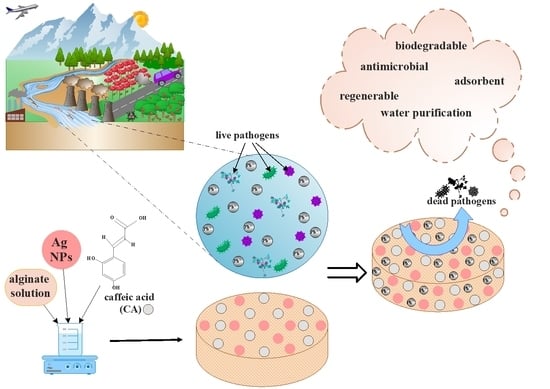
The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Methods
3. Results and Discussion
3.1. FTIR Spectroscopy and Microscopy
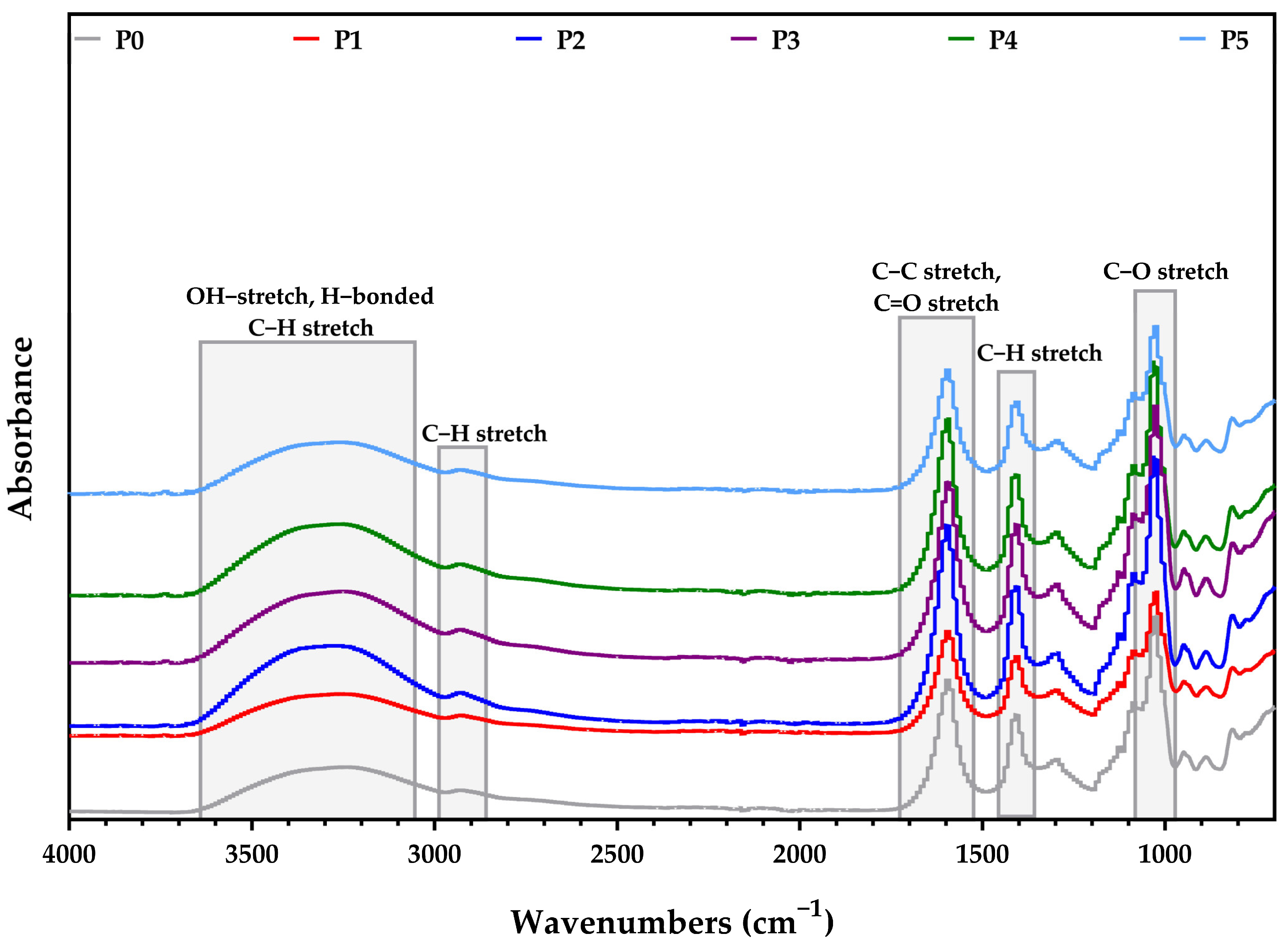
3.1.1. FTIR Spectroscopy
3.1.2. FTIR Microscopy
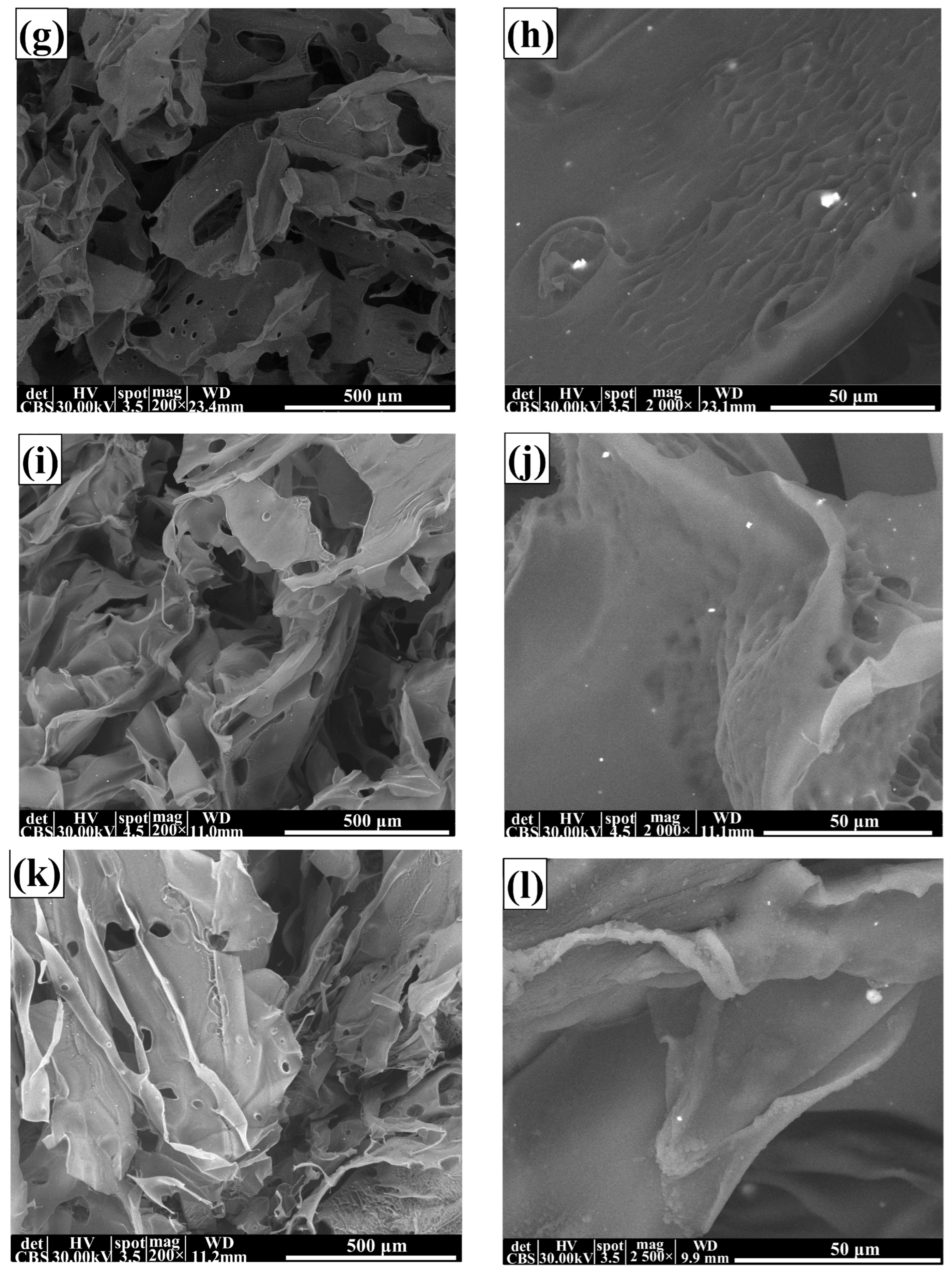
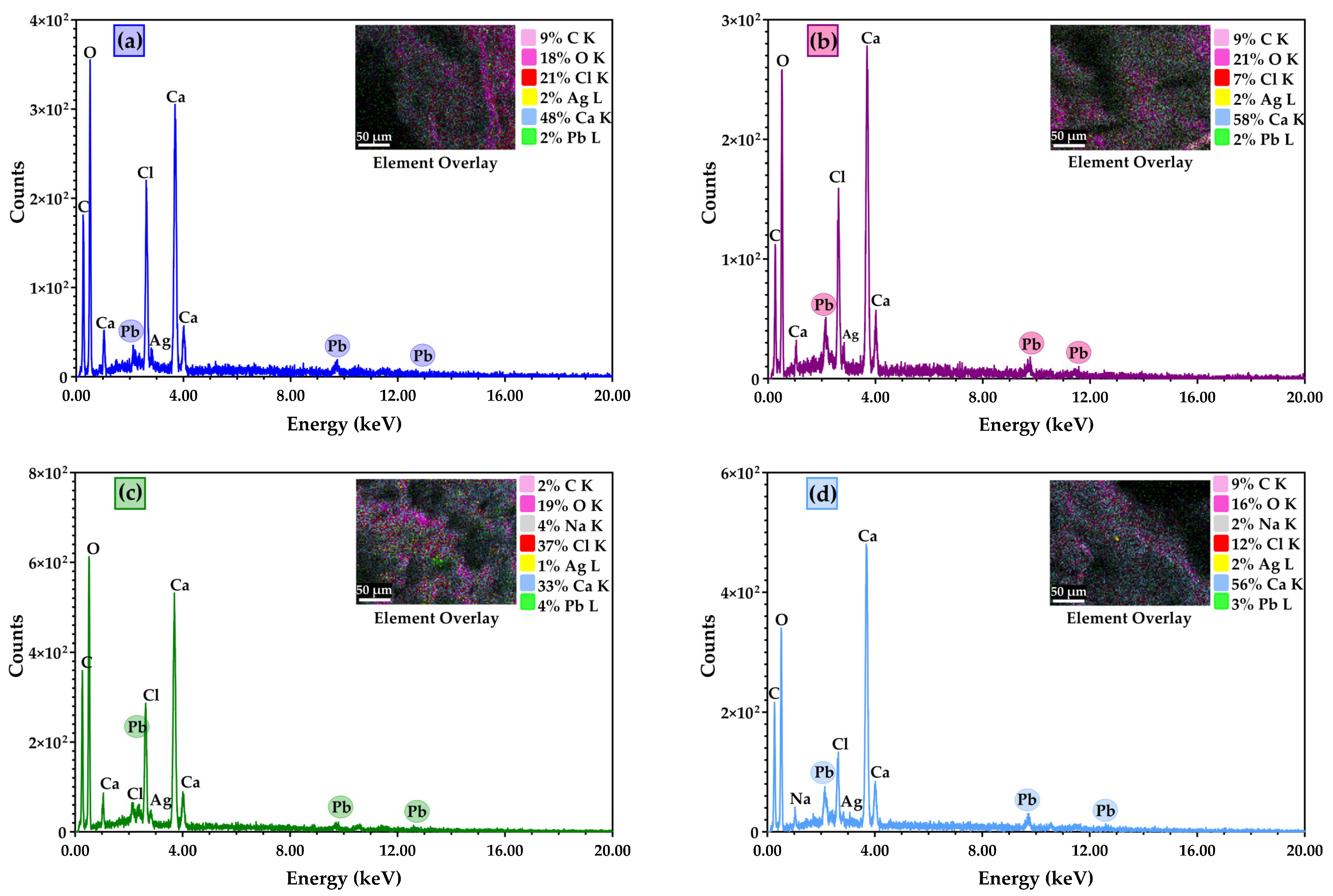
3.2. Scanning Electron Microscopy (SEM) Characterisation
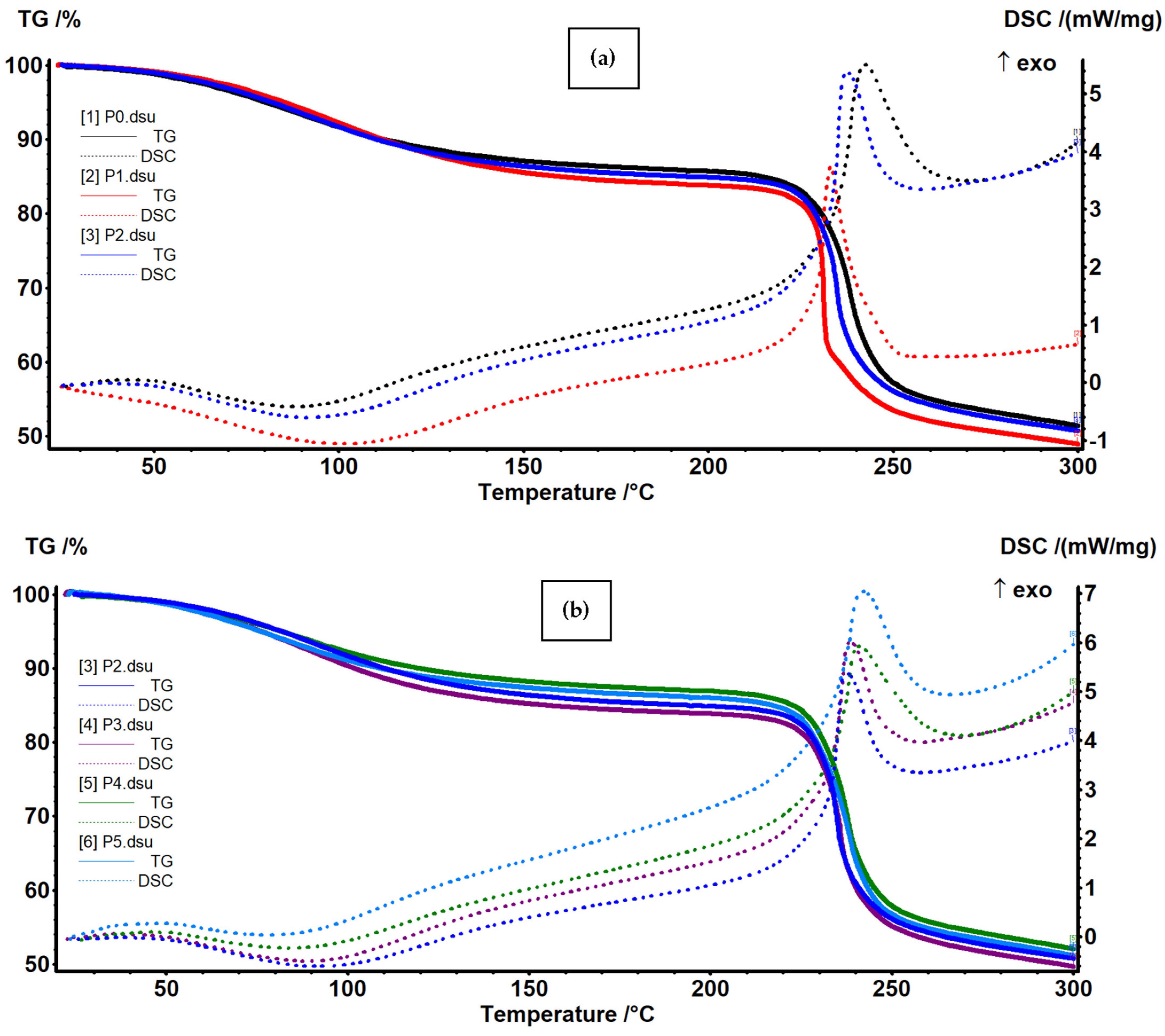
3.3. Thermal (TG-DSC) Analysis
3.4. Controlled Release of Polyphenol
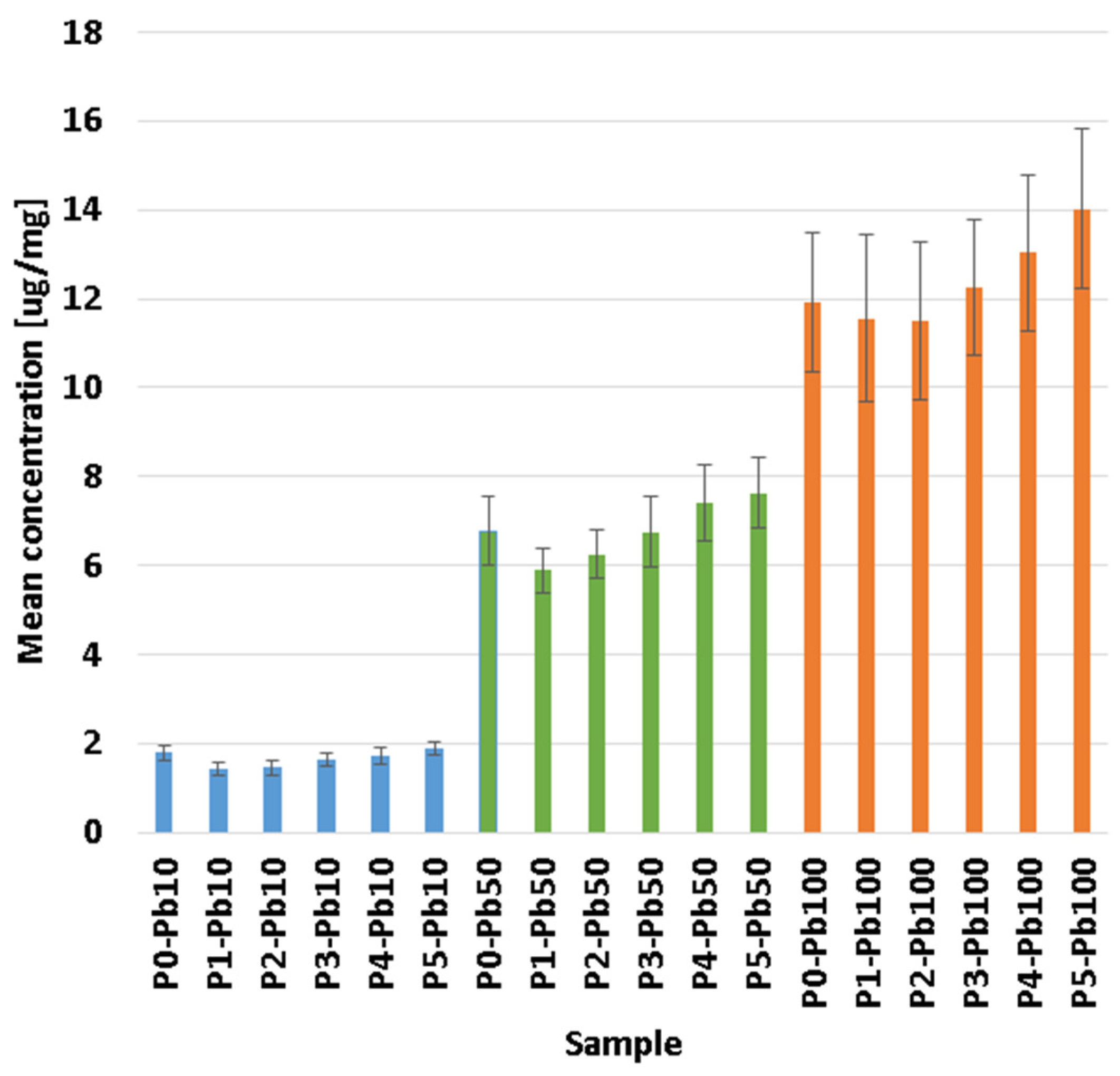
3.5. Removal Capacity of Lead Ions
3.6. Regeneration and Reusability Study
3.7. Sweeling/Adsorption Capacity
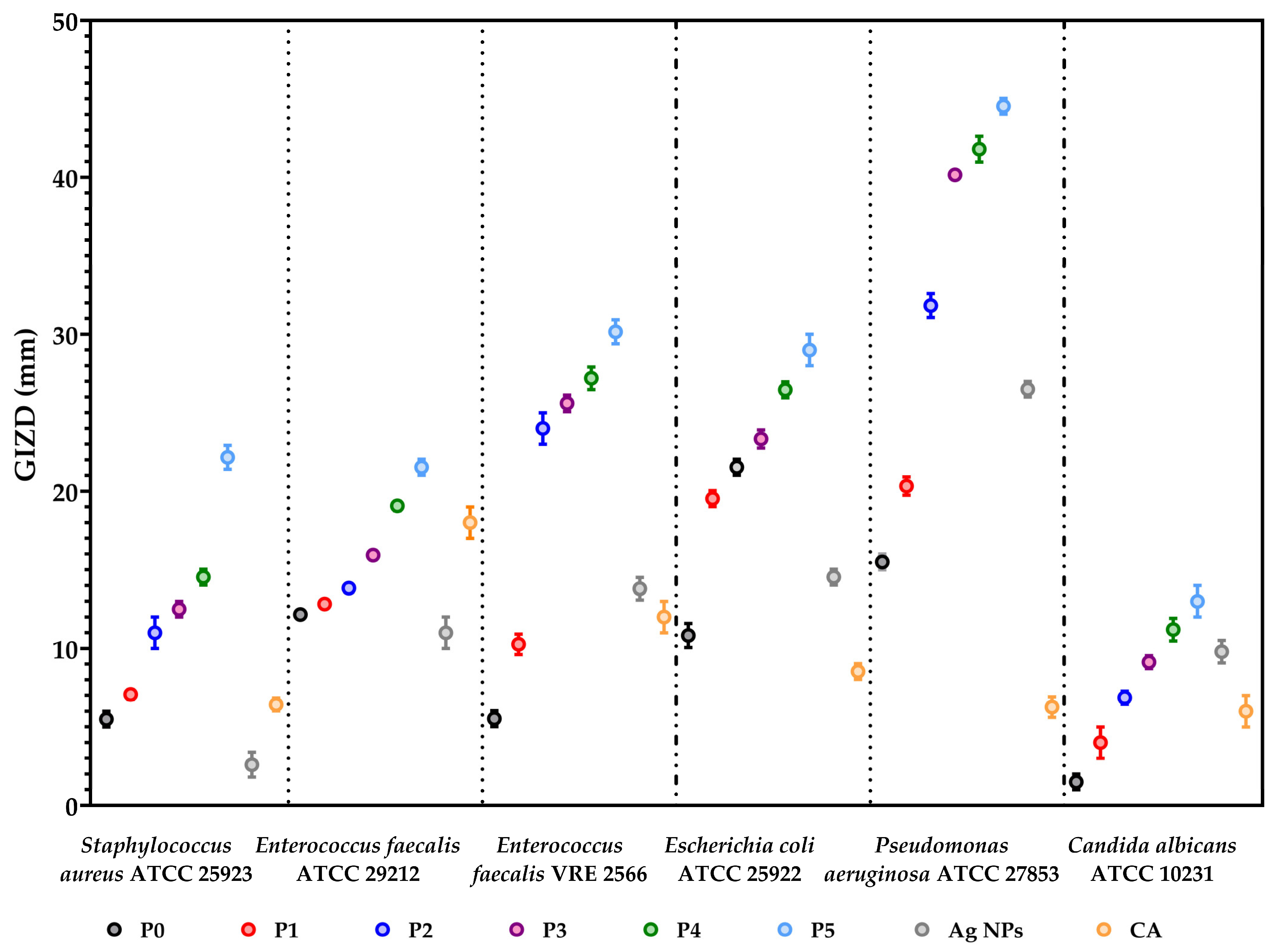
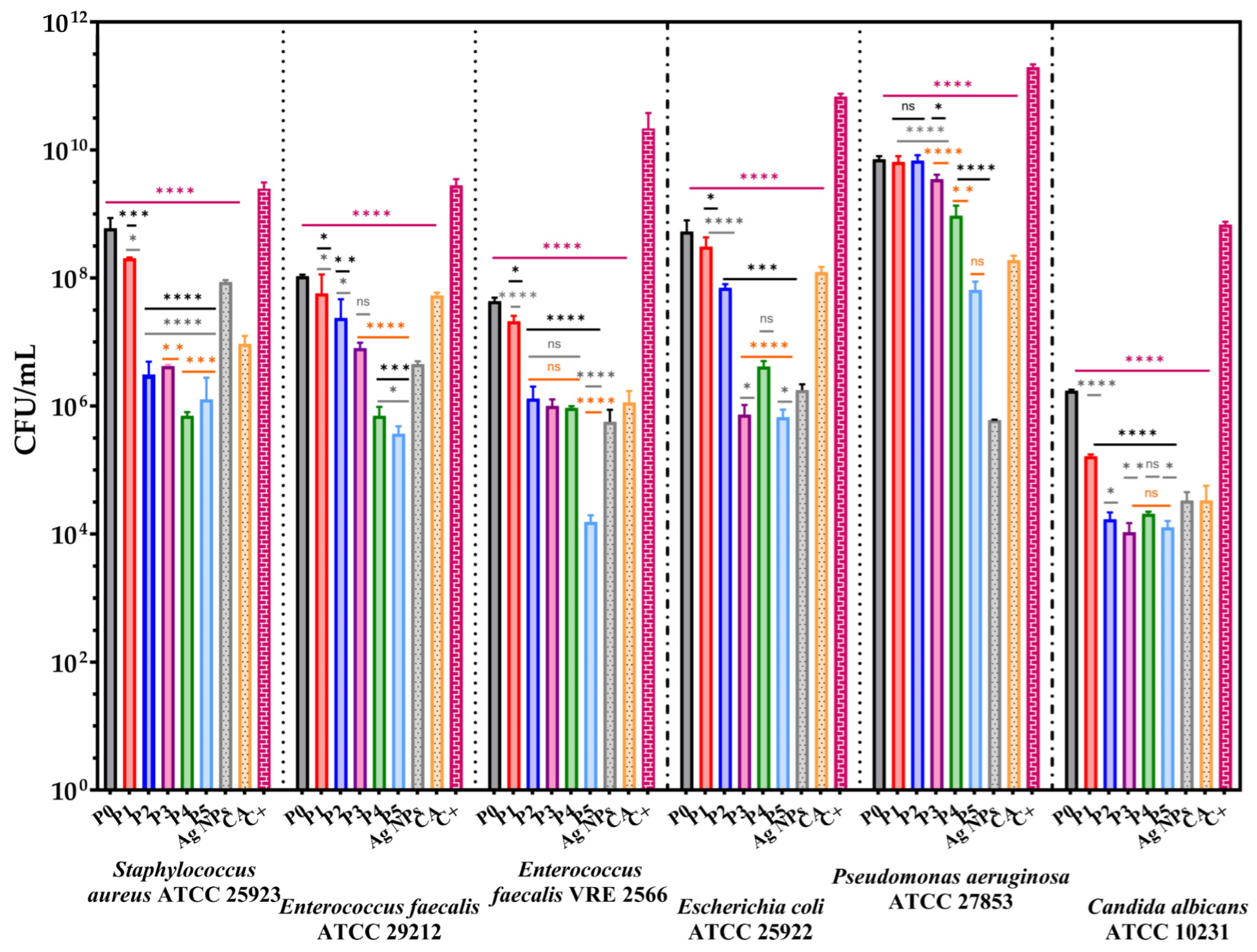
3.8. The Antimicrobial Assessments
3.8.1. Qualitative Evaluation of the Antimicrobial Activity
3.8.2. Quantitative Evaluation of the Anti-Adherence Capacity of the Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zare, E.N.; Motahari, A.; Sillanpaa, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced cu(ii) and pb(ii) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Al-Ashkar, E.A.; El-Ghaffar, M.A.A. Preparation and characterization of eco-friendly poly(p-phenylenediamine) and its composite with chitosan for removal of copper ions from aqueous solutions. Trans. Nonferrous Met. Soc. China 2015, 25, 3808–3819. [Google Scholar] [CrossRef]
- Mihaly, M.; Comanescu, A.F.; Rogozea, E.A.; Meghea, A. Nonionic microemulsion extraction of ni (ii) from wastewater. Mol. Cryst. Liq. Cryst. 2010, 523, 63–72. [Google Scholar] [CrossRef]
- Bhaumik, M.; Agarwal, S.; Gupta, V.K.; Maity, A. Enhanced removal of Cr(vi) from aqueous solutions using polypyrrole wrapped oxidized MWCNTS nanocomposites adsorbent. J. Colloid Interface Sci. 2016, 470, 257–267. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Wang, L.; Li, Z. Amino-functionalized mofs combining ceramic membrane ultrafiltration for Pb (ii) removal. Chem. Eng. J. 2016, 306, 619–628. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Lin, D.; Saroj, D.P.; Naz, I.; Khan, Z.M.; Sultan, M.; Ali, M. Encapsulated green magnetic nanoparticles for the removal of toxic Pb(2+) and Cd(2+) from water: Development, characterization and application. J. Environ. Manag. 2019, 234, 273–289. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Iflazoglu Mutlu, S.; Seven, I.; Arkali, G.; Ozer Kaya, S.; Kanmaz, O.E. Protective role of yeast beta-glucan on lead acetate-induced hepatic and reproductive toxicity in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 53668–53678. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, L.; Liang, Y.; Peng, D.; Aschner, M.; Jiang, Y. Signal transduction associated with lead-induced neurological disorders: A review. Food Chem. Toxicol. 2021, 150, 112063. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Shah, S.S.; Bhattacharya, J.; Subramaniam, K.; Pradeep Singh, N.D. Adsorption of Pb(ii) from aqueous solution using a magnetic chitosan/graphene oxide composite and its toxicity studies. Int. J. Biol. Macromol. 2018, 115, 1142–1150. [Google Scholar] [CrossRef]
- Wells, E.M.; Liu, Y.; Rolle-McFarland, D.; Mostafaei, F.; Zheng, W.; Nie, L.H. In vivo measurement of bone manganese and association with manual dexterity: A pilot study. Environ. Res. 2018, 160, 35–38. [Google Scholar] [CrossRef]
- Meng, H.; Wang, L.; He, J.; Wang, Z. The protective effect of gangliosides on lead (Pb)-induced neurotoxicity is mediated by autophagic pathways. Int. J. Environ. Res. Public Health 2016, 13, 365. [Google Scholar] [CrossRef]
- Agraz-Cibrian, J.M.; Delgado-Rizo, V.; Segura-Ortega, J.E.; Maldonado-Gomez, H.A.; Zambrano-Zaragoza, J.F.; Duran-Avelar, M.J.; Vibanco-Perez, N.; Fafutis-Morris, M. Impaired neutrophil extracellular traps and inflammatory responses in the peritoneal fluid of patients with liver cirrhosis. Scand. J. Immunol. 2018, 88, e12714. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Facile one pot green synthesis of chitosan-iron oxide (Cs-Fe2O3) nanocomposite: Removal of Pb(ii) and Cd(ii) from synthetic and industrial wastewater. J. Clean. Prod. 2018, 186, 342–352. [Google Scholar] [CrossRef]
- Filipoiu, D.C.; Bungau, S.G.; Endres, L.; Negru, P.A.; Bungau, A.F.; Pasca, B.; Radu, A.F.; Tarce, A.G.; Bogdan, M.A.; Behl, T.; et al. Characterization of the toxicological impact of heavy metals on human health in conjunction with modern analytical methods. Toxics 2022, 10, 716. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(iii) and Pb(ii) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef]
- Spoiala, A.; Ilie, C.I.; Dolete, G.; Croitoru, A.M.; Surdu, V.A.; Trusca, R.D.; Motelica, L.; Oprea, O.C.; Ficai, D.; Ficai, A.; et al. Preparation and characterization of chitosan/TiO(2) composite membranes as adsorbent materials for water purification. Membranes 2022, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.-I.; Dolete, G.; Trușcă, R.-D.; Motelica, L.; Oprea, O.-C.; Ficai, D.; Ficai, A.; Andronescu, E.; Dițu, L.-M. The development of antimicrobial chitosan/zno nanocomposite membranes for water purification. Rev. Română De Mater. Rom. J. Mater. 2022, 52, 17–25. [Google Scholar]
- Ahmad, R.; Mirza, A. Adsorption of Pb(ii) and Cu(ii) by alginate-au-mica bionanocomposite: Kinetic, isotherm and thermodynamic studies. Process Saf. Environ. Prot. 2017, 109, 1–10. [Google Scholar] [CrossRef]
- Enache, D.F.; Vasile, E.; Simonescu, C.M.; Culita, D.; Vasile, E.; Oprea, O.; Pandele, A.M.; Razvan, A.; Dumitru, F.; Nechifor, G. Schiff base-functionalized mesoporous silicas (MCM-41, HMS) as Pb(ii) adsorbents. Rsc Adv. 2018, 8, 176–189. [Google Scholar] [CrossRef]
- Enache, D.F.; Vasile, E.; Simonescu, C.M.; Razvan, A.; Nicolescu, A.; Nechifor, A.C.; Oprea, O.; Patescu, R.E.; Onose, C.; Dumitru, F. Cysteine-functionalized silica-coated magnetite nanoparticles as potential nano adsorbents. J. Solid State Chem. 2017, 253, 318–328. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Dragne, M.; Stanica, N.; Munteanu, C.; Preda, S.; Oprea, O. Effect of surfactant concentration on textural, morphological and magnetic properties of CoFe2O4 nanoparticles and evaluation of their adsorptive capacity for Pb(ii) ions. Ceram. Int. 2015, 41, 13553–13560. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Ficai, A.; Ficai, D.; Trusca, R.; Dolete, G.; Andronescu, E.; Turculet, S.C. Chitosan/graphene oxide nanocomposite membranes as adsorbents with applications in water purification. Materials 2020, 13, 1687. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Lateef, S.A. Review of adsorptive desulfurization process: Overview of the non-carbonaceous materials, mechanism and synthesis strategies. Fuel 2021, 294, 120273. [Google Scholar] [CrossRef]
- Kamar, F.H.; Nechifor, A.C.; Nechifor, G.; Al-Musawi, T.J.; Mohammed, A.H. Aqueous phase biosorption of Pb(ii), Cu(ii), and Cd(ii) onto cabbage leaves powder. Int. J. Chem. React. Eng. 2017, 15, 1–13. [Google Scholar]
- Choi, N.-C.; Cho, K.-H.; Kim, M.-S.; Park, S.-J.; Lee, C.-G. A hybrid ion-exchange fabric/ceramic membrane system to remove As(v), Zn(ii), and turbidity from wastewater. Appl. Sci. 2020, 10, 2414. [Google Scholar] [CrossRef]
- Yoon, S.; Cho, K.-H.; Kim, M.; Park, S.-J.; Lee, C.-G.; Choi, N.-C. Selenium removal from aqueous solution using a low-cost functional ceramic membrane derived from waste cast iron. Water 2023, 15, 312. [Google Scholar]
- Monier, M.; Abdel-Latif, D.A.; Mohammed, H.A. Synthesis and characterization of uranyl ion-imprinted microspheres based on amidoximated modified alginate. Int. J. Biol. Macromol. 2015, 75, 354–363. [Google Scholar] [PubMed]
- Simonescu, C.M.; Mason, T.J.; Calinescu, I.; Lavric, V.; Vinatoru, M.; Melinescu, A.; Culita, D.C. Ultrasound assisted preparation of calcium alginate beads to improve absorption of Pb + 2 from water. Ultrason. Sonochem. 2020, 68, 105191. [Google Scholar] [CrossRef] [PubMed]
- Mousa, N.E.; Simonescu, C.M.; Patescu, R.E.; Lavric, V.; Culita, D.C. Regeneration of calcium alginate and chitosan coated calcium alginate sorbents to be reused for lead (ii) removal from aqueous solutions. Rev. Chim. 2017, 68, 1992–1996. [Google Scholar] [CrossRef]
- Cordova, B.M.; Jacinto, C.R.; Alarcon, H.; Mejia, I.M.; Lopez, R.C.; de Oliveira Silva, D.; Cavalheiro, E.T.G.; Venancio, T.; Davalos, J.Z.; Valderrama, A.C. Chemical modification of sodium alginate with thiosemicarbazide for the removal of Pb(ii) and Cd(ii) from aqueous solutions. Int. J. Biol. Macromol. 2018, 120, 2259–2270. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles-An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2018, 49, 318–356. [Google Scholar] [CrossRef]
- Mihaly, M.; Lacatusu, I.; Enesca, I.A.; Meghea, A. Hybride nanomaterials based on silica coated C-60 clusters obtained by microemulsion technique. Mol. Cryst. Liq. Cryst. 2008, 483, 205–215. [Google Scholar] [CrossRef]
- Xiangliang, P.; Jianlong, W.; Daoyong, Z. Biosorption of Pb(ii) by pleurotus ostreatus immobilized in calcium alginate gel. Process Biochem. 2005, 40, 2799–2803. [Google Scholar]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef]
- Bee, A.; Talbot, D.; Abramson, S.; Dupuis, V. Magnetic alginate beads for Pb(ii) ions removal from wastewater. J. Colloid Interface Sci. 2011, 362, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Ugur Nigiz, F. Graphene oxide-sodium alginate membrane for seawater desalination through pervaporation. Desalination 2020, 485, 114465. [Google Scholar] [CrossRef]
- Aburabie, J.; Nassrullah, H.; Hashaikeh, R. Fine-tuning of carbon nanostructures/alginate nanofiltration performance: Towards electrically-conductive and self-cleaning properties. Chemosphere 2023, 310, 136907. [Google Scholar] [CrossRef]
- Aburabie, J.H.; Puspasari, T.; Peinemann, K.-V. Alginate-based membranes: Paving the way for green organic solvent nanofiltration. J. Membr. Sci. 2020, 596, 117615. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Yan, S.; Yin, X.; Chen, J. Development of alginate hydrogel modified multifunctional filtration membrane with robust anti-fouling property for efficient water purification. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 582, 123891. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Kanellopoulos, N.K. Prediction of binary adsorption isotherms of Cu(2+), Cd(2+) and Pb(2+) on calcium alginate beads from single adsorption data. J. Hazard. Mater. 2009, 162, 1347–1354. [Google Scholar] [CrossRef]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial chitosan based formulations with impact on different biomedical applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, P.; Wang, M.; Wu, G.; Sun, J.; Zhang, Y.; Dong, C.; Wu, A.G. Highly efficient removal of toxic Pb2+ from wastewater by an alginate-chitosan hybrid adsorbent. J. Chem. Technol. Biotechnol. 2018, 93, 2691–2700. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.C.; Vasile, B.S.; Ficai, A.; Andronescu, E.; Ficai, D.; Holban, A.M. Antibacterial activity of solvothermal obtained zno nanoparticles with different morphology and photocatalytic activity against a dye mixture: Methylene blue, rhodamine b and methyl orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the alcohols on the zno synthesis and its properties: The photocatalytic and antimicrobial activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Plotniece, A.; Sobolev, A.; Supuran, C.T.; Carta, F.; Bjoerkling, F.; Franzyk, H.; Yli-Kauhaluoma, J.; Augustyns, K.; Cos, P.; De Vooght, L.; et al. Selected strategies to fight pathogenic bacteria. J. Enzym. Inhib. Med. Chem. 2023, 38, 2155816. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Q.; Luo, H.Y.; Rong, H.W.; Wu, S.H.; Zheng, Z.X.; Chen, B.Y. Calcium alginate gels-functionalized polyurethane foam decorated with silver nanoparticles as an antibacterial agent for point-of-use water disinfection. Int. J. Biol. Macromol. 2023, 231, 123289. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Ene, V.L.; Vasile, B.S.; Andronescu, E.; Holban, A.M. Antibacterial biodegradable films based on alginate with silver nanoparticles and lemongrass essential oil-innovative packaging for cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Spoiala, A.; Ficai, D.; Ficai, A.; Craciun, L.; Titu, M.A.; Andronescu, E. Towards synthesis-derived applications of silver nanoparticles. Adv. Mater. Tech. Env. 2021, 5, 337–356. [Google Scholar]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-based synergistic antimicrobial composites. A critical review. Nanomaterials 2021, 11, 1687. [Google Scholar] [CrossRef] [PubMed]
- Stavinskay, O.; Laguta, I.; Kuzema, P.; Skorochod, I.; Roy, A.; Kurdish, I. Preparation of composite based on caffeic acid and fumed silica and evaluation of its antioxidant and antimicrobial properties Br. Chem. J. Mold. 2022, 17, 43–49. [Google Scholar] [CrossRef]
- Zeren, S.; Sahin, S.; Sumnu, G. Encapsulation of Caffeic Acid in Carob Bean Flour and Whey Protein-Based Nanofibers via Electrospinning. Foods 2022, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Kepa, M.; Miklasinska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smolen-Dzirba, J.; Wasik, T.J. Antimicrobial potential of caffeic acid against staphylococcus aureus clinical strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.A.; Voicu, G.; Oprea, O.C.; Ficai, A.; Andronescu, E. New mesoporous silica materials loaded with polyphenols: Caffeic acid, ferulic acid and p-coumaric acid as dietary supplements for oral administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef]
- Enaru, B.; Socaci, S.; Farcas, A.; Socaciu, C.; Danciu, C.; Stanila, A.; Diaconeasa, Z. Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals 2021, 14, 946. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/caffeic acid hybrid materials: Synthesis, spectroscopic characterization, and bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Espindola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Craioveanu, M.G.; Stoica, L.; Constantin, C.; Oprea, O. Cr(iii)aq separation by flotation with multipolar collector. Sep. Sci. Technol. 2020, 55, 346–357. [Google Scholar] [CrossRef]
- Boilet, L.; Cornard, J.P.; Lapouge, C. Determination of the chelating site preferentially involved in the complex of lead(ii) with caffeic acid: A spectroscopic and structural study. J. Phys. Chem. A 2005, 109, 1952–1960. [Google Scholar] [CrossRef]
- Sharma, S.; Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Fabrication of antibacterial silver nanoparticle—Sodium alginate–chitosan composite films. Rsc Adv. 2012, 2, 5837–5843. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Bellala, S.; Chintha, M.; Sake, A.; Subbarao, S.M.C.; Lai, W.F. Synthesis and properties of alginate-based nanoparticles incorporated with different inorganic nanoparticulate modifiers for enhanced encapsulation and controlled release of favipiravir. Arab. J. Chem. 2023, 16, 104751. [Google Scholar] [CrossRef]
- Lemnaru Popa, G.M.; Trusca, R.D.; Ilie, C.I.; Tiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Ditu, L.M. Antibacterial activity of bacterial cellulose loaded with bacitracin and amoxicillin: In vitro studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Laz, R.V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690. [Google Scholar] [CrossRef]
- Cotar, A.I.; Grumezescu, A.M.; Andronescu, E.; Voicu, G.; Ficai, A.; Ou, K.-L.; Huang, K.-S.; Chifiriuc, M.C. Nanotechnological solution for improving the antibiotic efficiency against biofilms developed bygram-negative bacterial strains. Lett. Appl. NanoBioSci. 2013, 2, 97–104. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; p. 352. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Trusca, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative antimicrobial chitosan/ZnO/Ag NPs/citronella essential oil nanocomposite-potential coating for grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Spoiala, A.; Ilie, C.I.; Trusca, R.D.; Oprea, O.C.; Surdu, V.A.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Ditu, L.M. Zinc oxide nanoparticles for water purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Stojkovska, J.; Obradović, B.; Mišković-Stanković, V. Alginate hydrogel microbeads incorporated with Ag nanoparticles obtained by electrochemical method. Mater. Chem. Phys. 2012, 133, 182–189. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparations and characterization of alginate/silver composite films: Effect of types of silver particles. Carbohydr. Polym. 2016, 146, 208–216. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Alvarez-Ramirez, J.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Hernandez-Marin, N.Y. Characterization of corn starch-calcium alginate xerogels by microscopy, thermal, XRD, and FTIR analyses. Starch-Starke 2021, 73, 2000282. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil—A novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb(ii) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 119585. [Google Scholar] [CrossRef]
- Li, K.; Wu, G.; Wang, M.; Zhou, X.; Wang, Z. Efficient removal of lead ions from water by a low-cost alginate-melamine hybrid sorbent. Appl. Sci. 2018, 8, 1518. [Google Scholar] [CrossRef]
- Belalia, F.; Djelali, N.E. Investigation of swelling/adsorption behavior of calcium alginate beads. Rev. Roum. Chim. 2016, 61, 747–754. [Google Scholar]
- Lopusiewicz, L.; Macieja, S.; Sliwinski, M.; Bartkowiak, A.; Roy, S.; Sobolewski, P. Alginate biofunctional films modified with melanin from watermelon seeds and zinc oxide/silver nanoparticles. Materials 2022, 15, 2381. [Google Scholar] [CrossRef]
- Bibi, A.; Ur-Rehman, S.; Akhtar, T.; Akhter, K.; Rafique, S.; Faiz, R. Synthesis of alginate-based nanocomposites: A novel approach to antibacterial films. Chem. Pap. 2022, 76, 3425–3435. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, C.; Zhai, S.R.; An, Q.D. Nanosilver anchored alginate/poly(acrylic acid/acrylamide) double-network hydrogel composites for efficient catalytic degradation of organic dyes. Front. Chem. Sci. Eng. 2023, 17, 1–13. [Google Scholar] [CrossRef]
- Kanagaraj, S.S.P.; Rajaram, S.K.; Ahamed, M.; Subedhar, S.; Sankar, K.; Innasimuthu, G.M.; Karuppiah, P. Antimicrobial activity of green synthesized biodegradable alginate–silver (Alg-Ag) nanocomposite films against selected foodborne pathogens. Appl. Nanosci. 2021, 13, 651–662. [Google Scholar] [CrossRef]
- Susilowati, E.; Mahardiani, L.; Hardini, R.D. The effect of silver nanoparticles toward properties and antibacterial activity of silver-alginate nanocomposite films. Front. Sustain. Food Syst. 2022, 6, 293. [Google Scholar] [CrossRef]
- Mahapatra, A.; Padhi, N.; Mahapatra, D.; Bhatt, M.; Sahoo, D.; Jena, S.; Dash, D.; Chayani, N. Study of biofilm in bacteria from water pipelines. J. Clin. Diagn. Res. JCDR 2015, 9, DC09–DC11. [Google Scholar] [CrossRef]
- Maes, S.; Vackier, T.; Nguyen Huu, S.; Heyndrickx, M.; Steenackers, H.; Sampers, I.; Raes, K.; Verplaetse, A.; De Reu, K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019, 19, 77. [Google Scholar] [CrossRef]
- Vanaja, M.; Gnanajobitha, G.; Paulkumar, K.; RajeshKumar, S.; Malarkodi, C.; Annadurai, G. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J. Nanostruct. Chem. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Kedziora, A.; Wieczorek, R.; Speruda, M.; Matolinova, I.; Goszczynski, T.M.; Litwin, I.; Matolin, V.; Bugla-Ploskonska, G. Comparison of antibacterial mode of action of silver ions and silver nanoformulations with different physico-chemical properties: Experimental and computational studies. Front. Microbiol. 2021, 12, 659614. [Google Scholar] [CrossRef]
- Pavlikova, N. Caffeic acid and diseases-mechanisms of action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Lee, W.-F.; Tsao, K.-T. Effect of silver nanoparticles content on the various properties of nanocomposite hydrogels by in situ polymerization. J. Mater. Sci. 2010, 45, 89–97. [Google Scholar] [CrossRef]
- Sharmin, N.; Pang, C.; Sone, I.; Walsh, J.L.; Fernandez, C.G.; Sivertsvik, M.; Fernandez, E.N. Synthesis of sodium alginate-silver nanocomposites using plasma activated water and cold atmospheric plasma treatment. Nanomaterials 2021, 11, 2306. [Google Scholar] [CrossRef] [PubMed]
- Zakia, M.; Koo, J.M.; Kim, D.; Ji, K.; Huh, P.; Yoon, J.; Yoo, S.I. Development of silver nanoparticle-based hydrogel composites for antimicrobial activity. Green Chem. Lett. Rev. 2020, 13, 34–40. [Google Scholar] [CrossRef]







| Code | Sample Name | Alginate (g) | Ag NPs (mg) | Ag NPs (%) | Caffeic Acid (mg) | Caffeic Acid (%) | Water (mL) |
|---|---|---|---|---|---|---|---|
| P0 | Alg 3% | 1.5 | 0 | 0 | 0 | 0 | 50 |
| P1 | Alg/Ag NPs1 | 1.5 | 5 | 0.33 | 0 | 0 | 50 |
| P2 | Alg/Ag NPs2 | 1.5 | 10 | 0.66 | 0 | 0 | 50 |
| P3 | Alg/Ag NPs/CA1 | 1.5 | 5 | 0.33 | 2 | 0.13 | 50 |
| P4 | Alg/Ag NPs/CA2 | 1.5 | 5 | 0.33 | 10 | 0.66 | 50 |
| P5 | Alg/Ag NPs/CA3 | 1.5 | 5 | 0.33 | 20 | 1.31 | 50 |
| Sample/Assignment | P0 | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|---|
| O-H and H-bonded (alcohols, phenols) | 3253 | 3252 | 3272 | 3253 | 3260 | 3254 |
| C-H stretch (aromatic) | - | - | - | 3050 | 3054 | 3055 |
| C-H stretch (alkanes) | 2923 | 2927 | 2927 | 2930 | 2925 | 2925 |
| C=O stretch | - | - | - | 1640 | 1645 | 1645 |
| C-C stretch | 1593 | 1596 | 1596 | 1592 | 1596 | 1595 |
| C-O stretch (alcohols, carboxylic acids, esters, ethers) | 1031 | 1027 | 1027 | 1025 | 1028 | 1027 |
| Sample | RT-210 °C | 210–270 °C | 270–360 °C | 360–600 °C | Residual Mass |
|---|---|---|---|---|---|
| P0 | 14.72% | 31.41% | 8.14% | 20.38% | 20.61% |
| P1 | 16.54% | 32.33% | 6.07% | 19.89% | 21.41% |
| P2 | 15.47% | 31.43% | 7.17% | 20.72% | 21.93% |
| P3 | 16.46% | 31.42% | 7.72% | 20.05% | 20.52% |
| P4 | 13.54% | 31.85% | 16.96% | 11.39% | 20.10% |
| P5 | 14.39% | 31.85% | 18.72% | 9.90% | 20.00% |
| Sample | CaCl2 1% | CaCl2 10% | ||
|---|---|---|---|---|
| Amount Pb in Solution [µg] | Amount Pb in Membrane [µg] | Amount Pb in Solution [µg] | Amount Pb in Membrane [µg] | |
| P1 | 103.42 ± 0.28 | 133.01 ± 12.80 | 195.71 ± 1.73 | 41.05 ± 13.20 |
| P2 | 98.09 ± 0.26 | 120.12 ± 13.30 | 187.62 ± 1.66 | 31.01 ± 2.90 |
| P3 | 102.59 ± 0.10 | 128.11 ± 32.40 | 201.01 ± 1.63 | 29.12 ± 11.70 |
| P4 | 108.37 ± 0.15 | 114.03 ± 14.40 | 211.57 ± 1.59 | 11.21 ± 10.30 |
| P5 | 115.48 ± 0.03 | 131.05 ± 12.40 | 227.65 ± 1.41 | 19.02 ± 7.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoială, A.; Ilie, C.-I.; Dolete, G.; Petrișor, G.; Trușcă, R.-D.; Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.-C.; Dițu, M.-L. The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification. Membranes 2023, 13, 591. https://doi.org/10.3390/membranes13060591
Spoială A, Ilie C-I, Dolete G, Petrișor G, Trușcă R-D, Motelica L, Ficai D, Ficai A, Oprea O-C, Dițu M-L. The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification. Membranes. 2023; 13(6):591. https://doi.org/10.3390/membranes13060591
Chicago/Turabian StyleSpoială, Angela, Cornelia-Ioana Ilie, Georgiana Dolete, Gabriela Petrișor, Roxana-Doina Trușcă, Ludmila Motelica, Denisa Ficai, Anton Ficai, Ovidiu-Cristian Oprea, and Mara-Lia Dițu. 2023. "The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification" Membranes 13, no. 6: 591. https://doi.org/10.3390/membranes13060591
APA StyleSpoială, A., Ilie, C.-I., Dolete, G., Petrișor, G., Trușcă, R.-D., Motelica, L., Ficai, D., Ficai, A., Oprea, O.-C., & Dițu, M.-L. (2023). The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification. Membranes, 13(6), 591. https://doi.org/10.3390/membranes13060591