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2239–2251. [Google Scholar] [CrossRef]
- Forrest, J.A.; Dalnoki-Veress, K. The glass transition in thin polymer films. Adv. Colloid Interface Sci. 2001, 94, 167–195. [Google Scholar] [CrossRef]
- Forrest, J.A. A decade of dynamics in thin films of polystyrene: Where are we now? Eur. Phys. J. E 2002, 8, 261–266. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of film thickness on the gas-permeation characteristics of glassy polymer membranes. Ind. Eng. Chem. Res. 2007, 46, 2342–2347. [Google Scholar] [CrossRef]
- Shishatskii, A.M.; Yampol’skii, Y.P.; Peinemann, K.V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]
- Pfromm, P.H. The impact of physical aging of amorphous glassy polymers on gas separation membranes. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Pinnau, I., Freeman, B.D., Eds.; John Wiley & Sons: Chichester, UK, 2006; Chapter 11; pp. 293–306. [Google Scholar]
- Castro-Munoz, R.; Fila, V.; Dung, C.T. Mixed matrix membranes based on PIMs for gas permeation: Principles, synthesis, and current status. Chem. Eng. Commun. 2017, 204, 295–309. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of graphene and graphene oxide nanoplatelets on the gas permselectivity and aging behavior of poly (trimethylsilyl propyne)(PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending Aging in Super Glassy Polymer Membranes. Angew. Chem. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Konstas, K.; Doherty, C.M.; Kanehashi, S.; Ozcelik, B.; Kentish, S.E.; Hill, M.R. Tailoring Physical Aging in Super Glassy Polymers with Functionalized Porous Aromatic Frameworks for CO2 Capture. Chem. Mater. 2015, 27, 4756–4762. [Google Scholar] [CrossRef]
- Kitchin, M.; Teo, J.; Konstas, K.; Lau, C.H.; Sumby, C.J.; Thornton, A.W.; Doonan, C.J.; Hill, M.R. AIMs: A new strategy to control physical aging and gas transport in mixed-matrix membranes. J. Mater. Chem. A 2015, 3, 15241–15247. [Google Scholar] [CrossRef]
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked Additives for Ageless Gas-Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 1998–2001. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Liao, K.S.; Chung, T.S. Highly permeable and aging resistant 3D architecture from polymers of intrinsic microporosity incorporated with beta-cyclodextrin. J. Membr. Sci. 2017, 523, 92–102. [Google Scholar] [CrossRef]
- Bakhtin, D.; Bazhenov, S.; Polevaya, V.; Grushevenko, E.; Makaev, S.; Karpacheva, G.; Volkov, A. Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile. Membranes 2020, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Baker, G.L. Cross-linking of poly [1-(trimethylsilyl)-1-propyne] membranes using bis (aryl azides). J. Polym. Sci. B Polym. Phys. 1998, 36, 959–968. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Borisov, I.L.; Bakhtin, D.S.; Rybakova, A.N.; Khotimskiy, V.S.; Molchanov, S.P.; Volkov, V.V. High-permeance crosslinked PTMSP thin-film composite membranes as supports for CO2 selective layer formation. Green Energy Environ. 2016, 1, 235–245. [Google Scholar] [CrossRef]
- Shao, L.; Samseth, J.; Hägg, M.B. Crosslinking and stabilization of nanoparticle filled poly (1-trimethylsilyl-1-propyne) nanocomposite membranes for gas separations. J. Appl. Polym. Sci. 2009, 113, 3078–3088. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Legkov, S.A.; Khotimskiy, V.S.; Levin, I.S.; Borisov, I.L.; Maksimov, A.L.; Volkov, V.V.; Karakhanov, E.A.; Volkov, A.V. Aging of thin-film composite membranes based on PTMSP loaded with porous aromatic frameworks. J. Membr. Sci. 2018, 554, 211–220. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Bondarenko, G.N.; Vasilevskii, V.P.; Maksimov, A.L.; Volkov, A.V. Stabilization of Gas Transport Properties of Composite Membranes with a Thin PTMSP Selective Layer by Adding Porous Aromatic Framework Nanoparticles and Simultaneous Polymer Crosslinking. Pet. Chem. 2018, 58, 790–796. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Maksimov, A.L.; Volkov, A.V. Composite Membranes Based on the Poly(1-trimethylsylyl-1-propine): Influence of the Porous Aromatic Frameworks Produced from the Friedel-Crafts Reaction and Introduced into the Polymer Matrix. Russ. J. Appl. Chem. 2020, 93, 252–257. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Malakhov, A.O.; Polevaya, V.G.; Kulikov, L.A.; Grekhov, A.M.; Bazhenov, S.D.; Volkov, A.V. Behavior of Polytrimethylsilylpropyne-Based Composite Membranes in the Course of Continuous and Intermittent Gas Permeability Measurements. Russ. J. Appl. Chem. 2021, 94, 616–623. [Google Scholar] [CrossRef]
- Peter, J.; Peinemann, K.V. Multilayer composite membranes for gas separation based on crosslinked PTMSP gutter layer and partially crosslinked Matrimid® 5218 selective layer. J. Membr. Sci. 2009, 340, 62–72. [Google Scholar] [CrossRef]
- Shao, L.; Samseth, J.; Hägg, M.B. Crosslinking and stabilization of nanoparticle filled PMP nanocomposite membranes for gas separations. J. Membr. Sci. 2009, 326, 285–292. [Google Scholar] [CrossRef]
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. High performance organic solvent nanofiltration membranes: Development and thorough testing of thin film composite membranes made of polymers of intrinsic microporosity (PIMs). J. Membr. Sci. 2012, 401, 222–231. [Google Scholar] [CrossRef]
- Hou, R.; Ghanem, B.S.; Smith, S.J.; Doherty, C.M.; Setter, C.; Wang, H.; Pinnau, I.; Hill, M.R. Highly permeable and selective mixed-matrix membranes for hydrogen separation containing PAF-1. J. Mater. Chem. A 2020, 8, 14713–14720. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Doherty, C.M.; Smith, S.J.; Hou, R.; Wang, H.; Carta, M.; Yoon, H.; Park, J.; Freeman, B.D.; et al. Tailoring molecular interactions between microporous polymers in high performance mixed matrix membranes for gas separations. Nanoscale 2020, 12, 17405–17410. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Konstas, K.; Doherty, C.M.; Wood, C.D.; Mulet, X.; Xie, Z.; Ng, D.; Hill, M.R.; Shao, L.; Lau, C.H. Hyper-Cross-Linked Additives that Impede Aging and Enhance Permeability in Thin Polyacetylene Films for Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 14401–14408. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Hou, R.; Konstas, K.; Akram, A.; Lau, C.H.; Hill, M.R. Control of Physical Aging in Super-Glassy Polymer Mixed Matrix Membranes. Acc. Chem. Res. 2020, 53, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Golubev, G.S.; Borisov, I.L.; Litvinova, E.G.; Khotimsky, V.S.; Bakhtin, D.S.; Pastukhov, A.; Davankov, V.A.; Volkov, V.V. A novel hybrid material based on polytrimethylsilylpropyne and hypercrosslinked polystyrene for membrane gas separation and thermopervaporation. Pet. Chem. 2017, 57, 498–510. [Google Scholar] [CrossRef]
- Volkov, A.V.; Bakhtin, D.S.; Kulikov, L.A.; Terenina, M.V.; Golubev, G.S.; Bondarenko, G.N.; Legkov, S.A.; Shandryuk, G.A.; Volkov, V.V.; Khotimskiy, V.S.; et al. Stabilization of gas transport properties of PTMSP with porous aromatic framework: Effect of annealing. J. Membr. Sci. 2016, 517, 80–90. [Google Scholar] [CrossRef]
- Borisov, I.; Bakhtin, D.; Luque-Alled, J.M.; Rybakova, A.; Makarova, V.; Foster, A.B.; Volkov, V.; Volkov, A. Synergistic enhancement of gas selectivity in thin film composite membranes of PIM-1. J. Mater. Chem. A. 2019, 7, 6417–6430. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Malakhov, A.O.; Volkov, A.V.; Kulikov, L.A.; Petrova, I.V.; Borisov, I.L.; Bazhenov, S.D. Mitigating of Thin-Film Composite PTMSP Membrane Aging by Introduction of Porous Rigid and Soft Branched Polymeric Additives. Membranes 2023, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Sorption, Transport, and Structural Evidence for Enhanced Free Volume in Poly(4-methyl-2-pentyne)/Fumed Silica Nanocomposite Membranes. Chem. Mater. 2003, 15, 109–123. [Google Scholar] [CrossRef]
- Hill, A.J.; Pas, S.J.; Bastow, T.J.; Burgar, M.I.; Nagai, K.; Toy, L.G.; Freeman, B.D. Influence of methanol conditioning and physical aging on carbon spin-lattice relaxation times of poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 2004, 243, 37–44. [Google Scholar] [CrossRef]
- Volkov, A.V.; Tsarkov, S.E.; Gokzhaev, M.B.; Bondarenko, G.N.; Legkov, S.A.; Kukushkina, Y.A.; Volkov, V.V. Nanofiltration and sorption of organic solvents in poly(1-trimethylsilyl-1-propyne) samples of different microstructures. Petrol. Chem. 2012, 52, 598. [Google Scholar] [CrossRef]
- Yu, M.; Foster, A.B.; Scholes, C.A.; Kentish, S.E.; Budd, P.M. Methanol Vapor Retards Aging of PIM-1 Thin Film Composite Membranes in Storage. ACS Macro Lett. 2023, 12, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Abdulhamid, M.A.; Lai, H.W.; Wang, Y.; Jin, Z.; Teo, Y.C.; Ma, X.; Pinnau, I.; Xia, Y. Microporous polyimides from ladder diamines synthesized by facile catalytic arene–norbornene annulation as high-performance membranes for gas separation. Chem. Mater. 2019, 31, 1767–1774. [Google Scholar] [CrossRef]
- Ma, X.; Lai, H.W.; Wang, Y.; Alhazmi, A.; Xia, Y.; Pinnau, I. Facile Synthesis and Study of Microporous Catalytic Arene-Norbornene Annulation–Tröger’s Base Ladder Polymers for Membrane Air Separation. ACS Macro Lett. 2020, 9, 680–685. [Google Scholar] [CrossRef]
- Lai, H.W.; Benedetti, F.M.; Ahn, J.M.; Robinson, A.M.; Wang, Y.; Pinnau, I.; Xia, Y. Hydrocarbon ladder polymers with ultrahigh permselectivity for membrane gas separations. Science 2022, 375, 1390–1392. [Google Scholar] [CrossRef] [PubMed]
- Yavari, M.; Le, T.; Lin, H. Physical aging of glassy perfluoropolymers in thin film composite membranes. Part I. Gas transport properties. J. Membr. Sci. 2017, 525, 387–398. [Google Scholar] [CrossRef]
- Yavari, M.; Maruf, S.; Ding, Y.; Lin, H. Physical aging of glassy perfluoropolymers in thin film composite membranes. Part II. Glass transition temperature and the free volume model. J. Membr. Sci. 2017, 525, 399–408. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Mitra, T.; Adams, D.J.; Cooper, A.I.; Budd, P.M. Ultrahigh-permeance PIM-1 based thin film nanocomposite membranes on PAN supports for CO2 separation. J. Membr. Sci. 2018, 564, 878–886. [Google Scholar] [CrossRef]
- Liu, M.; Nothling, M.D.; Webley, P.A.; Jin, J.; Fu, Q.; Qiao, G.G. High-throughput CO2 capture using PIM-1@ MOF based thin film composite membranes. Chem. Eng. J. 2020, 396, 125328. [Google Scholar] [CrossRef]
- Ogieglo, W.; Puspasari, T.; Ma, X.; Pinnau, I. Sub-100 nm carbon molecular sieve membranes from a polymer of intrinsic microporosity precursor: Physical aging and near-equilibrium gas separation properties. J. Membr. Sci. 2020, 597, 117752. [Google Scholar] [CrossRef]
- Fan, S.T.; Tan, M.; Liu, W.T.; Li, B.J.; Zhang, S. MOF-layer composite polyurethane membrane increasing both selectivity and permeability: Pushing commercial rubbery polymer membranes to be attractive for CO2 separation. Sep. Purif. Technol. 2022, 297, 121452. [Google Scholar] [CrossRef]
- Bakhtin, D.S. Development of Gas Separation Membranes Based on PTMSP with Enhanced Stability of Characteristics in Time. Ph.D. Thesis, A. V. Topchiev Institute of Petrochemical Synthesis, Moscow, Russia, 2023; pp. 125–128. (In Russian). [Google Scholar]
- Mitra, T.; Bhavsar, R.S.; Adams, D.J.; Budd, P.M.; Cooper, A.I. PIM-1 mixed matrix membranes for gas separations using cost-effective hypercrosslinked nanoparticle fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, L.A.; Bakhtin, D.S.; Polevaya, V.G.; Balynin, A.V.; Maksimov, A.L.; Volkov, A.V. Friedel-Crafts Synthesis of New Porous Aromatic Frameworks for Stabilizing Gas Transport Properties of Highly Permeable Glassy Polymers. Russ. J. Appl. Chem. 2019, 92, 199–207. [Google Scholar] [CrossRef]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Baranchikov, A.E.; Karpacheva, G.P. IR radiation assisted preparation of KOH-activated polymer-derived carbon for methylene blue adsorption. J. Environ. Chem. Eng. 2019, 7, 103514. [Google Scholar] [CrossRef]
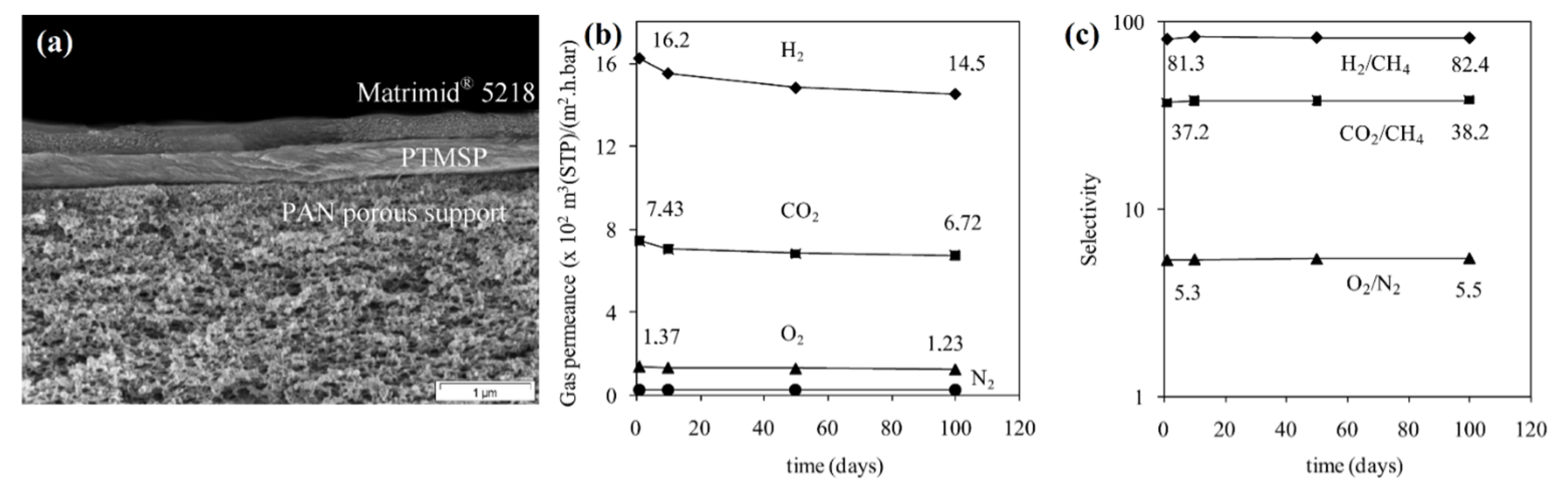

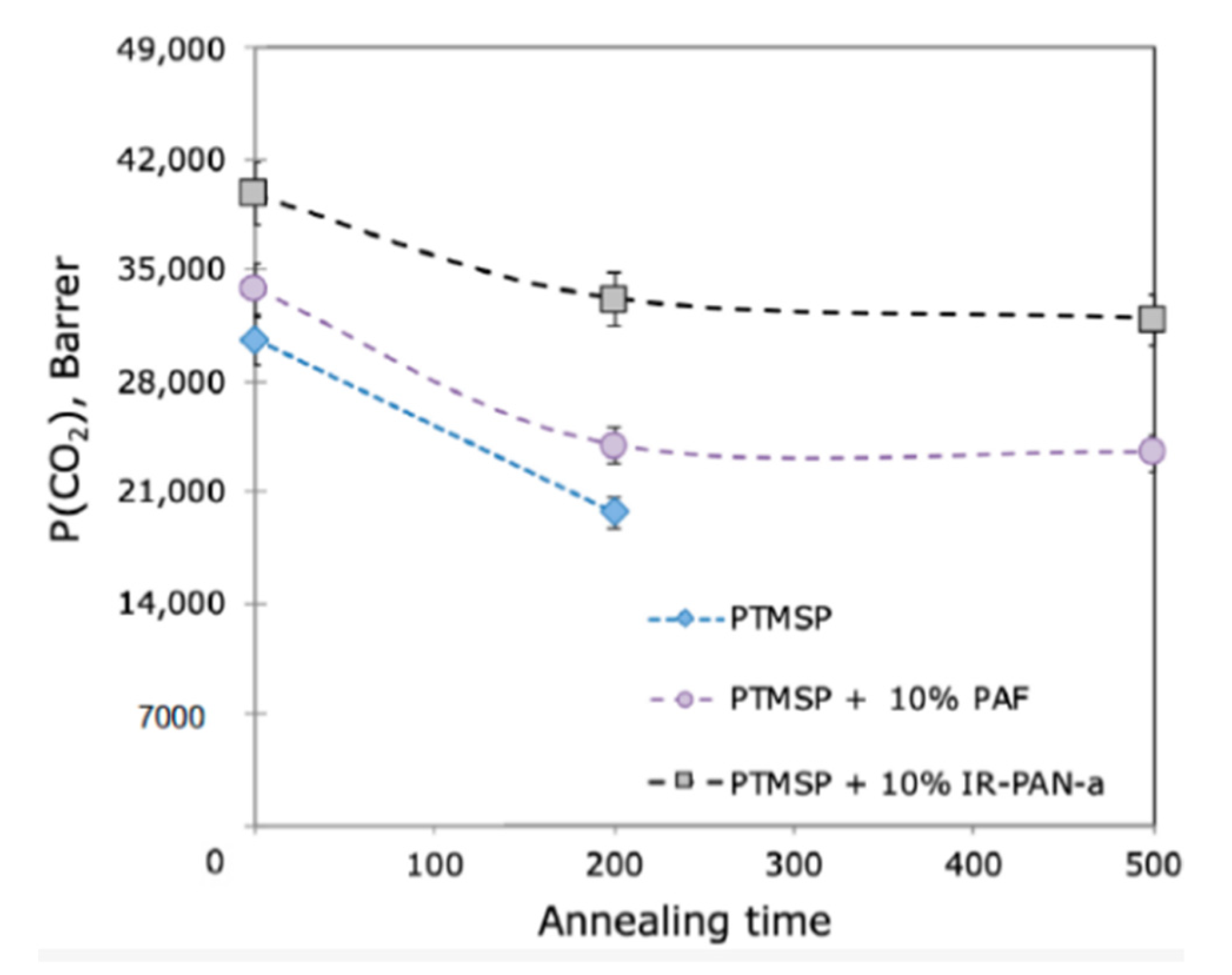
| Selective Layer | Selective Layer Thickness, µm | CO2 Permeance of Fresh As-Cast Membrane, GPU | Aging Time (Ambient Conditions) | CO2 Permeance Q/Q0, % | CO2/N2 Ideal Selectivity α/α0, % | Reference |
|---|---|---|---|---|---|---|
| PTMSP+10% PAF-11 | 1.7 | 1700 | >600 days | 8.5 | 314 | [64] |
| 6.8 | 6500 | 5.5 | 137 | |||
| PIM-1/C-HCP | 2.0 | 22,000 | 100 days | 37 | 150 | [90] |
| PIM-1 | 0.3 | 8000 | 90 days | 3.7 | 96 | [78] |
| PIM-1 | 0.7 | 4300 | 56 days | 11 | 166 | [91] |
| PIM-1/MOF-74-Ni | 5000 | 24 | 90 | |||
| PIM-1/NH2-UiO-66 | 7500 | 12 | 100 | |||
| PTMSP + PEI | 1.2 | 15,100 | >425 days | 23 | 139 | [60] |
| PTMSP + PEI + 10% IR-PAN-a | 23,700 | 17 | 158 | |||
| PTMSP + PEI + 20% IR-PAN-a | 1.8 | 24,700 | 26 | 133 | ||
| PTMSP + PEI + 30% IR-PAN-a | 24,100 | 22 | 111 | |||
| PTMSP + PEI + 10% IR-PAN-aM | 20,900 | 30 | 107 | |||
| PTMSP + PEI + 20% IR-PAN-aM | 1.0 | 24,500 | 27 | 110 | ||
| PTMSP + PEI + 30% IR-PAN-aM | 25,100 | 27 | 114 | |||
| Carbon molecular sieves (PDMS pyrolysis) precursor | 0.087 | 239 | 45 days | 9.6 | 50 | [92] |
| Carbon molecular sieves (PDMS pyrolysis) 500 °C | 0.069 | 294 | 9.9 | 110 | ||
| Carbon molecular sieves (PDMS pyrolysis) 600 °C | 0.082 | 320 | 0.9 | 166 | ||
| Carbon molecular sieves (PDMS pyrolysis) 700 °C | 0.072 | 8 | 17.5 | 250 | ||
| PU/PIM-1 | 30 | 11 | 60 days | 82 | - | [93] |
| PTMSP | 1.0 | 27,700 | 450 days | 14 | - | [79] |
| PTMSP + PEI | 1.1 | 21,000 | 30 | - | ||
| PTMSP + PEI + 20% PAF-11 | 1.0 | 34,400 | 42 | - | ||
| PTMSP + PEI + 30% PAF-11 | 1.1 | 40,600 | 43 | - |
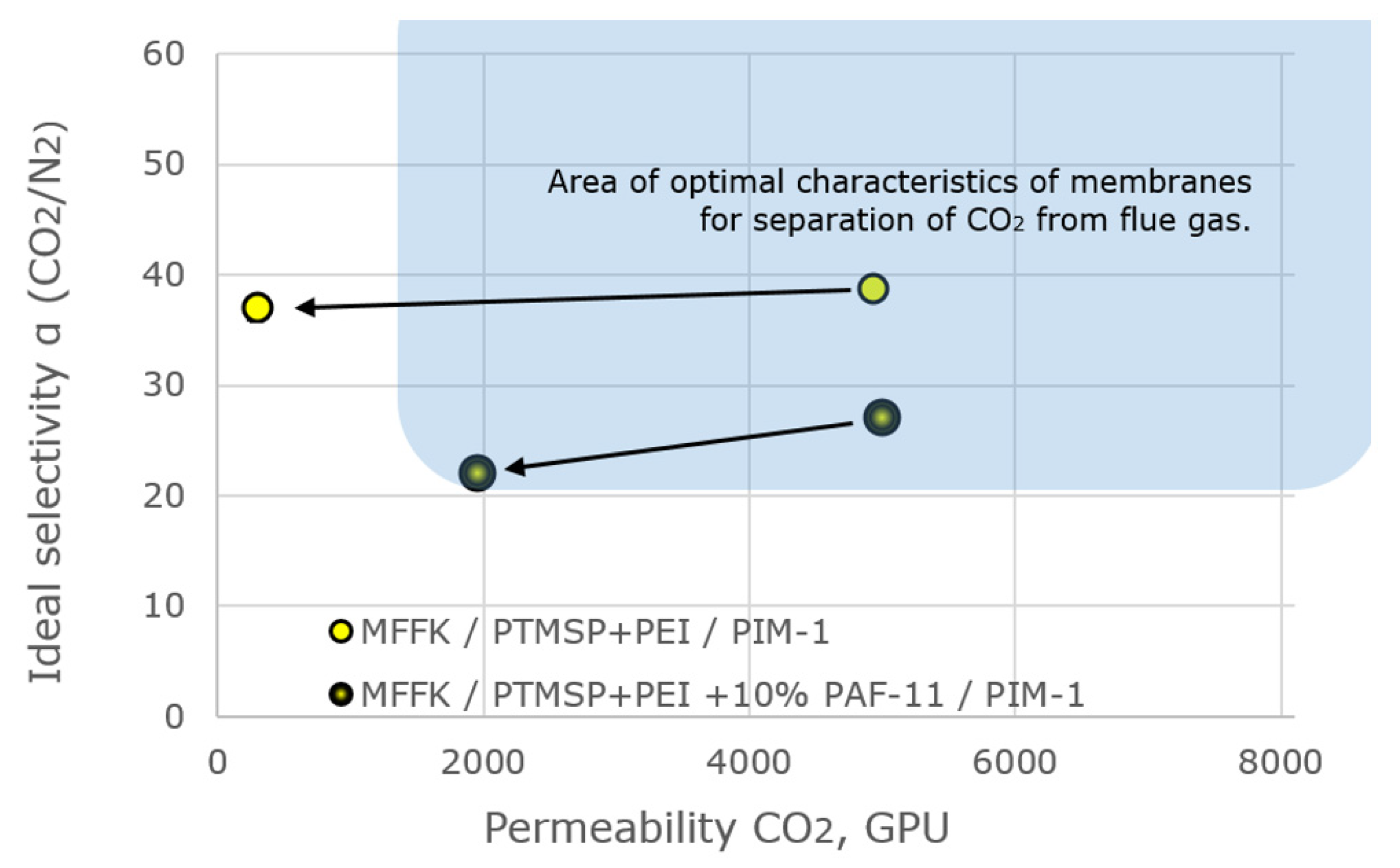
| Membrane | Time Since Formation, Days | Permeanse, GPU | Ideal Selectivity α (CO2/N2) | |
|---|---|---|---|---|
| N2 | CO2 | |||
| MFFK/gutter layer − PTMSP + cross-linked PEI/PIM-1 | 0 | 127 | 4930 | 38.8 |
| 94 | 8 | 297 | 37.1 | |
| MFFK/gutter layer − PTMSP + cross-linked − PEI + 10% PAF-11/PIM-1 | 0 | 185 | 5000 | 27.0 |
| 95 | 96 | 1970 | 20.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtin, D.S.; Sokolov, S.E.; Borisov, I.L.; Volkov, V.V.; Volkov, A.V.; Samoilov, V.O. Mitigation of Physical Aging of Polymeric Membrane Materials for Gas Separation: A Review. Membranes 2023, 13, 519. https://doi.org/10.3390/membranes13050519
Bakhtin DS, Sokolov SE, Borisov IL, Volkov VV, Volkov AV, Samoilov VO. Mitigation of Physical Aging of Polymeric Membrane Materials for Gas Separation: A Review. Membranes. 2023; 13(5):519. https://doi.org/10.3390/membranes13050519
Chicago/Turabian StyleBakhtin, Danila S., Stepan E. Sokolov, Ilya L. Borisov, Vladimir V. Volkov, Alexey V. Volkov, and Vadim O. Samoilov. 2023. "Mitigation of Physical Aging of Polymeric Membrane Materials for Gas Separation: A Review" Membranes 13, no. 5: 519. https://doi.org/10.3390/membranes13050519
APA StyleBakhtin, D. S., Sokolov, S. E., Borisov, I. L., Volkov, V. V., Volkov, A. V., & Samoilov, V. O. (2023). Mitigation of Physical Aging of Polymeric Membrane Materials for Gas Separation: A Review. Membranes, 13(5), 519. https://doi.org/10.3390/membranes13050519










